Abstract
Activation of the innate immune system is commonly accompanied by a set of behavioural, psychological and physiological changes known as ‘sickness behaviour’. In animals, infection-related sickness symptoms are significantly increased by exposure to psychosocial stress, suggesting that psychological and immune stressors may operate through similar pathways to induce sickness. We used a double-blind, randomised, placebo-controlled design to examine the effect of acute psychological stress on immune and subjective mood responses to typhoid vaccination in 59 men. Volunteers were assigned to one of four experimental conditions in which they were either injected with typhoid vaccine or saline placebo, and then either rested or completed two challenging behavioural tasks. Typhoid vaccine induced a significant rise in participants’ serum levels of interleukin-6 (IL-6) and this response was significantly larger in the stress versus rest conditions. Negative mood increased immediately post-tasks, an effect also more pronounced in the vaccine/stress condition. In the vaccine/stress group, participants with larger IL-6 responses had heightened systolic blood pressure responses to tasks and elevated post-stress salivary levels of the noradrenaline metabolite 3-methoxy-phenyl glycol (MHPG) and cortisol. Our findings suggest that, as seen in animals, psychological and immune stressors may act synergistically to promote inflammation and sickness behaviour in humans.
Keywords: Psychological stress, Vaccination, Cytokines, IL-6, Sickness response, Synergistic
1. Introduction
Chronic inflammatory and infectious diseases are commonly accompanied by a set of cognitive and affective symptoms, including confusion, fatigue, psychomotor retardation, impaired memory, decreased motivation, anxiety and depression (Dantzer et al., 2007; Evans et al., 2005). Until recently, these symptoms were considered simply as a detrimental consequence of illness per se. However, emerging evidence in humans and animals indicates that this ‘sickness behaviour’ is in fact a highly organised adaptive response specifically designed to combat infection, and promote survival (Dantzer et al., 2007; Hart, 1988). The inflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α), released from macrophages during the early innate immune response, are thought to play a pivotal role in sickness behaviour by communicating peripheral inflammation to the brain (Dantzer et al., 2008; Raison et al., 2006). Circulating cytokines can signal the brain through a number of routes including activation of vagal afferent fibers projecting to the nucleus of the solitary tract and higher viscerosensory centers, cytokine-specific transport molecules expressed on brain endothelium, and circumventricular organs lacking the blood–brain barrier (Dantzer et al., 2008; Raison et al., 2006). On reaching the brain, cytokine signals can be amplified through a central cytokine network that has profound effects on neurotransmitter metabolism, neuroendocrine function, synaptic plasticity and behaviour (Hayley et al., 2005; Raison et al., 2006).
Circulating cytokines are elevated in patients with inflammatory-related diseases such as rheumatoid arthritis, cardiovascular disease and cancer, and have been shown to correlate with the severity of depressive symptoms in these patients (Irwin and Miller, 2007; Lesperance et al., 2004; Zautra et al., 2004). Cytokine levels are also elevated in the circulation of adults with mild-moderate as well as major depression (Irwin and Miller, 2007; Raison et al., 2006). In rodents, peripheral injection with IL-1β or bacterial lipopolysaccharide (LPS) (a potent stimulator of cytokine release) reliably induces sickness behaviours including social withdrawal, immobility and anorexia (Dantzer et al., 2007). Similarly, sickness-related symptoms including depressed mood, fatigue and psychomotor slowing are frequently observed in human cancer and Hepatitis C patients receiving immunotherapy with IFN-α (a potent inducer of IL-6) (Capuron and Miller, 2004).
Traditionally, cytokine-induced sickness symptoms were thought to occur solely through activation of the immune system by a pathogen or inflammatory disease condition. However, increasing evidence suggests that psychological stressors can provoke sickness behaviour through a similar mechanism (Anisman and Merali, 2003; Simmons and Broderick, 2005). Psychological stress exacerbates a number of chronic inflammatory conditions including asthma, arthritis and cardiovascular disease (Black and Garbutt, 2002; Kemeny and Schedlowski, 2007) and chronic stress is a key risk factor for anxiety and depressive illness (Kendler et al., 1999). Acute psychological stressors have been shown to increase circulating levels of inflammatory cytokines and negative mood symptoms in healthy humans (Steptoe et al., 2007). Similarly, in animals, exposure to a novel environment, physical restraint and foot-shock stress increase both circulating and central expression of inflammatory cytokines, and induce sickness symptoms (Dantzer et al., 2007). Importantly, recent evidence suggests that psychological (or heterotypic) stressors can upregulate innate inflammatory and behavioural responses to immune pathogens. In rats, prior exposure to inescapable tail shock induced a more rapid and larger inflammatory cytokine response to peripheral LPS injection (Johnson et al., 2002). Similarly, social disruption stress synergistically increased inflammatory cytokine and sickness responses to peripheral administration of LPS, IFNα or the viral analogue Poly I:C in three separate studies of mice (Anisman et al., 2007; Gandhi et al., 2007; Gibb et al., 2008). These findings lend further support to the idea that psychological and immune stressors operate through similar biological pathways to induce sickness behaviour. However, so far no studies have investigated this phenomenon in humans.
We set out to address this issue by examining the effect of acute psychological stress on immune and subjective mood responses to typhoid (Salmonella typhi) vaccination in humans. Salmonella typhi polysaccharide vaccine is a mild inflammatory stimulus, previously shown by our group and others to increase circulating levels of inflammatory cytokines and induce negative mood states in healthy volunteers (Hingorani et al., 2000; Strike et al., 2004; Wright et al., 2005). Unlike other experimental models of inflammation, it does not provoke fever or feelings of malaise that could potentially confound responses measured (Hingorani et al., 2000; Strike et al., 2004; Wright et al., 2005). We hypothesised that acute psychological stress would upregulate inflammatory cytokine responses to typhoid vaccination and that this mechanism would provoke a larger increase in negative mood in volunteers subject to both psychological and immune stress.
2. Methods
2.1. Participants
Fifty-nine male student volunteers between 18 and 30 years of age were recruited from University College London. Volunteers were screened by structured interview to ensure that they were healthy, had no previous history of any relevant physical or psychiatric illness, were taking no medication and were non-smokers. Volunteers who had received typhoid vaccine in the past 3 years or any other vaccine in the previous 6 months were excluded. Participants were advised not to consume caffeinated beverages or alcohol and to refrain from excessive exercise during the 12 h prior to testing. They were also advised not to take aspirin, ibuprofen, or antibiotics for 14 days prior to testing. All participants gave their informed consent and the study was approved by the joint UCL/UCLH Committee on the Ethics of Human Research.
2.2. General procedure
The study was performed in a double-blind, randomised, placebo-controlled manner. Participants were assessed individually in the morning in a light- and temperature-controlled laboratory, and all sessions commenced at 09h00. Measures of weight, height and waist circumference were obtained using standardised methods and body fat mass was estimated using a Bodystat 1500 bioelectrical impedance body composition analysis device (Bodystat, Douglas, Isle of Man). A baseline blood sample (10 ml) was drawn into a serum-separator Vacutainer tube (BD Vacutainer Systems, Oxford, UK) by separate venipuncture using a 21-gauge butterfly needle, then participants were randomly assigned to one of four experimental conditions (15 vaccine and stress; 15 placebo and stress; 14 vaccine and rest; 15 placebo and rest), by an investigator who did not assist with participant testing at any stage. Injections of either Salmonella typhi capsular polysaccharide vaccine (0.025 mg in 0.5 ml, Typhim Vi, Aventis Pasteur MD) or control saline placebo (0.5 ml) were administered intramuscularly into the non-dominant deltoid muscle at 09h45 by a trained nurse or physician, and participants rested for 30 min. At the end of the rest period, participants either continued to rest or completed two 5 min challenging mental tasks. They were blinded to whether they would rest or perform tasks until this time point. The first task was a computerized color-word interference (Stroop) task, involving the successive presentation of target color words printed in an incongruous color. Participants were asked to press a computer key that corresponded to the position at the bottom of the screen of the name of the color in which the target word was printed. The second task was a simulated public speaking exercise. Participants were presented with a hypothetical scenario in which they had been wrongly accused of shoplifting, and were instructed to give a speech in their defense by addressing the camera directly in front of them. After listening to a description of the scenario and instructions read out by the experimenter, participants were provided with a written copy of the scenario and given two minutes to prepare their speech. They were told that their speech would be video recorded and later judged for efficacy and fluency. The experimenter remained in the room with the participant during delivery of their speech, and instructed them when to start and stop. Following completion of the second task (10h45, 1 h post-vaccination), participants rested quietly for the remainder of the session. They were asked to rate subjective feelings of stress on a 7-point scale from 1 = low to 7 = high at baseline (towards the end of the rest period), following each of the tasks and then at 30, 60 and 120 min post-task. Ratings of task difficulty, controllability and involvement were also made after each task on 7-point scales, and a second blood sample was drawn as described above, at 120 min post-tasks.
2.3. Psychological measures
Subjective ratings of mood and symptoms of illness were obtained throughout the session using a modified 36-item version of the Profile of Mood States (POMS), as described previously (McNair et al., 1981; Wright et al., 2005). Six high-loading items were taken from the vigour, tension-anxiety, depression–dejection, and confusion scales of the original POMS, and five items were taken from the fatigue scale. Four extra items were added to assess symptoms associated with mild infection (fever, aching joints, nausea, and headache). Participants were asked to rate how they felt at that moment on a 5-point scale from 0 = not at all to 4 = extremely. Ratings were obtained at baseline (towards the end of the rest period), immediately post-tasks, and at 30 min, 60 min and 120 min post-tasks. Body temperature was also measured at these time points using a sublingual digital thermometer. Total negative mood score was calculated (as recommended in the POMS manual) by summing all negative items (tension, depression, confusion and fatigue). Overall mood scores could range from 0 to 96, with higher scores indicating a more negative mood.
2.4. Cardiovascular and neuroendocrine measures
To investigate the biological pathways mediating the effects of stress on inflammation and mood, measures of autonomic nervous and hypothalamic-pituitary–adrenal (HPA) axes activity were also included. Blood pressure (BP) and heart rate were monitored throughout the session using a Portapres-2, a portable version of the Finapres continuous BP monitoring device that shows good reproducibility and accuracy in a range of settings (TNO-TPD Biomedical Instrumentation, Amsterdam, Holland). Five-minute recordings of BP and heart rate were made at baseline (towards the end of the rest period), during each of the tasks, and then at 25–30 min, 55–60 min and 115–120 min post-tasks. HPA axis activity was assessed by salivary cortisol levels and catecholamine responses were indexed by salivary 3-methoxy-phenylglycol (MHPG), the major metabolite of norepinephrine (Reuster et al., 2002). Salivary MHPG concentrations closely reflect both central (cerebrospinal fluid, CSF) and plasma MHPG levels and are used as an indicator of noradrenergic activity (Reuster et al., 2002; Yang et al., 1997). Saliva samples were collected using Salivettes (Sarstedt Inc., Leicester, UK) at baseline (towards the end of the rest period), immediately after the second task, and at 15 min, 30 min, 60 min and 120 min post-tasks, then stored at −80 °C prior to analysis. Salivary cortisol was measured by an enzyme-linked immunosorbent assay (ELISA) (SLV-2930, DRG International, Inc., USA) at Kurume University, Japan. The limit of detection of this assay was 0.53 ng/ml, with intra- and inter-assay coefficients of variation (CVs) of 2.61% and 3.63%, respectively. Salivary MHPG was assessed using gas chromatography mass spectrometry (Hitachi-M80B, Hitachi, Japan) at Kurume University, Japan, as described previously (Yajima et al., 2001). The limit of detection of this assay was 0.55 ng/ml, with intra- and inter-assay CVs of 3.95% and 5.70%, respectively. All samples were analysed in duplicate.
2.5. Interleukin-6 measures
Whole blood samples (10 ml) were left upright to clot for 30 min, then centrifuged at 1250g for 10 min at room temperature. Serum was removed, aliquoted and frozen at −80 °C prior to analysis. Serum concentrations of IL-6 were assessed in duplicate samples by a high-sensitivity two-site ELISA from R&D Systems (Oxford, UK). The limit of detection of this assay was 0.09 pg/ml, with intra- and inter-assay CVs of 4.69% and 4.66%, respectively.
2.6. Statistical analyses
The characteristics of participants in the four groups were compared using analysis of variance for continuous measures, and χ2 tests for categorical variables. Subjective stress and cardiovascular responses were analysed using repeated measures analysis of variance, with group (vaccine/placebo and stress/rest) as between-person factors and trial (baseline, Stroop, Speech, recovery 1 (30 min), recovery 2 (60 min) and recovery 3 (120 min)) as the within-person factor. The repeated measures analysis of cortisol and MHPG involved six trials (baseline, task, and 15, 30, 60 and 120 min post-task), while the analysis of serum IL-6 only involved baseline and 120 min post-task measures. The analyses of total negative mood and vitality included vaccine and stress grouping factors and five trials (baseline, immediately post-task, and 30, 60 and 120 min post-task). The Greenhouse–Geisser correction of degrees of freedom was applied when sphericity assumptions were violated, but raw degrees of freedom are presented in the Results section. Failed blood sampling or assay or equipment problems resulted in some loss of data, so analyses were carried out with the complete sample of 59 for subjective measures, 58 for cardiovascular variables and MHPG, 56 for cortisol, and 54 individuals for IL-6. The distributions of serum IL-6 and MHPG were skewed, so were log transformed before analysis, but the distribution of other variables was satisfactory. Post-hoc analyses were carried out using Tukey’s least significant difference (LSD) test.
3. Results
3.1. Participant characteristics
Sample characteristics and baseline measures are summarised in Table 1. The four experimental groups did not differ significantly in age, anthropometric measures, or baseline levels of cardiovascular activity, neuroendocrine activity or serum interleukin-6. There were also no significant differences in mood scores between groups. Participants’ body temperature was normal and none of the participants reported any appreciable somatic symptoms.
Table 1.
Sample characteristics and baseline measures
| Vaccine/Stress (n = 15) | Vaccine/Rest (n = 14) | Placebo/Stress (n = 15) | Placebo/Rest (n = 15) | |
|---|---|---|---|---|
| Age (years) | 22.5 ± 3.2 | 22.5 ± 2.5 | 21.4 ± 3.0 | 22.9 ± 4.4 |
| BMI (kg/m2) | 22.3 ± 2.4 | 22.5 ± 3.4 | 22.9 ± 1.9 | 23.1 ± 2.9 |
| Waist circumference (cm) | 81.0 ± 6.9 | 80.0 ± 7.3 | 81.7 ± 4.7 | 84.0 ± 9.6 |
| Body fat (%) | 14.4 ± 4.0 | 14.7 ± 3.3 | 12.1 ± 3.1 | 13.2 ± 4.9 |
| Systolic BP (mmHg) | 115.6 ± 13.7 | 112.8 ± 14.1 | 119.0 ± 10.5 | 117.4 ± 10.7 |
| Diastolic BP (mmHg) | 69.6 ± 6.6 | 68.6 ± 8.1 | 68.4 ± 8.0 | 67.3 ± 6.2 |
| Heart rate (bpm) | 73.4 ± 11.8 | 71.5 ± 9.1 | 72.1 ± 9.8 | 68.5 ± 8.5 |
| Cortisol (nmol/L) | 5.19 ± 1.8 | 5.26 ± 1.8 | 6.57 ± 5.0 | 4.49 ± 1.2 |
| Log MHPG (ng/mL) | 1.99 ± 0.4 | 1.93 ± 0.5 | 1.89 ± 0.4 | 1.69 ± 0.4 |
| Log IL-6 (pg/ml) | 0.64 ± 0.3 | 0.64 ± 0.2 | 0.71 ± 0.3 | 0.58 ± 0.3 |
| Total negative mood | 11.06 ± 9.7 | 9.86 ± 4.4 | 10.53 ± 5.5 | 11.93 ± 5.2 |
| Body temperature (°C) | 36.3 ± 1.1 | 36.2 ± 0.6 | 36.4 ± 0.5 | 36.4 ± 0.5 |
| Somatic symptoms | 0.40 ± 0.6 | 0.50 ± 1.2 | 0.33 ± 0.5 | 0.67 ± 0.9 |
3.2. Subjective, cardiovascular and neuroendocrine stress responses
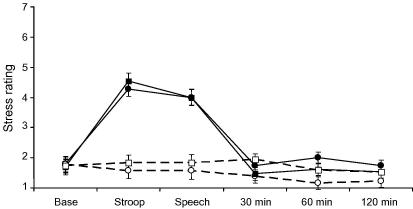
There was a significant stress by trial interaction in the analysis of subjective stress ratings (F(5,26) = 47.3, p < 0.001). As can be seen in Fig. 1, stress ratings increased in response to tasks, returning to baseline levels during the post-task recovery period. There was no difference in subjective stress responses to tasks between vaccine and placebo conditions, and no effect of vaccine alone on subjective stress ratings. Overall, the speech task was rated as more difficult than the Stroop task, with average ratings of 5.27 ± 2.0 versus 4.00 ± 1.6 (F(1,28) = 13.7, p < 0.001), while performance was rated as worse (means 2.57 ± 1.0 versus 4.33 ± 1.0, F(1,28) = 33.8, p < 0.001). There was no difference between vaccine and placebo conditions in subjective ratings of task difficulty or performance.
Fig. 1.
Subjective stress ratings at baseline (Base), during each of the tasks (Stroop, Speech) and at 30, 60 and 120 min post-tasks. Comparison of stress (solid lines) and rest (dotted lines) conditions in participants receiving typhoid vaccine (circles) or saline placebo (squares). Data are presented as means ± SEM.
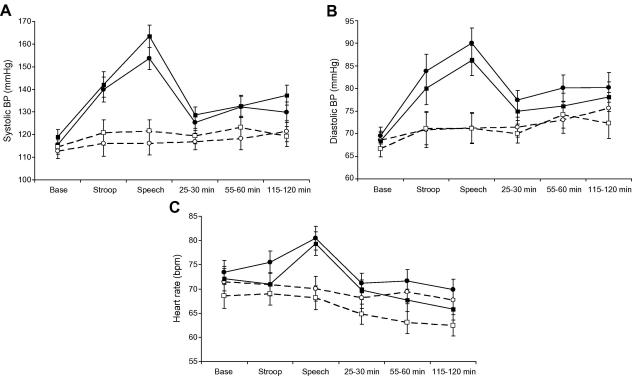
As can be seen in Fig. 2 (A and B), systolic and diastolic BP increased in response to behavioural tasks, returning towards baseline during the post-task period. Repeated measures analysis of variance showed significant stress condition by trial interactions for systolic BP (F(5,27) = 27.8, p < 0.001) and diastolic BP (F(5,27) = 18.6, p < 0.001). There were no significant differences in the magnitude of systolic or diastolic BP responses to tasks between vaccine and placebo groups. Vaccine alone in the absence of stress did not cause any increase in systolic or diastolic BP. However, there was a small progressive increase in systolic and diastolic BP across trials in the two rest groups (F(5,13) = 2.93 and 7.99, respectively, p < 0.05). Analysis of heart rate also produced a significant stress condition by trial interaction (F(5,27) = 9.78, p < 0.001). Heart rate did not increase significantly during the Stroop task, but did rise in response to the Speech task (Fig. 2C). Again there were no significant differences in heart rate responses between vaccine and placebo groups in either stress or rest conditions.
Fig. 2.
Systolic blood pressure (A), diastolic blood pressure (B), and heart rate (C) at baseline (Base), during each of the tasks (Stroop, Speech) and at 25–30, 55–60 and 115–120 min post-tasks. Comparison of stress (solid lines) and rest (dotted lines) conditions in participants receiving typhoid vaccine (circles) or saline placebo (squares). Data are presented as means ± SEM.
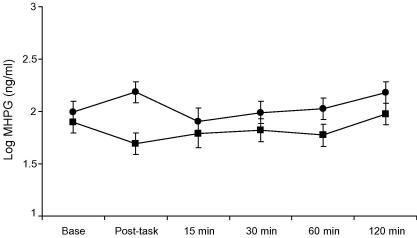
The analysis of MHPG showed a main effect of trial (F(5,27) = 2.51, p = 0.044) together with a significant vaccine by stress condition by trial interaction (p = 0.022). Separate analyses indicated that the vaccine by trial interaction was significant under stress conditions (p = 0.028) but not rest conditions (p = 0.77). The responses to stress in the vaccine and placebo conditions are shown in Fig. 3. Participants in the vaccine/stress group showed a mean increase of log MHPG of 0.19 (SD 0.24) ng/ml, compared with a decrease of −0.20 (SD 0.39) ng/ml in the placebo/stress group (p = 0.002). Cortisol showed a main effect of trial (F(5,26) = 8.81, p < 0.001) due to a progressive reduction across trials, with no difference between groups (data not shown).
Fig. 3.
Salivary 3-methoxy-phenylglycol (MHPG) concentrations before (Base), immediately following tasks (post-task) and at 15, 30, 60 and 120 min post-tasks. Comparison of MHPG stress responses in participants receiving typhoid vaccine (circles) or saline placebo (squares). MHPG was assessed using gas chromatography mass spectrometry. Distributions of MHPG were skewed, so were log transformed prior to analyses. Data are presented as log means ± SEM.
3.3. Interleukin-6 responses
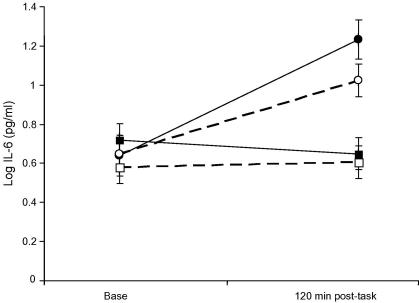
Analysis of serum IL-6 (which was logged before analysis) showed a significant vaccine by stress condition by trial interaction (F(1,50) = 5.51, p = 0.023). IL-6 increased in response to the vaccine, but remained stable in the placebo conditions (Fig. 4). Post-hoc tests using Tukey’s LSD confirmed that the increase in IL-6 was significantly greater in the vaccine/stress compared to vaccine/rest conditions (p = 0.04), with the rise averaging 0.90 (SD 0.28) and 0.59 (SD 0.43) in the two groups. Stress had no effect on IL-6 in the absence of vaccine in this study.
Fig. 4.
Serum interleukin-6 (IL-6) concentrations before (Base) and 120 min post-tasks (120 min post-task). Comparison of stress (solid lines) and rest (dotted lines) conditions in participants receiving typhoid vaccine (circles) or saline placebo (squares). IL-6 was measured using a high-sensitivity two-site ELISA. Distributions of IL-6 were skewed, so were log transformed prior to analyses. Data are presented as log means ± SEM.
3.4. Mood and somatic symptom responses, and relationship with IL-6
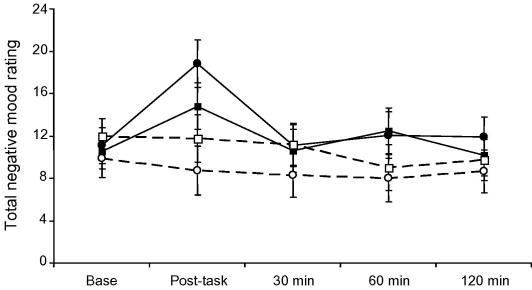
Analysis of total negative mood scores from the Profile of Mood States questionnaire showed a significant stress condition by trial interaction (F(4,22) = 6.25, p < .001), together with a vaccine by stress condition by trial cubic effect (F(1,55) = 4.62, p = .036). These effects are illustrated in Fig. 5. The stress condition by trial interaction was due to an increase in negative mood immediately post-tasks in the two stress groups. This increase was greater in the vaccine/stress compared to placebo/stress groups. In post-hoc analyses, the difference between vaccine and placebo responses to stress was significant (p = 0.05). Analysis of the individual POMS scales making up total negative mood revealed significant differences in acute responses for confusion (p = 0.01) and fatigue (p = 0.003), but not for tension–anxiety, anger, or depression. Confusion increased between baseline and post-task by 54.6% in the vaccine/stress compared with 7.6% in the placebo/stress condition. In the case of fatigue, there was an 8.6% rise in vaccine/stress and 33% reduction in the placebo/stress condition. There were no significant changes in somatic symptom ratings (fever, aching joints, nausea and headache) or body temperature throughout the study, and no differences related to vaccine or stress conditions. Interleukin-6 responses were not related to total negative mood ratings immediately post-stress, in any of the groups. However, in the vaccine/stress group only, absolute levels of IL-6 at 2 h post-stress correlated with increases in fatigue at 2 h (r = 0.57, p = 0.027).
Fig. 5.
Total negative mood scores at baseline (Base), immediately following tasks (post-task) and at 30, 60 and 120 min post-tasks. Comparison of stress (solid lines) and rest (dotted lines) conditions in participants receiving typhoid vaccine (circles) or saline placebo (squares). Data are presented as means ± SEM.
3.5. Relationship between cardiovascular and neuroendocrine measures, cytokine responses and mood
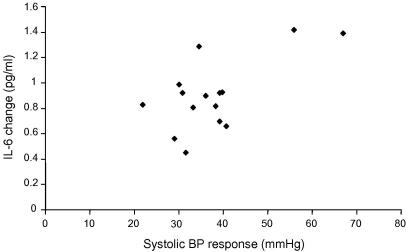
There was no association between IL-6 and systolic blood pressure at baseline. However, in the vaccine/stress condition, the IL-6 response correlated with systolic blood pressure responses to the speech task (r = 0.65, p = .009) (Fig. 6). In the vaccine/stress group, there was also a significant association between absolute levels of IL-6 at 2 h post-stress and systolic blood pressure at 2 h (r = 0.44, p = .023). There was no significant relationship between IL-6 measures and blood pressure responses in either of the placebo conditions, and no association between IL-6 and measures of diastolic blood pressure or heart rate.
Fig. 6.
Scatter plot illustrating the relationship between systolic blood pressure responses to the Speech task and changes in serum interleukin-6 (IL-6) levels, in the vaccine/stress group.
In the vaccine/stress group only, the increase in IL-6 at 2 h was associated with larger increases in MHPG between baseline and 1 h post-stress, independently of baseline MHPG. (r = 0.60, p = 0.023) and with higher cortisol levels at 2 h post-stress (r = 0.70, p = 0.005), independently of baseline cortisol. These effects were not seen in any of the other three groups. There was no significant relationship between any of the cardiovascular or neuroendocrine measures and mood ratings.
4. Discussion
Here we investigated whether acute psychological stress could upregulate inflammatory cytokine and mood responses to a peripheral immune stimulus, Salmonella typhi vaccine, in men. To the best of our knowledge, this is the first study to directly investigate a synergistic effect of psychological and immune stressors on sickness behaviour in healthy humans. We predicted that acute stress would enhance inflammatory cytokine responses to typhoid vaccine in men and that this mechanism would lead to exaggerated infection-related sickness symptoms.
In line with our hypotheses, typhoid vaccine but not placebo induced a significant increase in participants’ serum levels of the pro-inflammatory cytokine IL-6, and exposure to acute psychological stress enhanced the IL-6 response to vaccine. This is in agreement with previous reports in animals showing that acute psychological (or heterotypic) stressors upregulate inflammatory cytokine responses to immune pathogens (Anisman et al., 2007; Gandhi et al., 2007; Gibb et al., 2008; Johnson et al., 2002). Somewhat unexpectedly, circulating IL-6 levels were not altered in the placebo/stress group. Our group and others have previously shown that acute psychological stress increases circulating levels of IL-6 in healthy humans, reaching maximum levels at 2 h post-stress (Steptoe et al., 2007). However, participants in this study were all male, and there is evidence that women have larger IL-6 stress responses than men (Steptoe et al., 2007).
As predicted, we also found that acute psychological stress increased subjective ratings of negative mood in participants, and this effect was particularly marked in the group who had received typhoid vaccine versus placebo. The heightened mood response in the presence of both psychological and immune stress is concordant with previously reported synergistic effects of heterotypic stressors on sickness responses in animals (Anisman et al., 2007; Gandhi et al., 2007; Gibb et al., 2008; Simmons and Broderick, 2005). However, the kinetics of the mood response and the observed lack of mood change in participants receiving typhoid vaccine alone were somewhat unexpected, based on our previous research using the same experimental model. In two separate studies of healthy young men and women, we found that typhoid vaccine induced a sharp decline in mood between 1.5 and 3 h post-vaccination, with mood levels continuing to fall until 6 h post-vaccine (Strike et al., 2004; Wright et al., 2005). In contrast, in the current investigation, changes in negative mood occurred rapidly in the vaccine/stress group, peaking immediately post-stress (1 h post-vaccine) then returning to baseline levels by 30 min post-stress, and mood was unaltered in the vaccine/rest comparison group. Participants in the current study remained in the laboratory throughout the entire testing session, whereas in previous studies participants were free to return to their normal college schedule between assessments, and it is conceivable that this might have affected responses. Furthermore, animal evidence suggests that combined exposure to two heterotypic stressors may alter the dynamics of stress responses. Prior exposure to immobilisation stress in rats significantly increased circulating as well as central levels of IL-1β 1 h following LPS injection, but did not potentiate IL-1 β responses at later time points (Johnson et al., 2002). This is consistent with a rapid, transient effect of psychological stress on sickness responses to typhoid vaccine in humans.
Both circulating IL-6 levels and negative mood scores were highest in the vaccine/stress group, suggesting that psychological and immune stressors synergistically increased sickness behaviour through up-regulating this cytokine. However, there was no significant relationship between serum IL-6 levels and negative mood assessed immediately post-stress. Although IL-6 is a useful index of inflammation, it acts as part of a network of several different cytokines, and is induced primarily by interleukin-1β (IL-1β). It is thus conceivable that IL-6 does not directly affect mood, but instead reflects the production of another cytokine such as IL-1β that modulates mood. Supporting this, peripheral administration of IL-1β or bacterial LPS reliably induces a variety of sickness behaviours in animals, whereas administration of IL-6 alone does not directly induce sickness behaviour but rather potentiates the effects of IL-1 (Dantzer et al., 2007; Lenczowski et al., 1999). In line with the kinetics of our observed mood response, IL-1β expression precedes the IL-6 response to peripheral LPS (Dantzer et al., 2007), and monocyte–macrophage levels of IL-1β increase rapidly (within 30 min) following exposure to acute psychological stress, whereas stress-induced elevations in plasma IL-6 tend to be delayed (Brydon et al., 2004; Brydon et al., 2005; von Kanel et al., 2006). Unfortunately we do not have data on serum levels of IL-1β since it acts predominately in an autocrine/paracrine manner and is difficult to detect in healthy circulating blood (Dinarello, 1996). Notably, analyses of the POMS subscales revealed that in the vaccine/stress group only, post-stress IL-6 levels were associated with increases in fatigue at 2 h (3 h post-vaccine). This corresponds to our previous results in women showing that IL-6 responses to typhoid vaccine correlated with a decline in mood 3 h following vaccination (Wright et al., 2005), and suggests that IL-6 may play a more important role in later mood responses.
A number of pathways have been implicated in stressor-induced sickness responses. Psychological stress activates the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) axis, resulting in elevated blood pressure and heart rate and increased circulating levels of the ‘stress hormones’ catecholamines and glucocorticoids, which modulate inflammatory cytokine production through binding to their respective receptors on peripheral immune cells (Black and Garbutt, 2002). At the same time, peripheral cytokines (IL-1, IL-6, TNFα) released during infection and/or psychological stress, can feedback to the brain to exert potent effects on neuroendocrine function and neurotransmitter metabolism (Dantzer et al., 2007). In animals, cytokines and/or stressor-induced behavioural changes are associated with increased neuroendocrine activity, as well as altered neurotransmitter turnover in brain regions essential to the regulation of emotion, motor function and reward (Anisman and Merali, 2003; Dunn, 2006). Notably, these responses are significantly more pronounced in animals concurrently exposed to psychological and immune stressors (Anisman et al., 2007; Gandhi et al., 2007; Gibb et al., 2008; Merali et al., 1997; Song et al., 1999), suggesting that these pathways may mediate the synergistic effects of heterotypic stressors on sickness. In humans, hyperactivity of neuroendocrine pathways and disrupted neurotransmitter metabolism have been observed in clinically depressed people (Hayley et al., 2005) and in patients undergoing IFN-α therapy, where they correlate with development of specific sickness symptoms (Capuron and Miller, 2004). In the current study, there were no differences between vaccine and placebo conditions in either cardiovascular or neuroendocrine responses to stress. However, in the vaccine/stress group, participants with greater increases in IL-6 had larger systolic blood pressure responses to the Speech task, elevated post-stress salivary MHPG, and prolonged increases in systolic BP following tasks, suggesting that hyperactivity of the SNS may mediate the heightened inflammatory and mood responses in this group. Supporting this idea, catecholamines increase plasma levels of IL-6 in rodents, whereas in vivo blockade of the autonomic nervous system or pre-treatment with β-adrenergic receptor (β-AR) antagonists attenuates stress-induced increases in plasma IL-6 (Johnson et al., 2005; Mohamed-Ali et al., 2001) and prevents potentiation of LPS-induced macrophage cytokine production by foot-shock stress in animals (Broug-Holub et al., 1998). Whether β-AR antagonists would prevent the synergistic effects of typhoid vaccine and psychological stress observed in our study remains to be investigated.
Stress-induced enhancement of the innate immune response to infection and consequent sickness symptoms can be thought of as an evolutionary adaptive mechanism, designed to conserve energy and combat infection during times of adversity such as wounding due to a predator-prey encounter (Hart, 1988). However, in the context of ongoing inflammation, these effects may become detrimental. In humans, stressful life events have been linked to the onset and exacerbation of illness symptoms in a number of chronic inflammatory conditions where cytokines are elevated, including rheumatoid arthritis, cardiovascular disease, multiple sclerosis, asthma and psoriasis (Black and Garbutt, 2002; Kemeny and Schedlowski, 2007; Perry et al., 2007). Notably, depressive symptoms are prevalent in many of these conditions, and are predictive of increased disease morbidity and mortality (Evans et al., 2005; Irwin and Miller, 2007).
This investigation was carried out in a sample of healthy male university students, and results may not generalise to other populations. Blood was sampled at a single post-stress time point (2 h), making it difficult to determine when the peak cytokine response occurred, and a more detailed cytokine profile including earlier time points would have been useful. Cytokine and mood responses were relatively small. However, this is consistent with the fact that Salmonella typhi vaccine is a mild inflammatory stimulus compared to other endotoxin models (Krabbe et al., 2005; Wright et al., 2005). The correlation between IL-6 and systolic BP responses was dependent on extreme responders, so corroboration in a larger data set would be desirable. We also did not measure peripheral neurotransmitter levels and it will be important to carry out additional studies to investigate the potential contribution of these pathways, as well as the mechanisms underlying differences between our current findings and previous observations using the same inflammatory model.
Taken together, our results provide added insight into the biological mechanisms through which psychological and immune stressors interact to exacerbate sickness behaviour in humans. Improved understanding of these mechanisms may inform the development of new psychopharmacological interventions to combat the depressive symptoms that commonly accompany inflammatory disease, thereby improving patient outcomes.
Acknowledgments
This study was supported by the British Heart Foundation. We are grateful to Dr. Linda Perkins-Porras, Dr. Mimi Bhattacharyya, Bev Milne and Bev Murray for their assistance with vaccination.
References
- Anisman H., Merali Z. Cytokines, stress and depressive illness: brain–immune interactions. Ann. Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Anisman H., Poulter M.O., Gandhi R., Merali Z., Hayley S. Interferon-α effects are exaggerated when administered on a psychosocial stressor backdrop: cytokine, corticosterone and brain monoamine variations. J. Neuroimmunol. 2007;186:45–53. doi: 10.1016/j.jneuroim.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Black P.H., Garbutt L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Broug-Holub E., Persoons J.H., Schornagel K., Mastbergen S.C., Kraal G. Effects of stress on alveolar macrophages: a role for the sympathetic nervous system. Am. J. Respir. Cell Mol. Biol. 1998;19:842–848. doi: 10.1165/ajrcmb.19.5.3103. [DOI] [PubMed] [Google Scholar]
- Brydon L., Edwards S., Mohamed-Ali V., Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav. Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Brydon L., Edwards S., Jia H., Mohamed-Ali V., Zachary I., Martin J.F., Steptoe A. Psychological stress activates interleukin-1β gene expression in human mononuclear cells. Brain Behav. Immun. 2005;19:540–546. doi: 10.1016/j.bbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Capuron L., Miller A.H. Cytokines and psychopathology: lessons from interferon-α. Biol. Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Dantzer R., Bluthe R.M., Castanon N., Kelly K.W., Konsman J.-P., Laye S., Lestage J., Parnet P. Cytokines, sickness behavior, and depression. In: Ader R., editor. Psychoneuroimmunology. Elsevier; New York: 2007. pp. 281–318. [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dunn A.J. Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.L., Charney D.S., Lewis L., Golden R.N., Gorman J.M., Krishnan K.R., Nemeroff C.B., Bremner J.D., Carney R.M., Coyne J.C., DeLong M.R., Frasure-Smith N., Glassman A.H., Gold P.W., Grant I., Gwyther L., Ironson G., Johnson R.L., Kanner A.M., Katon W.J., Kaufmann P.G., Keefe F.J., Ketter T., Laughren T.P., Leserman J., Lyketsos C.G., McDonald W.M., McEwen B.S., Miller A.H., Musselman D., O’Connor C., Petitto J.M., Pollock B.G., Robinson R.G., Roose S.P., Rowland J., Sheline Y., Sheps D.S., Simon G., Spiegel D., Stunkard A., Sunderland T., Tibbits P., Jr., Valvo W.J. Mood disorders in the medically ill: scientific review and recommendations. Biol. Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Gandhi R., Hayley S., Gibb J., Merali Z., Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav. Immun. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Gibb J., Hayley S., Gandhi R., Poulter M.O., Anisman H. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav. Immun. 2008;22:573–589. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hayley S., Poulter M.O., Merali Z., Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Hingorani A.D., Cross J., Kharbanda R.K., Mullen M.J., Bhagat K., Taylor M., Donald A.E., Palacios M., Griffin G.E., Deanfield J.E., MacAllister R.J., Vallance P. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Miller A.H. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Johnson J.D., Campisi J., Sharkey C.M., Kennedy S.L., Nickerson M., Greenwood B.N., Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson J.D., O’Connor K.A., Deak T., Stark M., Watkins L.R., Maier S.F. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav. Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Kemeny M.E., Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav. Immun. 2007;21:1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski L.M., Prescott C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Krabbe K.S., Reichenberg A., Yirmiya R., Smed A., Pedersen B.K., Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav. Immun. 2005;19:453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lenczowski M.J., Bluthe R.M., Roth J., Rees G.S., Rushforth D.A., van Dam A.M., Tilders F.J., Dantzer R., Rothwell N.J., Luheshi G.N. Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am. J. Physiol. 1999;276:R652–R658. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- Lesperance F., Frasure-Smith N., Theroux P., Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am. J. Psychiatry. 2004;161:271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- McNair, D. M., Lorr, N., Droppleman, L. F., 1981. Manual for the Profile of Mood States
- Merali Z., Lacosta S., Anisman H. Effects of interleukin-1β and mild stress on alterations of norepinephrine, dopamine and serotonin neurotransmission: a regional microdialysis study. Brain Res. 1997;761:225–235. doi: 10.1016/s0006-8993(97)00312-0. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V., Flower L., Sethi J., Hotamisligil G., Gray R., Humphries S.E., York D.A., Pinkney J. β-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 2001;86:5864–5869. doi: 10.1210/jcem.86.12.8104. [DOI] [PubMed] [Google Scholar]
- Perry V.H., Cunningham C., Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuster T., Rilke O., Oehler J. High correlation between salivary MHPG and CSF MHPG. Psychopharmacol. (Berl) 2002;162:415–418. doi: 10.1007/s00213-002-1125-z. [DOI] [PubMed] [Google Scholar]
- Simmons D.A., Broderick P.A. Cytokines, stressors, and clinical depression: augmented adaptation responses underlie depression pathogenesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:793–807. doi: 10.1016/j.pnpbp.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Song C., Merali Z., Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience. 1999;88:823–836. doi: 10.1016/s0306-4522(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Hamer M., Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Strike P.C., Wardle J., Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. J. Psychosom. Res. 2004;57:189–194. doi: 10.1016/S0022-3999(03)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R., Kudielka B.M., Preckel D., Hanebuth D., Fischer J.E. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav.Immun. 2006;20:40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wright C.E., Strike P.C., Brydon L., Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav. Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yang R.K., Yehuda R., Holland D.D., Knott P.J. Relationship between 3-methoxy-4-hydroxyphenylglycol and homovanillic acid in saliva and plasma of healthy volunteers. Biol. Psychiatry. 1997;42:821–826. doi: 10.1016/s0006-3223(97)00055-3. [DOI] [PubMed] [Google Scholar]
- Yajima J., Tsuda A., Yamada S., Tanaka M. Determination of saliva free-3-methoxy-4-hydroxyphenylglycol in normal volunteers using gas chromatography mass spectrometry. Biogenic Amines. 2001;16:173–183. [Google Scholar]
- Zautra A.J., Yocum D.C., Villanueva I., Smith B., Davis M.C., Attrep J., Irwin M. Immune activation and depression in women with rheumatoid arthritis. J. Rheumatol. 2004;31:457–463. [PubMed] [Google Scholar]