Abstract
Glucocorticoids play an important biphasic role in modulating neural plasticity; low doses enhance neural plasticity and spatial memory behavior, whereas chronic, higher doses produce inhibition. We found that 3 independent measures of mitochondrial function—mitochondrial oxidation, membrane potential, and mitochondrial calcium holding capacity—were regulated by long-term corticosterone (CORT) treatment in an inverted “U”-shape. This regulation of mitochondrial function by CORT correlated with neuroprotection; that is, treatment with low doses of CORT had a neuroprotective effect, whereas treatment with high doses of CORT enhanced kainic acid (KA)-induced toxicity of cortical neurons. We then undertook experiments to elucidate the mechanisms underlying these biphasic effects and found that glucocorticoid receptors (GRs) formed a complex with the anti-apoptotic protein Bcl-2 in response to CORT treatment and translocated with Bcl-2 into mitochondria after acute treatment with low or high doses of CORT in primary cortical neurons. However, after 3 days of treatment, high, but not low, doses of CORT resulted in decreased GR and Bcl-2 levels in mitochondria. As with the in vitro studies, Bcl-2 levels in the mitochondria of the prefrontal cortex were significantly decreased, along with GR levels, after long-term treatment with high-dose CORT in vivo. These findings have the potential to contribute to a more complete understanding of the mechanisms by which glucocorticoids and chronic stress regulate cellular plasticity and resilience and to inform the future development of improved therapeutics.
Keywords: allostasis, Bcl-2, mitochondria, mood disorders, glucocorticoid receptor
Glucocorticoids, the adrenal steroid hormones secreted during stress, are necessary for survival of the organism. However, sustained elevations of glucocorticoids (such as those seen during chronic stress) are associated with numerous deleterious effects, including on neuronal survival (1). Accumulating data have shown an inverted “U”-shaped relationship between corticosterone (CORT) levels and performance on behavioral tasks such as spatial learning and memory (2) and passive avoidance tasks (3). In addition, long-term potentiation and primed burst potentiation also demonstrate an inverted “U”-shaped relationship with CORT levels (4). Furthermore, considerable data have shown that low doses of glucocorticoids have trophic actions on neuronal branching and survival (5), whereas higher doses are detrimental to neuronal survival (6). The role of these hormones in mediating both the protective, as well as the potentially damaging, effects of stress has led to the introduction of the terms “allostasis” (the process of maintaining homeostasis by active means) and “allostatic load” (the deleterious consequences of sustained allostasis) (7).
However, the precise cellular mechanisms underlying glucocorticoids' intriguing biphasic effects have not been fully elucidated. Some data suggest that—at least in the hippocampus—these effects are mediated by distinct intracellular receptor subtypes: the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR). The GR has a lower affinity for endogenous glucocorticoids and is therefore believed to be more important in the response to, and regulation of, the stress response when endogenous levels of glucocorticoids are high; in contrast, MRs are activated at lower glucocorticoid concentrations (8). However, it is unclear if this postulated mechanism fully explains the biphasic effects of glucocorticoids, particularly in areas outside the hippocampus, such as the frontal cortex.
It is notable that in addition to their well-characterized nuclear translocation in many cell lines, recent reports indicate that GRs also translocate to the mitochondria (9). Mitochondria have the well-known function of energy production through the Krebs tricarboxylic-acid cycle and oxidative phosphorylation (10). However, it is now clear that mitochondria play additional important roles in regulating intracellular calcium, cytoprotection, and neural plasticity (11). Thus, mitochondrial function plays an important role in both “here and now” synaptic function, as well as long-term structural plasticity—both of which are known to be regulated by glucocorticoids.
In the present study, we therefore sought to determine, in a systematic manner, the effects of both physiologic and pathophysiologic levels of glucocorticoids on various aspects of mitochondrial function. We found that glucocorticoids exert biphasic effects on neuronal mitochondrial function, with low levels potentiating various aspects of mitochondrial function, and chronically high levels attenuating function. These findings have the potential to contribute to a more complete understanding of the mechanisms by which glucocorticoids and chronic stress regulate cellular plasticity and resilience and to inform the future development of improved therapeutics.
Results
CORT Regulates Mitochondrial Oxidation in a Dose- and Time-Dependent Manner.
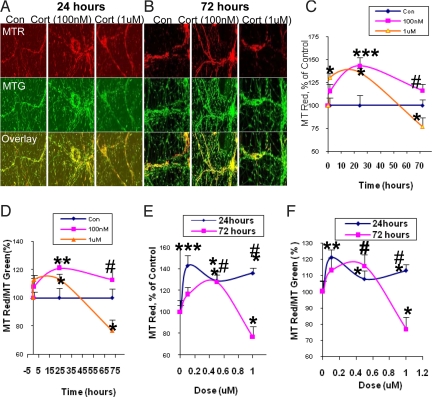
Mitochondrial oxidation is one of the important mitochondrial functions involved in ATP synthesis. We investigated mitochondrial oxidation after physiological (low, 100 nM, 500 nM) and pathological (high, 1 μM) doses of CORT using MitoTracker Red (MTR) and MitoTracker Green (MTG) staining. MTR dye will become a red fluorescent color only when it is oxidized. We measured both the fluorescent intensity of MTR and the ratio of MTR/MTG; this ratio was able to filter the bias caused by quenching of the fluorescence signals. After 1 day of treatment with 100 nM, 500 nM, and 1 μM of CORT, mitochondrial oxidation was significantly higher than control, by 143%, 129%, and 137%, respectively (Fig. 1A, C, and E). For the low-dose (100 nM) CORT treatment, the enhancement of mitochondrial oxidation was sustained for 3 days (Fig. 1 A-C, and E). However, after long-term (3 days) treatment with high-dose CORT, cortical neurons showed a significant reduction in mitochondrial oxidation (from 137% to 77%) (Fig. 1 B, C, and E). When the ratio of MTR to MTG was used to represent mitochondrial oxidation, the data showed a similar pattern (Fig. 1 D and F).
Fig. 1.
Mitochondrial oxidation has an inverted “U”-shaped relationship with CORT dose. Cortical neurons (10–12DIV) were treated with 100 nM, 500 nM, or 1 μM CORT for 1 h, 1 day, or 3 days and stained with MTR and MTG. Data were combined from 2–3 independent experiments and presented as mean ± SE (*one-way ANOVA ***P < 0.001, **P < 0.05,*P < 0.01 compared to control; # P < 0.05 compared to group of 1 μM and 72-hr treatment, n = 32–70). (A) The sample image of MTR and MTG staining after CORT treatment for 24 h. (B) The sample image of MTR and MTG staining after CORT treatment for 72 h. (C) Time course of mitochondrial oxidation by MTR. (D) Time course of mitochondrial oxidation by MTR/MTG ratio. (E) Dose-dependent curve for mitochondrial oxidation by MTR. (F) Dose-dependent curve for mitochondrial oxidation by MTR/MTG ratio.
Mitochondrial Membrane Potential Was Modulated by CORT in a Dose- and Time-Dependent Manner.
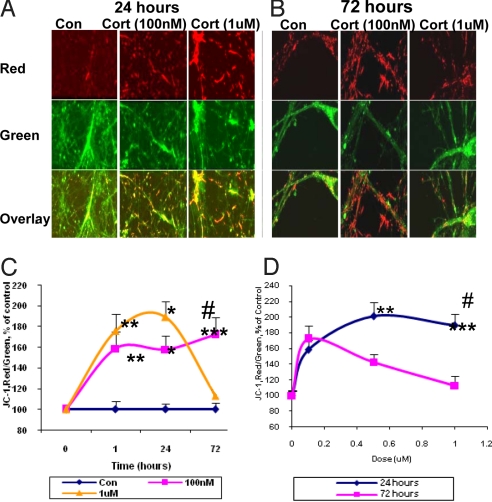
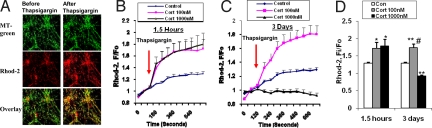
We also measured mitochondrial membrane potential by JC-1 staining in cultured cortical neurons [10 days in vitro (DIV)] after CORT treatment. We found that mitochondrial membrane potential was significantly increased after 1.5 hr treatment with 100 nM, 500 nM, or 1 μM of CORT, and this enhancement was sustained for 1 day (Fig. 2A, C, and D). Moreover, 100 nM and 500 nM of CORT continued to increase mitochondrial membrane potential after 3 days of treatment (Fig. 2 B-D). However, high-dose CORT (1 μM) decreased mitochondrial membrane potential after 3 days (Fig. 2 C and D). Mitochondria play a key function in buffering intracellular calcium (12). Therefore, we explored calcium holding capacity after treatment with CORT (100 nM, 1 μM) for 1.5 h or 3 days. The dihydrorhod-2 colocalized with the MTG signal showed by confocal image, suggesting that the calcium indicator dyhydrorhod-2 (Rhod-2) was localized in the mitochondria (Fig. 3 A). We calculated Fi/Fo (before and after thapsigargin stimulation), and found that calcium holding capacity was enhanced after 1.5 h of both low- and high-dose CORT treatment. However, after 72 h of treatment with CORT, the low dose showed significantly enhanced mitochondrial holding capacity. High doses of CORT (1 μM) reduced the calcium holding capacity of the neurons (Fig. 3 B-D). Indeed, the regulation of calcium holding capacity correlated with the pattern of Bcl-2 levels in the mitochondria.
Fig. 2.
CORT modulates membrane potential in a dose- and time-dependent manner. Data were combined from 2–3 independent experiments and presented as mean ± SE (*one-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001 compared to control, #P < 0.01 compared to 72 h and 1 μM CORT treatment, n = 34–90). (A) JC-1 staining image after 24 h treatment with 100 nM and 1 μM of CORT. (B) JC-1 staining image after 72 h treatment with 100 nM and 1 μM of CORT. (C) Time course of JC-1 staining after CORT treatment. (D) Dose-dependent curve for JC-1 staining after CORT treatment.
Fig. 3.
Modulation of mitochondrial calcium holding capacity by CORT in a time- and dose-dependent manner. Data are presented as mean ± SE (one-way ANOVA, n = 4, n = 223. *P < 0.05, **P < 0.01 compared to control, # P < 0.001 compared to the group of CORT 1 μM for 3 days). (A) Rhod-2 colocalizes with MTG in cortical neurons. (B) Mitochondrial calcium levels (Fi/Fo) in response to thapsigargin stimulation in cortical neurons after 1.5-hr treatment with CORT. (C) Mitochondrial calcium levels in response to thapsigargin stimulation in cortical neurons after 3-day treatment with CORT. (D) Comparison of calcium holding capacity 10 mins after thapsigargin stimulation in CORT-treated cortical neurons.
The Biphasic Effects of Glucocorticoids on Mitochondrial Function Were Accompanied by Similar Effects on Neuronal Viability.
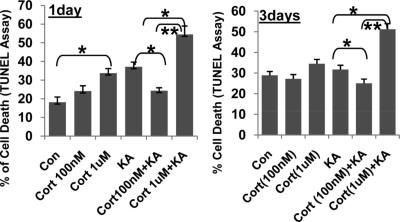
Because our findings suggested that CORT regulates mitochondrial function in a biphasic manner, we further investigated the impact of this alteration of mitochondrial function on neuronal viability. Cortical neurons (10–12 DIV) were treated with 100 nM or 1 μM of CORT for 1 or 3 days. After treatment with various concentrations of CORT, the cortical neurons were challenged with kainic acid (KA) (50 μM) for 12 h and TUNEL assay was performed to determine cell death. As has been previously demonstrated, KA treatment enhanced apoptosis of cortical neurons (13). As predicted, we found biphasic effects of CORT on KA-mediated toxicity; 1 day or 3 days of treatment with 100 nM of CORT had significant neuroprotective effects against KA-induced apoptosis. However, 1 day or 3 days of treatment with the higher dose of CORT (1 μM) enhanced the apoptotic effects of KA (Fig. 4).
Fig. 4.
The effect of low and high concentrations of CORT in KA-induced apoptosis. Cortical neurons (10–12DIV) were treated with CORT (100 nM, 1 μM) for 1 day in Neurobasal and 3 days in N2 plus Neurobasal. KA (50 μM) was then applied for 12 h to challenge the neurons. Neuronal apoptosis was determined by TUNEL assay (n = 2, n = 17–29 for each group. One-way ANOVA, *P < 0.05, **P < 0.01). Data are presented as mean ± SE.
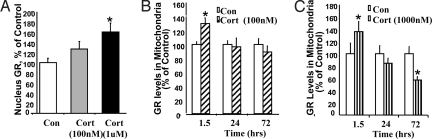
GRs Translocated to Mitochondria in a Dose- and Time-Dependent Manner.
We sought to determine the mechanism whereby glucocorticoids regulate mitochondrial functions. We postulated that GRs translocate to mitochondria and modulate mitochondrial function in cortical neurons. First, we set out to confirm the purity of the mitochondrial fraction, and found a significant 9-fold enrichment of the 57 kDa mitochondrial protein cytochrome oxidase subunit 1 (COX-1) in the mitochondrial fraction (Fig. S1A). When investigating whether the mitochondrial fractions were contaminated by nuclear or cytosol fractions, we found that the levels of both 70 kDa marker nuclear pore protein, and the cytosolic marker glyceraldehyde 3-phosphate dehydrogenase (GADPH), were low in mitochondrial fractions (Fig. S1 B and C), suggesting that the mitochondrial fraction was not contaminated by nuclear or cytosol fractions. We chose 100 nM, 500 nM, and 1 μM of CORT to cover a physiologically and pathologically relevant range in our in vitro experiments. We confirmed that in our experiments, GRs translocated into nucleus in response to CORT (Fig. 5A). We then systematically characterized the dose- and time-dependence of GR mitochondrial translocation after CORT treatment. Cortical neurons (10 DIV) were treated with a physiological dose (100 nM) and a supraphysiological/pathological dose (1 μM) for 1.5, 24, and 72 h. After 1.5 h of treatment, both low- and high-dose CORT enhanced mitochondrial localization of GRs by 130% and 136%, respectively (Fig. 5 B and C). However, after long-term treatment (3 days), GR content in mitochondria was reduced to 57% only in neurons treated with high doses of CORT (Fig. 5 B and C). In all of the lanes, the molecular weight of the protein band was 97 kDa, corresponding to the reported molecular size of the GR protein. We used subunit specific antibodies—anti-GRα and anti-GRβ—to determine the subtype and found that the GR band we recognized was at the same molecular weight as anti-GRα antibody (data not shown). The mitochondrial translocation of GRs was further confirmed by double immunostaining and confocal microscopy (Fig. S2 A and B). Because MRs are also a target for glucocorticoids, we further examined potential mitochondrial translocation of MRs after CORT stimulation. In contrast to the effects observed with GRs, we found that MRs did not translocate into mitochondria (Fig. S3).
Fig. 5.
GRs translocate into mitochondria in response to low and high concentrations of CORT. Data were combined from 2–3 independent experiments and presented as mean ± SE. (A) GR translocation into nucleus after CORT treatment after 1.5 h treatment (one-way ANOVA, P < 0.05, n = 18). (B) GR translocation into mitochondria after 100 nM CORT treatment (*Student's t test P < 0.05, n = 8–10). (C) GR translocation into mitochondria after 1 μM CORT treatment (*Student's t test P < 0.05, n = 8–10)
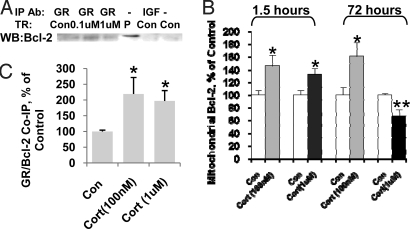
GRs Formed a Complex with Bcl-2, and Mitochondrial Bcl-2 Levels Were Regulated by CORT.
We thought it possible that GRs bind to and recruit Bcl-2 into mitochondria to regulate mitochondrial function. Indeed, immunoprecipitation with anti-GR antibody—but not the control anti-IGF Ab—was able to pull down Bcl-2 after low-dose (100 nM) and high-dose (1 μM) CORT treatment (Fig. 6A). Coimmunoprecipitation of GRs and Bcl-2 showed that, after CORT treatment for 30 mins, formation of the GR/Bcl-2 complex was significantly enhanced (Fig. 6B). Previous studies have shown that GRs form protein complexes with heat shock protein 90 (HSP90) after 5 mins of treatment with dexomethasone (14). We therefore investigated the dynamics of the formation of GR/Bcl-2 complex after 5 mins of treatment. After the neurons were treated with CORT (1 μM) for 5 mins, the GR/Bcl-2 complex was significantly decreased in cytosol fraction to 40.3 + 13.9% of the control (paired Student's t test, n = 3, P < 0.05) and significantly increased in mitochondrial fraction up to 253.7 + 13.7% of the control (paired Student's t test, n = 3, P < 0.05).
Fig. 6.
Bcl-2 coimmunoprecipitated with GRs and translocated into mitochondria after CORT treatment in a time- and dose-dependent manner. (A) Bcl-2 coimmunoprecipitates with GRs in cultured cortical neurons after 30-min treatment (lane1–3: IP with anti-GR antibody; lane 4: total protein from cortical neurons; lane 5: IP with anti-IGF antibody; lane 6: no antibody). (B) The formation of GR/Bcl-2 complexes was enhanced in cortical neurons after 30-min treatment with CORT (1 μM and 100 nM) (n = 6, n = 5–8, Student's t test P < 0.05). (C) Bcl-2 translocated into mitochondria after CORT treatment in cultured cortical neurons (n = 2–3, n = 8–14, Student's t test, **P < 0.01, *P < 0.05). Data are presented as mean ± SE.
Similar to the GR increase seen in mitochondrial fractions, we found that Bcl-2 levels in the mitochondria were enhanced after 1.5 h with the low (100 nM) and high (1 μM) dose of CORT by 146% and 132%, respectively (Fig. 6C). In the low-dose treatment group, this increase of mitochondrial Bcl-2 was sustained for 72 h by 168%. However, in the high-dose treatment group, Bcl-2 levels in mitochondria were significantly reduced by 68% in cortical neurons after 3 days of treatment (Fig. 6C). The whole cell expression levels of Bcl-2 did not change after short or long-term treatment, suggesting that the Bcl-2 increase in mitochondria was due to a Bcl-2 translocation into mitochondria in response to CORT treatment (data not shown). We further performed Bcl-2/GR double immunostaining to confirm the interaction of Bcl-2 and GR in response to CORT treatment. We found that after 1 h of treatment with CORT (1 μM), the colocalization of GR/Bcl-2 was significantly increased (Fig. S4). There were more Bcl-2 (green) puncta in the control group, and these did not colocalize with the GRs (red puncta). After CORT treatment, the number of GR/Bcl-2 colocalized puncta increased (Fig. S4).
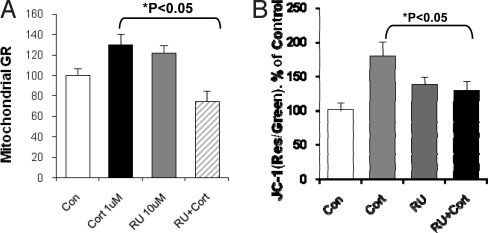
RU486, a GR Partial Agonist, Inhibited CORT-Induced GR Trafficking to Mitochondria and Changes in Mitochondrial Function.
RU486 is a ligand that acts both as a partial GR agonist and antagonist (15). To determine whether CORT's effects were mediated through GRs, we investigated whether RU486 blocked CORT-induced mitochondrial translocation of GRs or enhancement of mitochondrial potential. Cortical neurons were treated with CORT 1 μM, RU486 (10 μM), or CORT plus RU486 for 1.5 h. As expected due to its partial agonistic effects, treatment of the neurons with 10 μM RU486 induced a translocation of GRs from cytosol into mitochondria similar to that observed with CORT alone (Fig. 7A). However, RU486 blocked CORT-induced GR mitochondrial translocation; together, these data show that the effect is mediated through CORT binding to GRs (Fig. 7A). Next, we determined whether CORT-induced changes in mitochondrial function were altered by the GR inhibitor RU486. As before, RU486 treatment alone significantly increased membrane potential compared to control (Fig. 7B). However, cotreatment of the cells with 10-fold excess concentrations of RU486 (10 μM) and CORT (1 μM) resulted in a significant inhibitory effect on the mitochondrial membrane potential compared with CORT 1 μM alone (Fig. 7B). Thus, the antagonistic effects of RU486 were observed for the regulation of membrane potential, as well as for GR translocation to mitochondria, suggesting that these effects were mediated through GRs.
Fig. 7.
RU486 has an antagonist effect on CORT-induced GR translocation and mitochondrial potential in cortical neurons. (A) RU486 exerted antagonist effects on CORT-induced GR translocation into mitochondria (N = 2–3, n = 56. One-way ANOVA, *P < 0.05). (B) RU486 effect on CORT-induced increase in mitochondrial potential revealed by JC-1 staining (N = 3–4, n = 346. One-way ANOVA, *P < 0.01, **P < 0.05). Data are presented as mean ± SE.
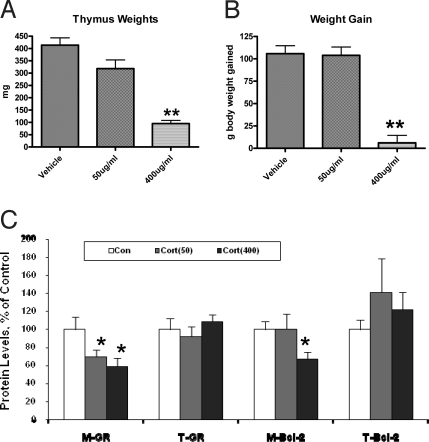
Chronic in Vivo CORT Administration Also Regulated Mitochondrial GR and Bcl-2 Levels in Rodent Prefrontal Cortex.
Finally, we sought to determine whether these in vitro biochemical changes also occurred in vivo. We treated the animals chronically for 21 days with 2 doses of CORT (50 μg/ml or 400 μg/ml in drinking water) or vehicle. We measured body weights and thymus weights to ensure adequacy of the chronic CORT treatment. The body weights of the animal and the weights of the thymus were indeed significantly lower in the group treated with 400 μg/ml of CORT. We found that in the prefrontal cortex, GR levels in mitochondria were significantly decreased by about 30–40% after chronic treatment with high- or low-dose CORT (50 μg/ml or 400 μg/ml); total cellular levels of GRs remained unchanged (Fig. 8). In addition, mitochondrial Bcl-2 levels were also significantly reduced by approximately 35% only in the group treated with high-dose CORT (400 μg/ml) (Fig. 8).
Fig. 8.
GR and Bcl-2 levels in mitochondria are decreased in mitochondrial fractions in prefrontal cortex after chronic stress and after CORT treatment. Western blot analysis of GR and Bcl-2 levels was performed in total homogenates (T) and mitochondrial fractions (M) from prefrontal cortex of CORT (50 μg/ml, 400 μg/ml)-treated animals. Western blot analysis of GR and Bcl-2 content was performed in total homogenates (T) and mitochondrial (M) fractions from prefrontal cortex. Data are presented as mean ± SE. (A) Thymus weights in CORT-treated animals (n = 8 animals per group; **one-way ANOVA, P < 0.01). (B) Body weights of CORT-treated animals (n = 8 animals per group, one-way ANOVA, ** P < 0.01) (C) Chronic CORT treatment significantly reduced mitochondrial GR and Bcl-2 levels (Control group n = 12–15; CORT 50 μg/ml, n = 8; CORT 400 μg/ml, n = 12–15, *one-way ANOVA, P < 0.05)
Discussion
In this study, we demonstrated for the first time that glucocorticoids exert biphasic effects on neuronal mitochondrial dynamics, with low levels potentiating and chronic high levels attenuating various aspects of mitochondrial function. Overall, the study had 5 key findings: (i) 3 independent mitochondrial measures—mitochondrial oxidation, membrane potential, and calcium holding capacity—demonstrated a biphasic response to glucocorticoids; (ii) the enhancement of mitochondrial function by low-dose CORT was associated with a neuroprotective effect against KA-induced neurotoxicity; in contrast, high-dose CORT enhanced KA neurotoxicity; (iii) GRs translocated into mitochondria in primary cortical neurons in a biphasic relationship with concentrations of CORT as well as time of treatment; (iv) GRs formed a complex with the antiapoptotic protein Bcl-2 and facilitated Bcl-2 translocation into mitochondria. The expression of Bcl-2 in mitochondria also showed a biphasic response to CORT dose and time; and (v) in rodents chronically treated with CORT, GR levels in mitochondria were significantly reduced, with high doses of CORT also reducing mitochondrial Bcl-2 levels.
Glucocorticoids Exert Biphasic Effects on Various Aspects of Neuronal Mitochondrial Function.
We undertook a series of studies to determine if—as hypothesized—the biphasic effects of glucocorticoids on long-term potentiation (LTP) and behavior are accompanied by similar effects on various aspects of critical mitochondrial functions. We found that short-term treatment with low or high doses of CORT enhanced mitochondrial oxidation, mitochondrial potential, and calcium holding capacity; these effects were sustained during longer-term treatment with low-dose CORT. In contrast, reductions in mitochondrial oxidation, mitochondrial potential, and calcium holding capacity were observed after longer-term treatment with high-dose CORT (Figs. 1, 2, and 3), which may provide a novel molecular mechanism for the biphasic effects of glucocorticoids on animal behavior during chronic stress.
Biphasic Regulation of Mitochondrial Function by Glucocorticoids Produces Similar Effects on Neuronal Viability.
As noted above, glucocorticoids exert biphasic effects on neuronal viability/resilience with low doses exerting neuroprotective effects but higher doses enhancing neurotoxicity (16, 17). In view of the critical role of mitochondrial function in regulating neuronal viability/resilience, we investigated the effects of glucocorticoids on KA-induced cell death. Strikingly similar to the effects seen on mitochondrial function, we found that treatment with low-dose CORT had a neuroprotective effect, whereas long-term and high-dose concentrations of CORT enhanced KA-induced neurotoxicity. In addition to triggering calcium increases in these cells, CORT also induces gene expression and other nongenomic effects (e.g., changing ERK and mitochondrial functions) (18), which may help to potentiate or prevent the apoptosis induced by other insults (i.e., KA). Together, the data suggest that regulation of mitochondrial function may play an important role in the biphasic effects of glucocorticoids on neuronal viability.
Regulation of Mitochondrial Function by CORT Is Mediated Through GRs.
To confirm that the effects of glucocorticoids were indeed mediated by GRs, we used the well-known GR partial agonist/antagonist, RU486 (19). In many tissues, RU486 exerts partial agonistic effects, while blocking the effects of glucocorticoids. Indeed, RU486-induced nuclear GR translocation has also been previously demonstrated (20). In this study, RU486 clearly had partial agonist and antagonist effects on CORT's effects on mitochondrial GR translocation and mitochondrial membrane potential (Fig. 7). Therefore, the effects of GR translocation on mitochondria and regulation of mitochondrial potential do indeed appear to be mediated through GRs.
CORT Regulates Mitochondrial GR and Bcl-2 Levels in a Biphasic Manner.
As noted above, glucocorticoids exert biphasic concentration/time-dependent effects on various aspects of synaptic and neural function. However, the mechanism(s) underlying these complex effects have not been fully elucidated. Cortisol concentrations in normal human serum vary from 137 nM to 283 nM (7.5 ± 2.6 μg/dl) (21); pathophysiologic states have been associated with elevated levels in the 420–779 nM (21.4+/−6.4 μg/dl) range (22). Therefore, we chose 100 nM, 500 nM, and 1 μM of CORT to represent both physiologic and pathophysiologic levels of cortisol. For the CORT treatment of cortical neurons, we used neurobasal medium, which does not contain cortisol binding globulin (CBG). Therefore, free CORT may be as high as 100 nM, 500 nM, and 1 μM. Because of CBG in serum, the free concentrations in vivo in the brain might be in the range of the lowest concentration (100 nM) used here. Our study intentionally investigated the effects of very high concentrations and, indeed, we found the biphasic effect that leads to cytotoxicity. Whether or not this happens in vivo under stress conditions remains unclear. However, the in vivo data showed that both low and high doses (50 μg/ml or 400 μg/ml) of CORT in drinking water reduced GRs in mitochondria with a biphasic time course, whereas only the highest, clearly supraphysiological, doses reduced Bcl-2 levels in the mitochondria.
The expression of the major cytoprotective protein, Bcl-2, is known to reduce reactive oxygen species (ROS) production, thus preventing permeability transition pore opening, and mitochondrial depolarization (23). Studies of isolated mitochondria have shown that Bcl-2 overexpression increases mitochondrial calcium uptake capacity (24), making the cells resistant to the deleterious influence of elevated intracellular calcium. Thus, Bcl-2 exerts significant effects on cellular calcium buffering, not only under the extreme conditions of calcium-induced apoptosis, but likely even during normal synaptic activity. This report is the first to show that GRs interact with Bcl-2 and translocate into mitochondria in response to CORT, thus regulating mitochondrial functions (vide infra; Figs. 5 and 6).
GR and Bcl-2 Levels in Mitochondria Were Decreased after Chronic Treatment with CORT in Vivo.
Our in vitro studies demonstrated that long-term and high-dose treatment with CORT down-regulated GR and Bcl-2 levels in mitochondria (Figs. 5 and 6), as well as mitochondrial function (Figs. 1, 2, and 3). As expected, we found that chronic treatment with high-dose and low-dose glucocorticoids attenuated prefrontal cortical mitochondrial GR levels. Most importantly, anti-apoptotic mitochondrial Bcl-2 levels were reduced after high-dose and chronic treatment with glucocorticoids in the mitochondrial fractions, suggesting an alteration of mitochondrial functions (Figs. 4 and 8). This reduction in anti-apoptotic mitochondrial Bcl-2 levels by glucocorticoids is consistent with previous in vivo findings that glucocorticoids potentiated the KA- and ischemia-induced injury to neurons (25, 26).
Conclusion
In conclusion, this study demonstrates for the first time that glucocorticoids exert major, biphasic effects on various facets of cortical mitochondrial dynamics. In addition to regulating mitochondrial activities, there may well be other mechanisms operative in the biphasic effects that glucocorticoids exert on neuronal function. For instance, concentration-dependent effects of glucocorticoids on MRs and GRs may explain the biphasic effects of glucocorticoids on hippocampal function (27). Consistent with the findings presented here, recent work has shown that glucocorticoid-mediated cell death was counteracted by the MR agonist aldosterone (27). It is possible that under low concentrations of CORT, both MRs and GRs mediate neuroprotective effects via different mechanisms. While the different effects of glucocorticoids on MRs and GRs are undoubtedly important for various neural functions, the dynamic regulation of mitochondrial function by glucocorticoids provides new evidence to explain the biphasic effects of glucocorticoids on various neural functions. Indeed, the regulation of mitochondrial dynamics that we observed during chronic high-dose glucocorticoid administration appears to represent an important component of the allostatic overload observed as a pathological consequence of chronic stress (1, 7). The possibility that agents that enhance mitochondrial function may be useful in countering the deleterious effects of excessive glucocorticoid secretion observed in diverse conditions is an exciting prospect for future investigation.
Materials and Methods
Preparation of Cortical Neuronal Cultures.
Cultures of cortical neurons were prepared according to a previously published procedure with minor modifications (28). Detailed methods are provided in the SI.
MTR and MTG Staining of Cortical Neurons.
The staining of MTR CM-H2XROS (Molecular Probes), and MTG (Molecular Probes) was performed according to the manufacturer's instructions (Molecular Probes). Detailed methods are provided in the SI.
JC-1 Staining of Cortical Neurons.
JC-1 staining was performed according to the manufacturer's instructions (Molecular Probes). Detailed methods are provided in the SI.
Determination of Mitochondrial Calcium by Rhod 2 Staining.
The procedure for Rhod 2 staining was performed according to the manufacturer's instructions (Invitrogen). Detailed methods are provided in the SI.
In Situ Cell Death Detection Assay.
We used the kit from Roche to detect and quantify apoptosis at the single cell level based on labeling of DNA strand breaks (TUNEL assay) according to the manufacturer's instructions. Detailed methods are provided in the SI.
Preparation of Mitochondrial Fractions from Cortical Neurons.
Preparation of mitochondrial fractions from cortical neurons followed a previously published procedure with minor modifications (29). Detailed methods are provided in the SI.
Western Blot Analysis.
Protein concentrations were determined by using the BCA protein assay kit (Pierce, Rockford, IL). Western blot analysis was similar to a previously published procedure with minor modifications (29, 30). Detailed methods are provided in the SI.
CORT Treatment.
All animal treatments, procedures, and care were approved by the NIMH Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (31). Animal treatment was similar to a previously published procedure with minor modifications (29, 32). Detailed methods are provided in the SI.
Preparation of Mitochondrial Fraction from Brain Tissue.
Preparation of mitochondrial fractions from rat brain tissue was similar to a previously published procedure with minor modifications (33). Detailed methods are provided in the SI.
Supplementary Material
Acknowledgments.
We thank Ioline Henter for outstanding editorial assistance. We acknowledge the support of the Intramural Research Program of the NIMH and the Stanley Medical Research Institute. B.S.M. acknowledges grant support from NIH MH41256.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812671106/DCSupplemental.
References
- 1.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold PE, McGaugh JL. In: Neuropeptide Influences on the Brain and Behavior. Miller LH, Sandman CA, Kastin AJ, editors. New York: Raven Press; 1977. pp. 127–143. [Google Scholar]
- 4.Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2005;27:244–249. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- 6.Sapolsky R. Stress, the Aging Brain and the Mechanisms of Neuron Death. Cambridge: MIT Press; 1992. [Google Scholar]
- 7.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 8.Joels M, De Kloet ER. Coordinative mineralocorticoid and glucocorticoid receptor-mediated control of responses to serotonin in rat hippocampus. Neuroendocrinology. 1992;55:344–350. doi: 10.1159/000126135. [DOI] [PubMed] [Google Scholar]
- 9.Koufali MM, Moutsatsou P, Sekeris CE, Breen KC. The dynamic localization of the glucocorticoid receptor in rat C6 glioma cell mitochondria. Mol Cell Endocrinol. 2003;209:51–60. doi: 10.1016/j.mce.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Huttemann M, Lee I, Samavati L, Yu H, Doan JW. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta. 2007;1773:1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Toescu EC, Verkhratsky A. Ca2+ and mitochondria as substrates for deficits in synaptic plasticity in normal brain ageing. J Cell Mol Med. 2004;8:181–190. doi: 10.1111/j.1582-4934.2004.tb00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh AB. Mitochondrial regulation of intracellular Ca2+ signaling: More than just simple Ca2+ buffers. News Physiol Sci. 2003;18:252–256. doi: 10.1152/nips.01458.2003. [DOI] [PubMed] [Google Scholar]
- 13.Stein-Behrens BA, et al. Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem. 1992;58:1730–1735. doi: 10.1111/j.1471-4159.1992.tb10047.x. [DOI] [PubMed] [Google Scholar]
- 14.Bartis D, et al. Intermolecular relations between the glucocorticoid receptor, ZAP-70 kinase, and Hsp-90. Biochem Biophys Res Commun. 2007;354:253–258. doi: 10.1016/j.bbrc.2006.12.211. [DOI] [PubMed] [Google Scholar]
- 15.Groyer A, Schweizer-Groyer G, Cadepond F, Mariller M, Baulieu EE. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987;328:624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- 16.Wenzel A, et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001;42:1653–1659. [PubMed] [Google Scholar]
- 17.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 18.Haller J, Mikics E, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuroendocrinol. 2008;29:273–291. doi: 10.1016/j.yfrne.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Bourgeois S, Pfahl M, Baulieu EE. DNA binding properties of glucocorticosteroid receptors bound to the steroid antagonist RU-486. EMBO J. 1984;3:751–755. doi: 10.1002/j.1460-2075.1984.tb01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jewell CM, et al. Immunocytochemical analysis of hormone mediated nuclear translocation of wild type and mutant glucocorticoid receptors. J Steroid Biochem Mol Biol. 1995;55:135–146. doi: 10.1016/0960-0760(95)00174-x. [DOI] [PubMed] [Google Scholar]
- 21.Davis HA, Gass GC, Bassett JR. Serum cortisol response to incremental work in experienced and naive subjects. Psychosom Med. 1981;43:127–132. doi: 10.1097/00006842-198104000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Pirich K, Vierhapper H. 24-hour serum concentration profile of cortisol in patients with Cushing's disease. Exp Clin Endocrinol. 1988;92:275–279. doi: 10.1055/s-0029-1210815. [DOI] [PubMed] [Google Scholar]
- 23.Zamzami N, et al. The thiol crosslinking agent diamide overcomes the apoptosis-inhibitory effect of Bcl-2 by enforcing mitochondrial permeability transition. Oncogene. 1998;26:1055–1063. doi: 10.1038/sj.onc.1201864. [DOI] [PubMed] [Google Scholar]
- 24.Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 26.Roozendaal B, et al. Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat Neurosci. 2001;4:1169–1171. doi: 10.1038/nn766. [DOI] [PubMed] [Google Scholar]
- 27.Crochemore C, et al. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005;10:790–798. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- 28.Du J, et al. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. J Cell Biol. 2003;163:385–395. doi: 10.1083/jcb.200305134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida A, Medina JM. A rapid method for the isolation of metabolically active mitochondria from rat neurons and astrocytes in primary culture. Brain Res Brain Res Protoc. 1998;2:209–214. doi: 10.1016/s1385-299x(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 30.Du J, et al. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J Neurosci. 2008;28:68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute of Laboratory Animal Research; Commission on Life Sciences; National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: Superintendent of Documents, U.S. Government Printing Office; 1996. p. 140. [Google Scholar]
- 32.Nacher J, Pham K, Gil-Fernandez V, McEwen BS. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 2004;126:503–509. doi: 10.1016/j.neuroscience.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Zini R, Simon N, Morin C, Thiault L, Tillement JP. Tacrolimus decreases in vitro oxidative phosphorylation of mitochondria from rat forebrain. Life Sci. 1998;63:357–368. doi: 10.1016/s0024-3205(98)00284-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.