Abstract
Cytochrome P450 epoxygenase catalyzes 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acids (EETs) from arachidonic acid (AA). In 1996 our group identified the expression of the cytochrome P450 2C11 epoxygenase (CYP epoxygenase) gene in astrocytes. Because of our finding an array of physiological functions have been attributed to EETs in the brain, one of the actions of EETs involves a predominant role in brain angiogenesis. Blockade of EETs formation with different epoxygenase inhibitors decreases endothelial tube formation in cocultures of astrocytes and capillary endothelial cells. The intent of this investigation was to determine if pharmacologic inhibition of formation of EETs is effective in reducing capillary formation in glioblastoma multiforme with a concomitant reduction in tumor volume and increase in animal survival time. Two mechanistically different inhibitors of CYP epoxygenase, 17-octadecynoic acid (17-ODYA) and miconazole, significantly reduced capillary formation and tumor size in glial tumors formed by injection of rat glioma 2 (RG2) cells, also resulting in an increased animal survival time. However, we observed that 17-ODYA and miconazole did not inhibit the formation of EETs in tumor tissue. This implies that 17-ODYA and miconazole appear to exert their antitumorogenic function by a different mechanism that needs to be explored.
Keywords: angiogenesis, CYP epoxygenase, glioblastoma multiforme
Introduction
In the last 10 years, we have demonstrated that epoxyeicosatrienoic acids (EETs) are made within astrocytes in the brain; promote blood vessel formation and angiogenesis and vasodilation in the rat brain. Epoxyeicosatrienoic acids are formed by the catalytic conversion of arachidonic acid (AA) via cytochrome P450 (CYP) 2C11 enzymes in rat astrocytes (Alkayed et al. 1996) and by CYP 4X1 in rat brain (Bylund et al. 2002). Four regioisomers of EETs are formed from AA namely: 5,6-, 8,9-, 11,12-, and 14,15-EET. All regioisoforms are capable of supporting angiogenesis, however, our previous findings suggest that 8,9 and 11,12-EET are the most potent (Zhang and Harder 2002). The ability of EETs to induce angiogenesis is not limited to the brain. Epoxyeicosatrienoic acids have been shown to be angiogenic in human lung microvascular endothelial cells and other vascular beds (Medhora et al. 2003; Michaelis et al. 2003; Zhang and Harder 2002). We have previously described that formation of EETs in astrocytes are induced by glutamate or neural activation (Alkayed et al. 1997; Peng et al. 2002, 2004). Inhibition of EETs with mechanistically different cytochrome P450 2C11 epoxygenase (CYP epoxygenase) inhibitors blocks formation of capillaries or endothelial tube like structures in vitro (Munzenmaier and Harder 2000; Zhang and Harder 2002) and inhibits the increase in cerebral blood flow in vivo in response to neural activation (Peng et al. 2002; Bhardwaj et al. 2000). The mechanism by which EETs induce endothelial mitogenic activity appears to involve the antagonism of tyrosine kinase pathway and occurs independent of VEGF (Zhang and Harder 2002). Given the central role of EETs in astrocyte-mediated angiogenesis the studies described here were designed to test the hypothesis that CYP-derived EETs are essential in the development of nutritive capillary growth in tumors, and that inhibition of CYP epoxygenase in the brain results in tumor suppression specifically related to the formation of glioblastoma multiforme.
It is estimated that there are over 350,000 persons in the United States diagnosed with a brain tumor of which, gliomas account for approximately 50%. Of the individuals with central nervous system tumors, approximately 10–15% are malignant. Brain tumors are the leading cause of death from childhood cancer up to age 19 years and is the second leading cause of cancer-related deaths in males 20 to 39 years old (Statistics obtained from: www.abc2.org/statistics.shtml). Malignant gliomas are very aggressive tumors of the central nervous system and are resistant to conventional therapies like radiation and chemotherapy (Rich and Bigner 2004).
Rat glioma 2 (RG2) cells are non-differentiated astrocytes which form aggressive tumors when injected into the forebrain of Fischer rats. In this study glioblastomas were formed experimentally in the forebrain of male rats via direct injection of RG2 cells. Two mechanistically different CYP enzyme inhibitors were used in the study: 17-octadecynoic acid (17-ODYA) and miconazole, both of which have been shown in previous publications to block formation of EETs in brain tissue (Alkayed et al. 1996; Bhardwaj et al. 2000; Peng et al. 2002).
Materials and Methods
Materials
Male 8–10 week old Fischer rats were purchased from Taconic Inc, Hudson, NY. All the animal studies were approved by the Institutional Animal Care Committee and were carried out according to the guidelines of the NIH. Rat glioma 2 cells were obtained from ATCC (Manassa, VA, USA); 17-ODYA from Biomol (Plymouth Meeting, PA, USA); miconazole and all other chemicals and reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Culture media, fetal bovine serum, and trypsin-EDTA (ethylenediaminetetraacetic acid) and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). Polyclonal CYP2C11 antibody was raised in rabbits in our laboratory against the synthetic peptides derived from both the amino and carboxy terminals of CYP2C11 (Alkayed et al. 1996). Horseradish peroxidase - conjugated goat anti rabbit IgG and goat anti mouse IgG were purchased from Bio-Rad (Hercules, CA, USA). β-Actin antibody was purchased from Sigma-Aldrich. ECL plus reagent was obtained from GE healthcare (Buckinghamshire, UK).
Methods
Rat glioma 2 cell culture conditions
The RG2 cell line used in this study was obtained from ATCC and cultured according to the manufacturer’s specification in Dulbecco’s modified Eagle’s medium containing 10% FBS and 1% penicillin- streptomycin and maintained at 37°C in a humidified incubator containing 5% CO2.
Development of brain tumor
Intracranial tumors were induced with RG2 cells in syngenic 8–10 week old male Fischer rats (Barth 1998). After RG2 cells reached 85–90% confluency they were lifted with 1X trypsin-EDTA solution and resuspended in artificial cerebrospinal fluid (aCSF; mmol/L: 2.9 KCl, 38 MgCl2, 1.99 CaCl2, 131.9 NaCl, 19 NaHCO3, 3.69 glucose, with pH adjusted daily to 7.4.) in concentrations of 106 cells/ml and kept on ice until implantation. Cells were orthotopically implanted in the right frontal lobe of Fischer rats. Briefly, anesthesia was induced using 4% isoflurane and maintained at 2% using a gas anesthesia mask for small rodents (model 51610, Stoelting, Wood Dale, IL, USA), and placed in prone position in stereotaxic apparatus (model 900; David Kopf Instruments, Tujunga, CA, USA). The head was fixed with ear bars and the operative field prepared with saline, alcohol and iodine solution. After midline incision of scalp skin, underlying tissue was removed and parietal bone was exposed. A small burr hole was drilled in the parietal bone using air powered low-speed dental drill and dura was exposed. Rat glioma 2 cells were resuspended in aCSF and 5 μl of cells were slowly injected using Hamilton microsyringe attached to the stereotaxic arm for more than 10 minutes in subcortical area of right frontal lobe using stereotaxic coordinates (1 mm anterior, 2.5 mm lateral and 4.0 to 4.5 mm deep from Bregma). After, completion of injection, the needle was held in place for an additional 5 min and slowly withdrawn to prevent backflow from the injection site. The opening in the parietal bone was filled with sterile bone wax and skin was sutured. After completion of injection, the surgical site was additionally treated with alcohol/iodine and animals received a single intramural injection of medetomidine hydrochloride (60 μg/kg) for recovery.
Topical/intratumoral application of drugs
To achieve a constant concentration of drug in developing tumors and nearby tissue we chose intratumoral application of drugs via mini osmotic pumps and brain infusion kit (Alzet, model 1002, Durect Corporation, Cupertini, CA, USA). Three days after initial surgery, animals were briefly anesthetized with isoflurane inhalation anesthesia and mini osmotic pumps were implanted subcutaneously through a skin incision in the middle of the skull, after which a pocket was formed over the neck and shoulder blades to hold the mini pump. The tip of infusion catheter was positioned 3.5 mm below the dura at the place of initial injection site for the delivery of RG2 cells and secured to the bone by vetbond. The animals were divided into three groups and all 3 groups were injected with RG2 cells. Three days after injection of tumor cells group 1 received vehicle (10% ethanol), group 2 received 17-ODYA and group 3 received miconazole via the implanted osmotic mini pumps. Miconazole and 17-ODYA stock solutions were made at a concentration of 3 mmol/L in ethanol and then diluted with aCSF to a final concentration of 0.3 mmol/L and filled into the pumps. The filled mini pumps were incubated overnight in sterile saline at 37°C and then implanted in the brain as described above. The pump delivered the CYP epoxygenase inhibitors at a rate of 0.5 μl/h for 14 days.
Measurement of tumor volume
Cancer cells from initial site of injection grew in all directions and formed tumors that were ellipsoid in shape. Volume of tumor was calculated by determining all 3 dimensions of tumor using the formula: 4/3π r1 × r2 × r3. (r1, r2 and r3 represent length, width and depth of tumor, respectively).
Determination of CYP activity
Fifteen to seventeen days after implantation of the RG2 cells, all three groups, that is, vehicle-, 17-ODYA-, and miconazole-treated rats were euthanized with pentobarbital (100 mg/kg intraperitoneally). The brain was removed and the tumor and normal tissues were excised out and flash frozen in liquid nitrogen and stored separately at −80 °C until further use. On the day of the analysis the samples were homogenized in 10 mmol/L phosphate buffer pH 7.7 containing 250 mmol/L sucrose, 1 mmol/L EDTA and protease inhibitors and the homogenate was centrifuged at 4°C for 20 min at 3000 g to remove the cell debris and the supernatant was used for the detection of CYP activity. The protein content of the homogenate was determined by the Bio-Rad protein assay. The homogenate containing 2 mg of total protein was incubated in 100 mmol/L phosphate buffer pH 7.4 containing 5 mmol/L MgCl2 1 mmol/L EDTA and 1 mmol/L nicotinamide adenine dinucleotide phosphate in the presence of NADPH regenerating system (10 mmol/L isocitrate and 0.4 U/ml isocitrate dehydrogenase and AA (40 μmol/L) at 37°C for 30 min. The reaction was stopped by the addition of 1 mol/L formic acid and the metabolites were extracted with diethylether after addition of 5 ng of internal standard (either 14,15-epoxyeicosa-5(Z)-enoic-methyl-sulfonylimide (EEZE) or a mixture of deuterated EETs and HETEs). The ether layer was dried under a stream of nitrogen gas and reconstituted with 50% acetonitrile and cleaned using an online reverse–phase high performance liquid chromatography trapping column followed by the separation of HETEs and EETs using an isocratic step gradient on a C18 reverse phase micro bore column (150 × 213 μm Thermo Hypersil-Keystone, Bellefonte, PA, USA) using a mobile phase consisting of acetonitrile/water/acetic acid (57:43:0.1) for 20 min to resolve the HETEs followed by acetonitrile/water/acetic acid (63:37:0.1) for 15 minutes to resolve EETs. Samples were ionized using negative ion electrospray, and the peaks monitored in the selective ion mass spectroscopy mode using an Agilent LSD ion trap mass spectrometer 1100 (Agilent Technologies, Palo Alto, CA, USA) in the core laboratories of Physiology and Pharmacology and Toxicology, Medical College of Wisconsin. The ratio of ion abundances in the peak of interest (HETEs and EETs; m/z 319) versus that corresponding to the closely eluting internal standard (EEZE; m/z 323) or the corresponding deuterated standards were used for the quantitation of HETEs and EETs in the samples.
Western blot analysis
The homogenates obtained from the normal and tumor tissues were analyzed by Western blotting for the expression level of CYP 2C11. Typically, 50 μg of total protein was separated by electrophoresis in a 10 % sodium dodecyl sulphate -polyacrylamide gel. The separated proteins were transferred to a nitrocellulose membrane. The membranes were blocked overnight in a blocking buffer (10% non fat dry milk in Tris-buffered saline containing 0.1% Tween-20; TBST) followed by incubation in primary antibody (anti-P450 2C11 at 1:5000 dilution in 2% non fat dry milk in TBST) for 2 h at room temperature. The membranes were washed three times in wash buffer (TBST) and then incubated in secondary antibody (goat anti-rabbit horseradish peroxidase at 1:5000 dilution in 2% non fat dry milk in TBST) for 30 minutes at room temperature. The membrane was then washed three times in TBST, and chemiluminescence detection was performed using ECL plus reagent (GE Healthcare) according to the manufacturer’s protocol. The membranes were then probed with anti-β-actin (1:5000 dilution) for 1 h followed by goat anti-mouse horseradish peroxidase secondary antibody (1:5000 dilution) and treatment with ECL reagent for loading control.
Visualization of tumor vasculature and comparison of capillary density Fluorescein isothiocyanate–conjugated dextran method
Tumor vasculature was visualized by whole animal perfusion with fluorescein isothiocyanate (FITC)-conjugated dextran, of molecular weight 148 kDa (Sigma Chemical Co., St. Louis, MO, USA). Briefly, before killing, animals were anesthetized with isoflurane and perfused via femoral vein with 25 mg FITC-conjugated dextran dissolved in 1 ml of saline solution (bolus injection). Dextran was allowed to circulate 30 to 45 secs before decapitation. The brain was quickly removed, snap frozen in dry ice and later sectioned with cryostat. Five micron thick sections were analyzed with epifluorescence and quantitated using MetaVue software (Meta Imaging Series version 6.1 CD).
Survival studies
Rats were implanted with RG2 cells and at day 3 they received vehicle, 17-ODYA, or miconazole diluted in aCSF via mini osmotic pumps. Rats were monitored daily for weight loss, signs of toxicity, and neurological dysfunctions. Rats were killed when they exhibited loss of mobility, abnormal breathing, lethargy or discharge from the eyes.
Statistical analysis
Mean values ± s.e. are presented. Differences between groups were analyzed using analysis of variance with Holm-Sidak post hoc test. A P-value <0.05 was considered to be statistically significant. For survival studies, Kaplan-Meier Survival Analysis was performed using multiple comparison procedures of Holm-Sidak method, and a P < 0.05 was considered significant.
Results
Formation and Characterization of Tumor
Intracranial tumors formed in all subject animals in about 11 days. Furthermore, all animals died from massive intracranial tumors suggesting that RG2 cells are a good model for induction of glioblastoma multiforme. No infection of surgical sites was noted. We observed that onset of symptoms such as body weight loss and neurological deficits were delayed in the groups treated with CYP inhibitors.
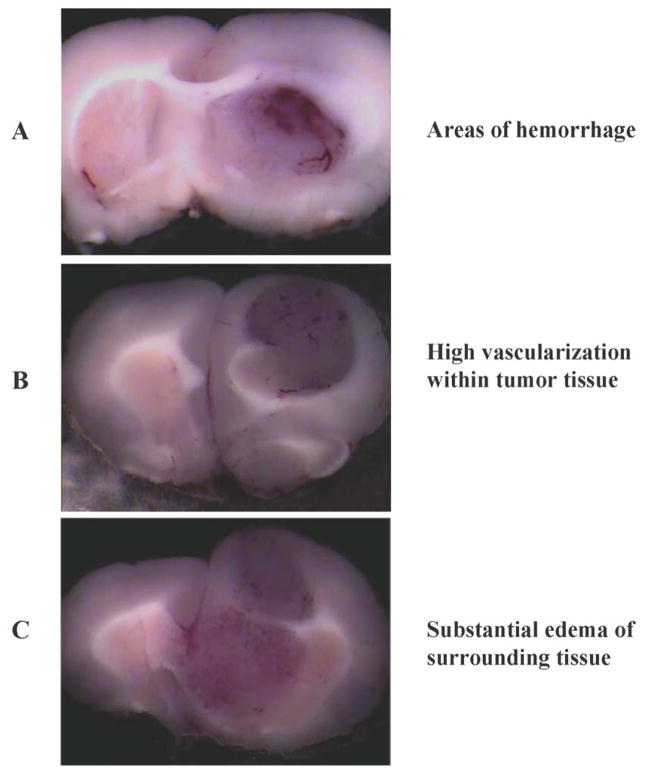
To determine invasiveness of developed tumors, fixed coronal sections were stained with cresyl violet, which allowed us to differentiate the boundaries between normal neuronal tissue versus the glioma under a light microscope (Roth et.al, 1999; Stojiljkovic et.al, 2003). Coronal sections of brain (Figure 1A to 1C) showed massive, highly vascularized intracranial tumors with multiple areas of hemorrhage. Occasionally, large feeding vessels were found on the periphery of tumors suggesting that even at this stage the tumors had developed their own vascular supply. Furthermore, significant edema of surrounding tissue was also noted with classical pathological signs including shifting of midline (butterfly effect). Results from cresyl violet staining are summarized in Figure 2A (control) clearly demonstrating invasive growth pattern of developing tumors.
Figure 1.
Pathological characteristics of tumors derived from RG2 cells (n = 6). (A) Depicts representative areas of hemorrhage, (B) Shows high vascularization within the tumor and (C) Substantial edema of the surrounding tissue.
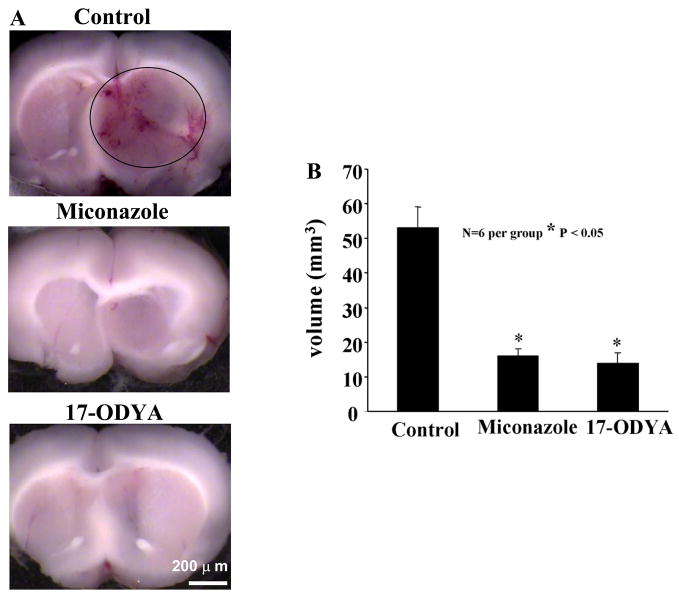
Figure 2.
(A) Inhibition of CYP epoxygenase activity with miconazole or 17-ODYA decreases tumor size in the Fischer rat. RG2 cells were implanted into the forebrain of male Fischer rats and 3 days later drug or vehicle was delivered using osmotic mini pumps. Animals were killed, perfusion fixed with formalin and frozen sections prepared for microscopy. (B) Depicts quantitative representation of tumor volume (n = 6 per group).
Tumor Volume
Comparison of tumor volume revealed significant reduction of tumor size in miconazole and 17-ODYA treated rats. Average tumor volume for control group (Figure 2A) was 53.2 ± 6 mm3 (n = 6). In contrast, average tumor volume for miconazole and 17-ODYA group were 16 ± 2mm3 (n = 6) and 14 ± 3mm3 (n = 6), respectively (Figure 2B and 2C). In addition, developed tumors in miconazole and 17-ODYA groups did not show hemorrhagic areas or significant peritumoral edema.
Assessment of Tumor Vasculature
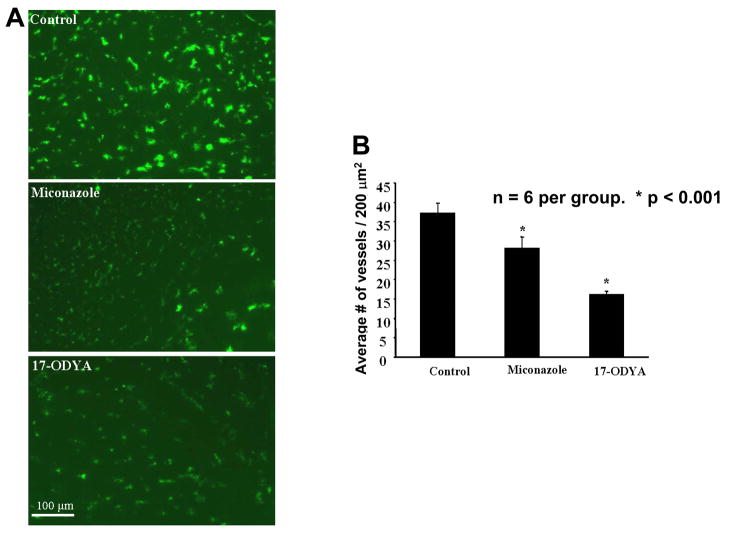
Numerous studies have shown that the tumor vasculature is highly heterogeneous and that a number of vessels are non functional (Rich and Bigner 2004; Cavallaro and Christofori 2000; Carmaliet and Jain 2000). To visualize the state of tumor vasculature, we perfused the brain with dextran conjugated FITC. Using this technique only patent and functional vessels were visualized. Figure 3 depicts the tumor vasculature after FITC-conjugated dextran staining. Permeability of the vasculature in the experimental groups is summarized in Figure 3A, which indicates that tumor capillaries are more permeable to FITC-conjugated dextran than normal vessels. Further analysis of tumor microcirculation suggests that density of capillaries is highly heterogeneous within tumor. Peripheral parts of tumor have significantly higher number of capillaries per 200 mm3 than central parts. A significant reduction in the number of vessels was observed in the miconazole and 17-ODYA treated groups compared to the control (Figure 3A) P <0.001, n = 6. Further the 17-ODYA group showed increased reduction compared to the miconazole group, P <0.05, n = 6.
Figure 3.
(A) Representative images of tumor vasculature captured following perfusion with FITC-conjugated dextran in the absence or presence of the CYP epoxygenase inhibitors. At the termination of the experiment, the animals were anesthetized, briefly perfused with FITC-conjugated dextran in a saline solution, the brains quickly removed and frozen sections prepared for fluorescence microscopy. The green spots represent blood vessels. (B) Bar graph representing the average number of blood vessels in 200 μm2 area (n=6 per group).
Survival Data
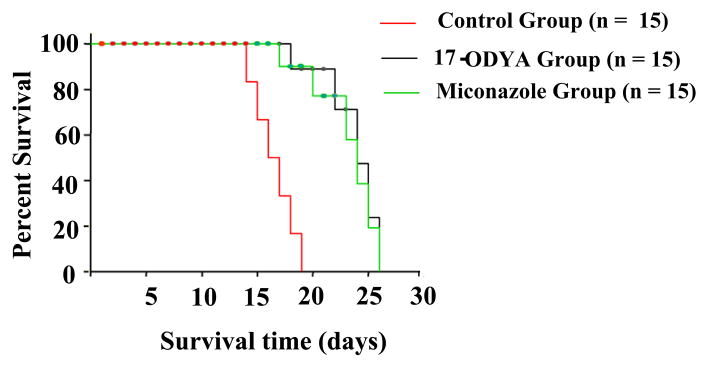
Median survival time for vehicle-treated tumor-developed animals (aCSF treatment, n = 15) was 16.5 ± 2 days after implantation. In contrast, median survival times for tumor-developed rats treated with 17-ODYA or miconazole group (n = 15) were 22 ± 2 and 23 ± 2 days respectively (Figure 4). These findings indicate that inhibition of CYP enzymes in brain reduced tumor size and increased survival time.
Figure 4.
Shows the survival time of rats in days of different treatment groups with glioblastoma multiforme. Red line denotes the control group with the least survival time compared to blue and black lines that are miconazole and 17-ODYA treated groups, respectively. The statistical significance of survival analysis were computed using multiple comparison procedures of Holm-Sidak method, n = 15 per group P <0.05.
CYP2C11 Expression in Tumor
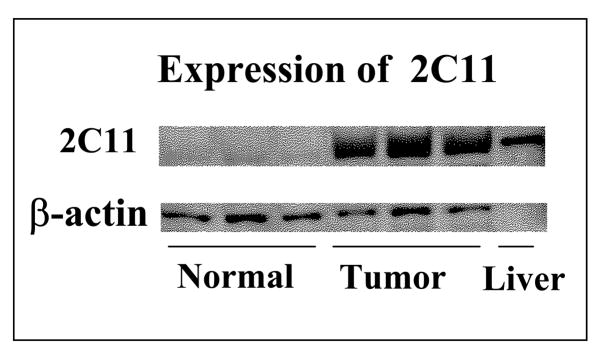
Examination of the expression level of CYP2C11 epoxygenase using antibody against CYP2C11 and Western blot analysis revealed increased expression of the protein for CYP2C11 epoxygenase in tumor brain tissues as compared to the control (Figure 5).
Figure 5.
Differential expression of CYP2C11 epoxygenase protein in normal and tumor brain samples as determined by western blot analysis using male rat liver microsomes as positive control.
Effect of 17-ODYA and Miconazole on CYP activity in Tumor Tissue
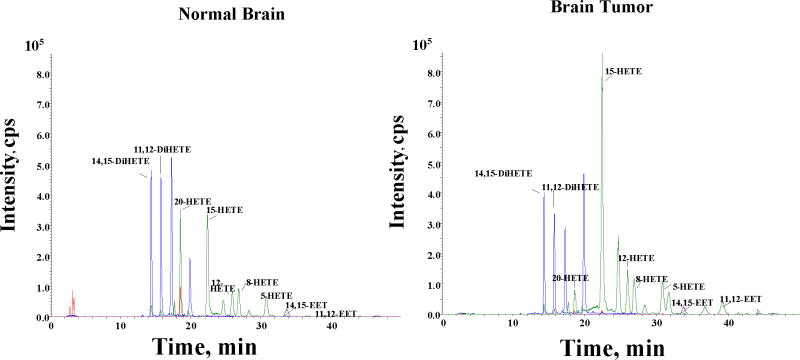
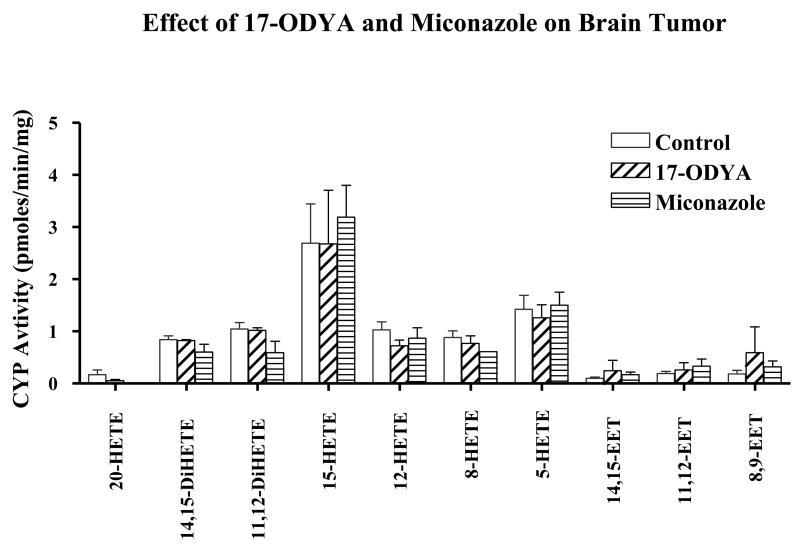
The fact that both tumor and normal brain tissues produce EETs (Figure 6) is suggestive of the contribution of the angiogenic EETs in promoting increased vascularity of the tumor tissue. We have also observed that both normal and tumor tissue metabolize AA to 5-, 8-, 12-,15-, and 20-hydroxyeicosatetraenoic acids (HETEs) (Figure 6). However, the observed inability of the two mechanistically different CYP enzyme inhibitors to reduce endogenous level of EETs and HETEs (Figure 7) appear to suggest the prevalence of preformed EETs and HETEs that are stored in esterified form in cell membrane phospholipid pools (Carroll et al. 1997; Fang et al. 1995; VanRollins et al. 1996) that could account for the observed unaltered level of EETs following CYP epoxygenase inhibition. This lack of effect of the CYP inhibitors may further suggest that the reduction in tumor size after the administration of the CYP inhibitors could be the result of the pharmacological actions of the CYP inhibitors themselves. These potential mechanisms of such actions of these CYP inhibitors are open for further investigation.
Figure 6.
Representative LC-MS profile of CYPAA metabolites in normal and tumor rat brain tissue samples. Normal brain and tumor core tissue were removed from rat brain and frozen in liquid nitrogen. The samples were homogenized and assayed for CYP activity as described in the methods section. Epoxyeicosatrienoic acids and Di-HETEs (8, 9- 11, 12- and 14,15-EETs) and HETEs ( 5-, 8-, 12-,15- and 20-HETE) were detected in both normal and tumor tissues.
Figure 7.
Effect of 17-ODYA and miconazole on CYP activity in normal and tumor samples. The brain tissue samples from the rats infused by implanted minipumps containing vehicle control, 17-ODYA (0.3 mmol/L) or miconazole (0.3 mmol/L) were removed and assayed for CYP activity by LC-MS as described in the methods section. There was no significant difference between the levels of CYP metabolites in control and treated tumor brain tissues.
Discussion
Glioblastoma-like tumors were induced with Fischer rat RG2 cells, which are one of the most potent gliosarcoma cell lines currently available to researchers. Tumors developed from RG2 cells closely mimic human tumors in growth pattern and resistance to therapy (Rich and Bigner 2004). To avoid one of the critical issues in cancer research involving immunological response from host, we used Fischer rats to match the RG2 cell host. Earlier findings from our laboratory and by others have shown that EETs generated by the action of CYP epoxygenases on AA are highly angiogenic in function (Munzenmaier and Harder 2000; Medhora et al, 2003; Zhang and Harder 2002). It is likely that these EETs could contribute to the increased vascularity in tumor tissue and thus promote tumor growth.
In the present study both 17-ODYA and miconazole were found to be equally effective in reducing tumor size and prolonging survival time. Our choice of two mechanistically different inhibitors of CYP enzyme-17-ODYA, an irreversible and non-selective suicide substrate inhibitor of CYP enzymes, and miconazole with strong selective inhibition of CYP2C epoxygenase family-is based on previously published studies by us and those of other investigators in this field (Alkayed et al, 1997, 1996; Campbell et al, 1996; Gebremedhin et al, 1992; Munzenmaier and Harder 2000; Roman 2002). We chose to deliver these two inhibitors directly into the developing tumor by the use of osmotic mini-pumps for the following reasons: (1) to achieve continuous and proper concentration of inhibitors in tumor, because of unknown pharmacodynamic properties of these drugs in the rat (e.g. binding to plasma proteins, rate of metabolism and excretion, crossing blood-brain barrier and so on). (2) Recent studies (Rich and Bigner, 2004) also showed significant advantage of continuous-versus-intermittent treatment if target of therapy are endothelial cells, especially undergoing proliferation. Intermittent schedules revealed that rapidly dividing cells (such as endothelial or cancer cells) can escape intended inhibition. (3) We used a concentration of 0.3 mmol/L even though the reported IC50 for these compounds is around 0.005 mmol/L in vitro. The dosage used is justified when taking into consideration the average volume of the tumor, which is typically around 50 mm3and the rate of delivery of the drug by the osmotic mini-pump at 0.5 μl/h. Furthermore in recent studies Jiang et al (2005) have reported the inhibition of proliferation of a number of carcinoma cell types (Tca-8113, A549, HepG2, and Ncl-H446) by 17-ODYA at concentrations of 0.05 to 0.1mmol/L. In the current study both 17-ODYA and miconazole were equally effective in reducing the overall tumor volume. However, the effect of 17-ODYA was more pronounced in reducing the tumor vasculature compared to miconazole.
In the present study we also found that expression of the CYP2C11 epoxygenase protein is markedly upregulated in tumor tissue compared to the normal tissue, which is consistent with a previous finding that CYP2J2 epoxygenase promotes the neoplastic phenotype of carcinoma cells and is up regulated in human tumors (Jiang et al, 2005). However, in our studies 17-ODYA and miconazole have no effect on EET and DHET (dihydroxy eicosatrienoic acids) levels, unlike Jiang’s study, where 17-ODYA inhibited the formation of 14,15-DHET (Jiang et al, 2005). This could be due to the different carcinoma cell type used and the overexpression of CYP2J2 epoxygenase in these cells. Similar studies reported by Guo et al (2005) further strengthen our observation that CYP inhibitors are antitumorogenic. In their study the authors have shown that human U251 glioma cells do not synthesize 20-HETE but produce other HETEs and EETs that were not inhibited by HET0016 (Guo et al, 2005). Our results are in agreement with these studies in that the 17-ODYA reduces tumor formation without affecting the EETs synthesis (Chen et al, 2005; Guo et al, 2006). Guo et al (2005) have reported that brain tumors developed by the injection of 9L gliosarcoma cells in Fischer rats were reduced in size by 80% by treating the rats with HET0016, a selective CYP4A inhibitor, when administered chronically twice daily by intraperitoneal injections at a concentration of 10 mg/kg per day in 10% lecithin in water for 2 weeks. It is interesting to note that the 9L rat gliosarcoma cells do not synthesize 20-HETE but synthesize 5-, 12-, 15-HETEs. Yet HET0016, a 20-HETE specific CYP4A inhibitor mitigates the tumor (Guo et al, 2006). One reason may be that these inhibitors are acting on other fatty acid substrates that may promote angiogenesis. Also, it is possible that the inhibitors are targeting the EETs in endothelial cells in the tumor vasculature, thus limiting angiogenesis. This is difficult to follow up given the absence of effective techniques for measurement of EETs levels in exclusively endothelial cells derived from tumor vasculature. Further studies are required to understand the mechanism of action of these compounds in attenuating the tumor growth.
The concept of targeting blood vessels that feed tumors as an approach to limit and/or mitigate tumor formation dates back to the early 1970’s (Folkman, 1971). However, it has only been within the last decade that major developments have been made to understand the molecular mechanisms involved in tumor-induced angiogenesis (Carmeliet and Jain 2000; Cavallaro and Christofori 2000). To this end, several anti-angiogenic agents are in development with some in Phase III clinical trials for aggressive tumors like small cell lung carcinoma (Blackhall and Shepherd 2004). Examples of these agents include matrix metalloproteinase inhibitors, angiogenic promoter antagonists, endogenous inhibitors (i.e., endostatin and angiostatin) and select novel agents (e.g., celecoxib, integrin antagonists and so on). In fact, given the significant number of pathways and steps involved in new blood vessel formation it is likely that tumor angiogenesis will continue to be an important target for cancer therapeutics.
Implementation of sophisticated brain imaging three decades ago significantly improved early diagnosis, however, prognosis has not changed, suggesting that new treatment approaches and or modalities are desperately needed for this devastating disease. The focus of most current therapies are geared toward cancer cells. However, all solid tumors are comprised not only of cancer cells but also contain vascular components and interstitial tissue. The vascular component of glioblastoma is extremely important because these cells also show some malignant features. More recently, microvascular proliferation was added as an independent prognostic factor for these tumors, suggesting that tumors with increased microvascular proliferation are more aggressive and resistant to therapy and survival (Rich and Bigner 2004). Because glioblastoma is resistant to many therapies, the current available treatment approach is radical or debulking surgery followed by chemotherapy. Even after an initial good response to therapy and clinical improvement, these tumors usually recur after a few months and show accelerated growth. In such circumstances, inclusion of 17-ODYA or miconazole, which in the present study was found to reduce tumor growth, as chemotherapeutic drugs after resection of the tumor may be beneficial in preventing reoccurrence.
In the present study miconazole or 17-ODYA treated rats survived approximately 5 to 7 days longer than control respectively. Similar survival rates have been observed with 9L gliosarcoma cell-induced brain tumors in Fischer rats treated with the CYP4A -hydroxylase inhibitor HET0016 (Guo et al, 2006). This survival time frame in rat is equivalent to 3 to 4 months life extension in humans. This could be a significant treatment option if the survival could be further extended with combinatorial treatment regimes such as partial resection followed by treatment with either 17-ODYA, miconazole, or combination of CYP inhibitors along with other antiangiogenic agents. In summary, our present observation that the CYP inhibitors miconazole and 17-ODYA reduced tumor size supports the possible use of these compounds in cancer therapy for the treatment of devastating disease such as glioblastoma multiforme.
Acknowledgments
This study was supported in part by grants HL33833-21, 2PO1 HL059996-06A1, and 5PO1 HL068769-04 from the National Institutes of Health, and by grant 3440-02P from the Department of Veterans Affairs Administration.
References
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, Koehler RC. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-D-aspartate. Am J Physiol Heart Circ Physiol. 2000;279:H1616–1624. doi: 10.1152/ajpheart.2000.279.4.H1616. [DOI] [PubMed] [Google Scholar]
- Blackhall FH, Shepherd FA. Angiogenesis inhibitors in the treatment of small cell and non-small cell lung cancer. Hematol Oncol Clin North Am. 2004;18:1121–1141. doi: 10.1016/j.hoc.2004.06.005. ix. [DOI] [PubMed] [Google Scholar]
- Bylund J, Zhang C, Harder DR. Identification of a novel cytochrome P450, CYP4X1, with unique localization specific to the brain. Biochem Biophys Res Commun. 2002;296:677–684. doi: 10.1016/s0006-291x(02)00918-x. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Balazy M, Huang DD, Rybalova S, Falck JR, McGiff JC. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 1997;51:1696–1702. doi: 10.1038/ki.1997.234. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Molecular mechanisms of tumor angiogenesis and tumor progression. J Neurooncol. 2000;50:63–70. doi: 10.1023/a:1006414621286. [DOI] [PubMed] [Google Scholar]
- Chen P, Guo M, Wygle D, Edwards PA, Falck JR, Roman RJ, Scicli AG. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am J Pathol. 2005;166:615–624. doi: 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, VanRollins M, Kaduce TL, Spector AA. Epoxyeicosatrienoic acid metabolism in arterial smooth muscle cells. J Lipid Res. 1995;36:1236–1246. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- Guo M, Roman RJ, Falck JR, Edwards PA, Scicli AG. Human U251 glioma cell proliferation is suppressed by HET0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine], a selective inhibitor of CYP4A. J Pharmacol Exp Ther. 2005;315:526–533. doi: 10.1124/jpet.105.088567. [DOI] [PubMed] [Google Scholar]
- Guo M, Roman RJ, Fenstermacher JD, Brown SL, Falck JR, Arbab AS, Edwards PA, Scicli AG. 9L gliosarcoma cell proliferation and tumor growth in rats are suppressed by N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine (HET0016), a selective inhibitor of CYP4A. J Pharmacol Exp Ther. 2006;317:97–108. doi: 10.1124/jpet.105.097782. [DOI] [PubMed] [Google Scholar]
- Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H215–224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR) Faseb J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3:430–446. doi: 10.1038/nrd1380. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Roth W, Isenmann S, Naumann U, Kugler S, Bahr M, Dichgans J, Ashkenazi A, Weller M. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265:479–483. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic M, Piperski V, Dacevic M, Rakic L, Ruzdijic S, Kanazir S. Characterization of 9L glioma model of the Wistar rat. J Neurooncol. 2003;63:1–7. doi: 10.1023/a:1023732619651. [DOI] [PubMed] [Google Scholar]
- VanRollins M, Kaduce TL, Fang X, Knapp HR, Spector AA. Arachidonic acid diols produced by cytochrome P-450 monooxygenases are incorporated into phospholipids of vascular endothelial cells. J Biol Chem. 1996;271:14001–14009. doi: 10.1074/jbc.271.24.14001. [DOI] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]