Abstract
The present functional magnetic resonance imaging (fMRI) study investigated whether age-related differences in the neural correlates of successful memory encoding are modulated by memory performance. Young (mean age 22 years; N = 16) and older (mean age 69 years; N = 32) subjects were scanned while making animacy decisions on visually presented words. Memory for the words was later assessed in a recognition test, allowing fMRI activity elicited by study words to be contrasted according to subsequent memory performance. Young and older adults exhibited equivalent subsequent memory effects (enhanced activity for later remembered items) in an extensive network that included left inferior prefrontal cortex and anterior hippocampus. In posterior cingulate cortex, reversed subsequent memory effects (greater activity for later forgotten items) were of greater magnitude in young subjects. A voxel-of-interest analysis conducted on left and right prefrontal subsequent memory effects revealed that the effects were distributed more bilaterally in older than in young subjects, replicating previous findings. This age-related difference was confined to older subjects with relatively poor recognition performance, who were also the only group to demonstrate statistically significant right prefrontal subsequent memory effects. The findings suggest that relative preservation of memory performance with increasing age does not depend upon right prefrontal “over-recruitment.”
Keywords: cognitive aging, compensation, episodic memory, hemispheric asymmetry reduction, prefrontal cortex
Introduction
Episodic memory—memory for unique events—declines markedly with increasing age, in contrast to short-term, semantic, and implicit memory, which show more modest age-related effects (Craik 1977; Light 1991; Craik and Jennings 1992; Nilsson 2003). A number of different explanations have been put forward for why episodic memory demonstrates such a marked age-related decline. These range from accounts proposing that the decline is just one expression of a more general decline in processing efficiency (e.g., Birren 1965; Craik and Byrd 1982; Cerella 1985; Salthouse 1985, 1996), to accounts that argue the decline reflects age-related changes in specific mnemonic processes (e.g., Jennings and Jacoby 1993; Naveh-Benjamin 2000; Howard et al. 2006; Prull et al. 2006). Proponents of these latter accounts have highlighted the uneven age-related reduction in the volume of brain regions associated with various cognitive processes (e.g., Raz 1996, 2000; Raz et al. 2005). For example, consistent age-related volume reductions are observed in both prefrontal cortex and the medial temporal lobe (e.g., Burke and Barnes 2006; Raz et al. 2007), regions that are particularly critical for episodic memory (Moscovitch et al. 2007).
Numerous studies employing functional neuroimaging methods have investigated whether age-related differences in episodic memory performance are accompanied by differences at the neural level. These studies have investigated age-related effects both at the time of encoding (e.g., Grady et al. 1995; Logan et al. 2002; Daselaar et al. 2003a; Grady et al. 2003; Morcom et al. 2003) and retrieval (e.g., Schacter et al. 1996; Cabeza et al. 2000; Schiavetto et al. 2002; Daselaar et al. 2003b; Morcom et al. 2003; Duverne et al. 2007; Velanova et al. 2007), generally reporting reliable age-related differences in both cases. Detailed findings differ across studies, but arguably the most consistent finding has been that, relative to younger subjects, older adults tend to demonstrate a pattern of “over-recruitment,” exemplified in several studies by a more bilateral pattern of memory-related activity than that evident in the young (e.g., Cabeza et al. 1997; Madden and Turkington 1999; Cabeza et al. 2002; Rosen et al. 2002; Maguire and Frith 2003; Morcom et al. 2003; Cabeza et al. 2004; Grady et al. 2005; Gutchess et al. 2005; Fernandes et al. 2006; van der Veen et al. 2006). In some studies, this pattern is accompanied by reduced memory-related activity in other regions activated in the young, prompting some authors to argue that over-recruitment reflects a compensatory response to an age-related decline in the functional integrity of these regions (e.g., Cabeza et al. 1997, 2002; Rosen et al. 2002; Cabeza et al. 2004; Grady et al. 2005; Gutchess et al. 2005; see also reviews by Cabeza 2002; Reuter-Lorenz and Lustig 2005). It has also been suggested, however, that over-recruitment (at least when in the form of a more bilaterally distributed pattern task-related activity) may be a reflection of age-related changes in neural function, such as decreased transcallosal inhibition, that have a detrimental rather than a compensatory impact on cognitive performance (see Buckner and Logan 2002; Logan et al. 2002).
An important question in relation to the functional significance of age-related over-recruitment concerns its relationship to task performance. Using functional magnetic resonance imaging (fMRI) with a blocked design, Rosen et al. (2002) operationalized encoding-related activity as the difference in activity associated with blocks of semantic versus nonsemantic judgments performed on visually presented words. They segregated their group of 14 older subjects according to mean score on 4 standard memory tests, and reported that the 7 highest scoring subjects had greater levels of encoding-related activity in right prefrontal cortex than either their lower-scoring counterparts, or a group of young subjects. This finding suggests that a more bilateral pattern of task-related activity is beneficial for memory performance. Cabeza et al. (2002) adopted a similar strategy in a retrieval study that used positron emission tomography (PET), employing a contrast between test blocks requiring either a source memory judgment or free recall. The high- and low-performing older subjects (Ns of 8 per subgroup) were segregated according to performance on a composite score on 4 standard memory tests. Whereas the contrast between recall and source tasks in both young and low-performing older groups revealed greater activity in right prefrontal cortex only, it revealed a bilateral prefrontal increase in activity in the high-performing older subjects. This finding too is consistent with the proposal that bilateral prefrontal recruitment is associated with relatively good memory performance. The impact of these 2 studies (Cabeza et al. 2002; Rosen et al. 2002) is, however, limited by the small sample sizes.
Findings contrasting with those of Cabeza et al. (2002) and Rosen et al. (2002) were reported by Persson et al. (2006) and Miller et al. (2008). Persson et al. (2006) compared nondemented older subjects in whom performance on a battery of memory tests had remained stable over the preceding few years with older subjects whose memories had significantly declined over the same time period (Ns = 20 per group). Using fMRI with a blocked design, they extracted the activity (relative to a fixation baseline) associated with engagement in a semantic classification task from 2 pairs of homotopic regions of interest in prefrontal cortex. A between-group difference was observed in right ventrolateral prefrontal cortex in the vicinity of Brodmann area (BA) 47. This took the form of greater activity in the declining than in the stable group. Miller et al. (2008) employed event-related fMRI to identify “subsequent memory effects” (greater activity for later remembered compared with later forgotten study items) associated with successful encoding of face-name pairs. Within their older group (N = 17), subjects performing below median on the later associative memory test demonstrated enhanced subsequent memory effects in right superior prefrontal cortex, along with a trend toward enhanced effects in the hippocampus. This pattern of over-recruitment in the low-performing older subjects was accompanied by a reduction in the magnitude of medial parietal “reversed” subsequent memory effects (i.e., greater activity for forgotten relative to remembered items). Both Persson et al. (2006) and Miller et al. (2008) interpreted the over-recruitment effects in their poorer performing older subgroups as a compensatory response to age-related decline in the functional integrity of the neural network subserving episodic encoding and associated cognitive operations.
In the present study, we also investigate the relationship between memory-related activity in prefrontal cortex and memory performance. The study takes as its starting point the findings of Morcom et al. (2003), who used fMRI to investigate the effects of age on the neural correlates of successful encoding of visually presented words. In that study subsequent memory effects common to young and older subjects were evident in several cortical regions, including the left inferior frontal gyrus and left hippocampal formation. The most impressive difference between age groups was with respect to the prefrontal cortex, where subsequent memory effects were considerably more strongly left lateralized in the younger subjects. Importantly, Morcom et al. (2003) manipulated retrieval performance by testing memory at 2 different poststudy intervals. The performance of the older group on the study items tested after the shorter interval was equivalent to that of the young subjects on items tested after the longer interval. The accompanying prefrontal subsequent memory effects still manifest age-related differences in lateralization, strongly suggesting that the differences were truly age-related, and not merely a reflection of disparate levels of memory performance in the 2 groups.
As was discussed by Morcom et al. (2003), the finding that prefrontal subsequent memory effects are more bilaterally distributed in older than in younger subjects is open to a range of interpretations. As already alluded to, the finding might reflect an adaptive or compensatory mechanism that boosts encoding beyond what could be achieved by the left hemisphere alone, consistent with the compensation hypothesis of Cabeza (2002). Alternatively, right-lateralized encoding effects might reflect a breakdown in functional cortical specialization, perhaps reflecting degradation of transcallosal inhibition (Buckner and Logan 2002; Logan et al. 2002). Another possibility is that the finding reflects differences in the cognitive operations engaged by young and older subjects during the processing of the study items. That is, bilateral subsequent memory effects might be a consequence of the employment of a processing strategy that happens to be supported by bilateral rather than unilateral prefrontal cortex (e.g., Morcom et al. 2003; Dennis et al. 2007).
One line of evidence that would help arbitrate between these competing accounts concerns the relationship between age-related shifts in the lateralization of subsequent memory effects and memory performance. The finding of a positive relationship between performance and bilateral effects would support the proposal that recruitment of the right hemisphere benefits encoding and ameliorates age-related memory impairment (cf. Cabeza et al. 2002; Rosen et al. 2002). Evidence for the reverse relationship would constitute evidence that bilateral encoding-related activity is not necessary for encoding to be effective in older individuals. Such a finding would be equally consistent with the proposals that recruitment of the right hemisphere is 1) detrimental to encoding (Logan et al. 2002), or 2) an adaptation to a relatively low level of mnemonic or more general cognitive functions (Miller et al. 2008).
Accordingly, we assessed fMRI subsequent memory effects in a sufficiently large sample of older subjects to allow the relationship between lateralization of subsequent memory effects and memory performance to be adequately characterized. By including a group of younger subjects we were also able to directly assess the generality of the findings from the original study of Morcom et al. (2003).
Methods
Participants
Sixteen young healthy adults (9 females) aged between 18 and 29 years (mean age: 22), and 32 community-dwelling older healthy adults (22 females) aged between 63 and 76 years (mean age: 69) participated in the experiment. Data collected from one additional older adult were excluded from all analyses because of excessive head movement during scanning. Data from one other older adult were excluded because of abnormal signal in frontal cortex. Young adults were recruited from the undergraduate and graduate student population of University of California, Irvine, and older adults were recruited from the surrounding community.
All subjects were right-handed, English native speakers, with a minimum of 12 years education and normal or corrected-to-normal vision. They were free from neurological, cardiovascular, and psychiatric disease and none was taking central nervous system–active medication. Nine of the included older subjects were taking antihypertensive medication. The study was approved by the Institution Review Board of the University of California, Irvine. Informed consent was obtained prior to participation in any experimental session.
Neuropsychological Testing
A battery of standardized neuropsychological tests was administered to all subjects in a separate session from the fMRI procedure. The battery assessed a range of cognitive functions known to either decline or to be maintained with age (see Duverne et al. 2007 for a detailed description). The Mini Mental State Examination was employed as a dementia screening measure. A nominal cut-off score of 26/30 was adopted, although no potential subject was rejected on the basis of this criterion. Long-term memory was assessed with the California Verbal Learning Test-II (Delis et al. 2000) and the Immediate and Delayed New York University (NYU) paragraph (Kluger et al. 1999). Short-term memory was assessed with the Digit Span Forward and Backward test of the Wechsler Adult Intelligence Scale-Revised (WAIS-R). General cognitive functions were further assessed with the Digit/Symbol Coding test of the WAIS-R, the Trail Making Test A and B, and letter fluency and category fluency tests. An estimate of full-scale IQ was obtained from the Wechsler Test of Adult Reading (Wechsler 2001). The Beck Depression Inventory was also administered. Data for one older subject were not obtained on the long-delay paragraph recall test because of a procedural error.
Experimental Stimuli
The experimental stimuli comprised 240 words representing different common objects. The words were randomly allocated to 3 sublists of 80 words each with the constraint that, within each sublist, half of the words represented animate objects and the other half inanimate objects. Sixteen sets of stimulus lists were created by assigning 2 of sublists to the “study item” condition and the third sublist to the “new item” condition. The assignment of sublists to each the old and new conditions was counterbalanced across the 16 sets of stimuli lists so that a given stimulus was equally likely to appear in each condition.
At study, 160 word stimuli were intermixed with 80 null (fixation only) events. At test, the 160 studied items were intermixed with 80 new unstudied items. For both study and test lists, the sequences of events were pseudorandomly ordered such that no more than 3 items belonging to the same word class occurred sequentially. Both study and test lists were divided into 2 blocks, and 2 filler items at the beginning of each block preceded the critical items. An additional 12 words were used to create 2 practice lists for the study task (see below). A further 6 additional items were used as new items for the practice session of the recognition memory task. The new items were intermixed with the 6 studied items from the second study practice list.
Experimental Tasks and Procedures
The experimental procedure closely resembled that of Morcom et al. (2003). It consisted of an incidental study task during which scanning took place, followed by a recognition memory test outside the scanner that started approximately 25 min after the end of the study task.
At study, subjects were informed that they would see words on the screen, presented one at a time, and that their task was to decide whether or not the word referred to or was part of an animate or an inanimate object. Half of the subjects were asked to press buttons with their left and right thumbs to signal animate and inanimate judgments, respectively, and the reverse response assignment was employed with the remaining subjects. Instructions emphasized the need for both speed and accuracy. There were 2 brief practice study sessions, one before entering the scanner and one in the scanner, so that subjects could familiarize themselves with the study task and environment. Subjects were not informed their memory for the words would be tested later. When positioned in the scanner subjects were able to view the experimental stimuli via a mirror directly above their eyes, responding via a hand-held button box. Prior to the study phase a 10-min structural scan was conducted, following which the second practice session was undertaken. The study task proper comprised 2 blocks of 122 trials each, during which the functional scans were acquired in a single session. A total of 160 critical words were shown, one at a time, in pseudorandom order, interspersed with the 80 null events. Two filler words were also presented at the beginning of each block. A white fixation cross was continuously present during the intertrial interval and throughout null trials. The presentation of each study word was preceded for 650 ms by a warning signal, which consisted in a change in the fixation symbol from white to red. The screen was then blanked for 150 ms, following which the word was shown for 300 ms. Words were presented in white uppercase Helvetica 30-point font on a black background and subtended an approximate vertical visual angle of 1° and a maximum horizontal visual angle of 4°. Stimulus presentation was followed by another blanked screen for 150 ms, and then the reappearance of the white fixation cross. Stimulus-onset asynchrony in the absence of intervening null trials was 3 s. Between the 2 study blocks, a short break occurred for a total duration of 30 s. Subjects saw the message “Short break” for 23 s, followed by the message “Get ready” for 5 s and then a white fixation point for 2 s. Subjects were required to remain immobile during the break.
After completing the study task, subjects were taken to another building where they were informed about the recognition memory task. It was explained that the words they had studied in the scanner would be shown again, intermixed with new words that they had not seen during the session. Subjects were required to judge whether each test word was old or new, and to indicate their degree of confidence associated with their judgment. Thus, one of 4 keys was pressed according to whether the word was 1) confidently judged to be old, 2) nonconfidently judged to be old, 3) confidently judged to be new, or 4) nonconfidently judged to be new. Responses were made with the middle and index fingers of the left and right hands, which rested on the q, w, o, and p keys of a keyboard placed on a table in front of the subject. The middle fingers were always used for confident responses. The assignment of old responses to the left or right hand was counterbalanced across subjects. Instructions were to respond as fast as possible without sacrificing accuracy. A brief practice was given to familiarize subjects with the task. Subjects then undertook 2 test blocks of 122 trials, each about 10 min in duration. Each trial consisted of a warming signal (red fixation point) for 650 ms, a 150-ms blank period and the stimulus presentation for 300 ms. All stimuli were presented visually on a computer screen, using the same format and font as at study. The words subtended an approximate visual angle of 1° x 4° and were displayed for 300 ms, after which the screen was blanked until a response was provided. The interval between each response and presentation of the subsequent test items was 3 s. A short rest was provided half-way through the test task.
Functional Data Acquisition
A Philips Achieva 3T MR scanner (Philips Medical Systems, Bothell, WA) was used to acquire both T1-weighted anatomical volume images (256 × 200 acquisition matrix, 1-mm3 voxels, 150 slices, axial acquisition, 3D Magnetization Prepared-Rapid Gradient Echo [MP–RAGE] sequence) and T2*-weighted echoplanar images (80 × 79 acquisition matrix, 3 mm × 3 mm in-plane resolution, 3-mm slice thickness with 1-mm interslice gap; ascending sequential acquisition, flip angle 70°, echo time 30 ms), with blood oxygenation level–dependent (BOLD) contrast, using a transmit/receive RF head coil. Each EPI volume comprised 30 axial slices positioned to give full coverage of the cerebrum and most of the cerebellum. Data were acquired in one session comprised of 400 volumes, with a repetition time (TR) of 2 s. BOLD signal was sampled with 3 s Stimulus Onset Asynchrony (SOA), using a 0.5 Hz sampling rate. The first 5 volumes were discarded to allow tissue magnetization to achieve a steady state.
fMRI Data Analysis
Data preprocessing and statistical analyses were performed with Statistical Parametric Mapping (SPM5, Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk/spm5.html; Friston et al. 1995) implemented in MATLAB R2006a (The Mathworks, Natick, MA). All volumes were realigned spatially to their mean, and these realigned volumes were then spatially normalized using a sample-specific template. The template was created by normalizing (Ashburner and Friston 1999) the first EPI volume of each subject's functional time series with reference to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain (Cocosco et al. 1997). The resulting normalized volumes were separately averaged within each age group (32 older subjects and 16 young subjects), and these 2 group-wise mean images were then averaged to generate a template that was equally weighted with respect to the 2 age groups. Volumes were resampled into 3-mm isotropic voxels prior to normalization. Normalized volumes were smoothed with an isotropic 10-mm full-width half-maximum Gaussian kernel to accommodate residual anatomical variation between subjects. T1 anatomical images were normalized using a procedure analogous to that applied to the EPI images. Prior to normalization, subjects' T1 images were coregistered to their mean EPI image and the images were resampled into 2-mm isotropic voxels.
Statistical analyses were performed in 2 stages of a mixed effects model. In the first stage, stimulus-elicited neural activity was modeled by a δ function at each stimulus onset, whereas the rest period separating the 2 study blocks was modeled with a 30-s duration boxcar function. These functions were convolved with 2 hemodynamic response functions (HRFs) to yield regressors in a General Linear Model (GLM) that modeled the BOLD response to each event-type. One function (the “early” function) was the canonical HRF as implemented in SPM (Friston et al. 1998). A second (“late”) function was generated by shifting the canonical HRF one TR (2 s) later in time, and was included to capture possible delayed responses. The late function was orthogonalized with respect to the early function using the Gram-Schmidt procedure so as to give priority to the early function (Andrade et al. 1999). Thus, variance common to the early and late functions was allocated to the early function, loadings on the orthogonalized late function accounting only for residual variance unexplained by the early function.
The design matrix of the GLM included 4 early and 4 late covariates that modeled events defined by subjects’ responses during the test phase. These events were 1) studied words attracting a confident “old” judgment at test (“Confident Hit”); 2) studied words attracting either a “new” judgment or a nonconfident “old” judgment (“Forgotten”); 3) events of no-interest (i.e., absent response, multiple responses, incorrect response at study, and fillers); 4) the 30-s break. The design matrix also included 6 covariates modeling movement-related variance (the 3 rigid-body translations and 3 rotations estimated by the realignment procedure) and a constant that modeled the mean over scans.
The time series in each voxel were high-pass filtered to 1/128 Hz to remove low-frequency noise and scaled within-session to a grand mean of 100 across both voxels and scans. Parameter estimates for events of interest were estimated using the aforementioned GLM. Nonsphericity of the error covariance was accommodated by an AR(1) model, in which the temporal autocorrelation was estimated by pooling over suprathreshold voxels (Friston et al. 2002). The parameters for each covariate and the hyperparameters governing the error covariance were estimated using Restricted Maximum Likelihood. Subsequent memory effects (operationalized by the contrast between Confident Hit and Forgotten items) were identified using voxel-wise linear contrasts of the parameter estimates. These contrasts were carried forward to a second stage in which subjects were treated as a random effect.
Results
Neuropsychological Data
Table 1 summarizes the demographic and neuropsychological data for the 2 age groups. As can be seen, the groups are well matched for education and estimated IQ, with the older group demonstrating the typical pattern of lower performance on tests of memory and speeded cognition. Table 2 shows the data from the older group segregated by median split on performance on the experimental task (see below; henceforth the “high-performing” and “low-performing” subgroups). The low-performing subgroup demonstrated significantly lower scores than their high-performing peers on one California Verbal Learning Test (CVLT) subtest (immediate recall), as well as on tests of letter and category fluency and symbol-digit substitution. With the exception of delayed paragraph recall (t(30) = 3.26, P ≤ 0.01), the memory test scores of the high-performing and young groups did not significantly differ. By contrast, scores of the low-performing subgroup were significantly lower than those of the young subjects on all memory tests (t(30) > 2.43, P ≤ 0.05) other than immediate paragraph recall.
Table 1.
Young and older adults' characteristics and raw scores (mean, standard deviation, and ranges) on the neuropsychological tests
| Young adults |
Older adults |
|||||
| Mean | Standard deviation | Ranges | Mean | Standard deviation | Ranges | |
| Age | 21.7 | 3.5 | 18–29 | 68.8 | 3.4 | 63–76 |
| Years of education | 15.6 | 2.3 | 13–22 | 16.2 | 2.2 | 12–21 |
| Mini Mental State Examination | 29.1 | 0.8 | 28–30 | 29.4 | 1.1 | 26–30 |
| CVLT immediate free recall** | 13.7 | 1.6 | 11–16 | 11.4 | 3.0 | 5–16 |
| CVLT immediate cued recall* | 14.1 | 1.4 | 12–16 | 12.9 | 2.2 | 7–16 |
| CVLT delayed free recall* | 13.8 | 1.3 | 11–16 | 12.2 | 2.7 | 6–16 |
| CVLT delayed cued recall* | 14.4 | 1.4 | 12–16 | 13.0 | 2.4 | 7–16 |
| NYU paragraph immediate recall | 8.0 | 3.0 | 5–15 | 7.4 | 2.2 | 2–11 |
| NYU paragraph delay recall* | 11.2 | 2.5 | 8–16 | 9.2 | 2.5 | 5–14 |
| Forward/backward digit span | 19.3 | 3.9 | 15–28 | 19.9 | 4.1 | 13–27 |
| Digit/symbol substitution test*** | 64.6 | 10.9 | 47–92 | 49.4 | 9.1 | 30–70 |
| Trail Making test A*** | 20.1 | 3.8 | 15–28 | 29.0 | 7.5 | 18–52 |
| Trail Making test B*** | 47.3 | 13.3 | 28–70 | 70.5 | 23.7 | 44–143 |
| Letter Fluency | 44.6 | 15.1 | 23–71 | 49.5 | 11.3 | 23–75 |
| Category fluency | 22.3 | 4.0 | 15–28 | 21.6 | 4.2 | 12–32 |
| Wtar FSIQ | 112.1 | 6.8 | 94–122 | 114.5 | 3.7 | 106–119 |
| Beck Depression inventory** | 5.1 | 3.6 | 1–12 | 2.5 | 2.6 | 0–13 |
Note: FSIQ, Wechsler Test of Adult Reading Full Scale Intellectual Quotient.
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
Table 2.
High- and low-performing older adults' characteristics and raw scores (mean, standard deviation, and ranges) on the neuropsychological tests
| High-performing older adults |
Low-performing older adults |
|||||
| Mean | Standard deviation | Ranges | Mean | Standard deviation | Ranges | |
| Age | 68.5 | 3.6 | 63–76 | 69.1 | 3.3 | 63–76 |
| Years of education | 16.2 | 2.5 | 12–21 | 16.2 | 2.00 | 12–20 |
| Mini Mental State Examination* | 29.9 | 0.3 | 29–30 | 28.9 | 1.4 | 26–30 |
| CVLT immediate free recall* | 12.4 | 2.1 | 9–15 | 10.4 | 3.5 | 5–16 |
| CVLT immediate cued recall | 13.5 | 1.5 | 11–16 | 12.3 | 2.6 | 7–16 |
| CVLT delayed free recall | 12.7 | 2.0 | 9–15 | 11.6 | 3.3 | 6–16 |
| CVLT delayed cued recall | 13.8 | 1.3 | 11–15 | 12.2 | 2.9 | 7–16 |
| NYU paragraph immediate recall | 9.6 | 2.6 | 4–10 | 8.9 | 2.3 | 2–11 |
| NYU paragraph delay recall | 8.4 | 2.2 | 5–14 | 8.2 | 1.7 | 5–12 |
| Forward/backward digit span | 19.1 | 3.3 | 15–25 | 20.7 | 4.7 | 13–27 |
| Digit/Symbol substitution test* | 52.7 | 8.9 | 30–70 | 46.0 | 8.3 | 33–63 |
| Trail Making test A | 27.7 | 8.6 | 18–52 | 30.3 | 6.2 | 20–43 |
| Trail Making test B | 68.9 | 22.4 | 44–126 | 72.1 | 25.6 | 47–143 |
| Letter fluency* | 52.7 | 8.9 | 23–75 | 46.0 | 8.3 | 34–59 |
| Category fluency* | 23.2 | 4.0 | 17–32 | 20.0 | 3.9 | 12–27 |
| Wtar FSIQ | 115.3 | 3.7 | 106–119 | 113.7 | 3.5 | 108–119 |
| Beck Depression inventory | 2.7 | 3.1 | 0–13 | 2.3 | 2.2 | 0–6 |
Note: FSIQ, Wechsler Test of Adult Reading Full Scale Intellectual Quotient.
P ≤ 0.05.
In addition to the dichotomous contrasts of the older subjects’ neuropsychological scores reported in Table 2, we also computed the correlations between the subjects’ experimental task performance and each of the scores. Out of the resulting 14 correlation coefficients, only 3 were significant, namely, the correlations with CVLT immediate free recall (r = 0.37, P ≤ 0.05, 2-tailed), symbol-digit substitution (r = 0.37, P ≤ 0.05) and category fluency (r = 0.42, P ≤ 0.05).
Task Performance
Study Task
Accuracy on the study task did not differ with age (mean accuracy of 0.97 in both age groups). This remained the case when the older group was split into the low- and high-performing subgroups. Table 3 shows reaction times (RT) associated with correctly judged study items according to age and whether the items were confidently recognized versus unconfidently recognized or missed on the subsequent memory test (these categories correspond to those employed to estimate fMRI subsequent memory effects, as described below). ANOVA (factors of age group and subsequent memory) revealed significant effects of age (F1,46 = 4.05, P ≤ 0.05) and subsequent memory (F1,46 = 12.42, P ≤ 0.001), but no interaction between these factors (P > 0.4). The 2 main effects reflect slower responding in the older than the younger group (mean RT difference 73 ms) and a small but reliable trend for slower study RTs to items that were confidently recognized on the later test (mean RT difference 16 ms). The size of the subsequent memory effect on study RT did not differ between the low- and high-performing older subgroups (P > 0.1).
Table 3.
Mean response time (and standard deviation) in the study phase for young, older adults, and high- and low-performing older adults
| Young adults | Older adults | High-performers | Low-performers | |
| Remembered items | 768 (137) | 837 (114) | 814 (97) | 861 (128) |
| Forgotten items | 748 (127) | 825 (111) | 809 (94) | 841 (127) |
Recognition Task
Performance on the recognition task is summarized in Table 4. Collapsed across confidence, there was a significant difference between the age groups for hit rate (t(46) = 3.92, P ≤ 0.001) but not for false alarm rate (P > 0.7), and a significant difference for the discrimination metric Pr (PHit − PFalseAlarm; t(46) = 3.12, P ≤ 0.01). When segregated according to response confidence, Pr for low-confidence responses was extremely low and did not differ between the age groups (P > 0.6). Pr for confident responses was both considerably higher and differed significantly with age (t(46) = 3.15, P ≤ 0.01).
Table 4.
Mean response time (and standard deviation) in the test phase for young, older adults, and high- and low-performing older adults
| Young adults | Older adults | High-performers | Low-performers | |
| Hit rate | 0.83 (0.06) | 0.70 (0.13) | 0.79 (0.06) | 0.60 (0.11) |
| False alarm rate | 0.24 (0.09) | 0.23 (0.11) | 0.22 (0.12) | 0.24 (0.11) |
| Pr | 0.59 (0.11) | 0.46 (0.11) | 0.57 (0.11) | 0.36 (0.08) |
| Pr confident | 0.57 (0.10) | 0.44 (0.16) | 0.56 (0.10) | 0.31 (0.08) |
| Pr nonconfident | 0.02 (0.09) | 0.03 (0.05) | 0.00 (0.05) | 0.05 (0.05) |
As described below, for the purposes of the fMRI analyses, study items were deemed successfully encoded only if they were confidently recognized on the later memory test. Accordingly, the older group was split into high- and low-performing subgroups according to a median split of Pr for confident recognition judgments (see Table 4). (The same pattern of results was obtained with analyses performed on high- and low-performing subgroups segregated on the basis of Pr collapsed across confident and nonconfident judgments.) Whereas the former subgroup's scores did not differ from those of the young subjects (P > 0.7), the scores of the low-performing subgroup were significantly lower (t(30) = 7.95, P ≤ 0.001).
fMRI Data
Following Morcom et al. (2003), subsequent memory effects were estimated by contrasting the activity elicited by study items that were confidently recognized on the later recognition memory test (henceforth “remembered” items) with the activity elicited by items that received either nonconfident “old” responses or were misclassified as “new” (henceforth, “forgotten” items). The analysis of these effects was conducted in 3 stages. First, regions demonstrating subsequent memory effects common to the 2 age groups were identified. The second stage identified regions where effects differed according to age. In the third stage, a voxel-of-interest approach was employed to compare the lateralization of prefrontal subsequent memory effects in the different groups, focusing on the low- and high-performing older subgroups.
Common Effects
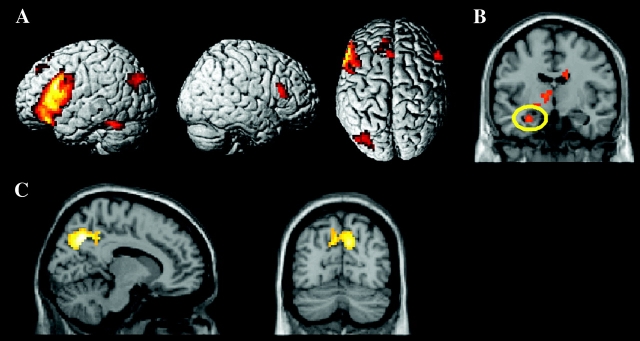
Subsequent memory effects common to the 2 age groups were identified by computing each side of the main effect of subsequent memory (remembered > forgotten and vice-versa), thresholded in each case at P ≤ 0.0005 with a 5 voxel extent threshold, and exclusively masking the resulting SPMs with the F contrast for the Group × Subsequent Memory interaction, thresholded at P ≤ 0.1. Thus, these analyses revealed regions where subsequent memory effects and “reversed” subsequent memory effects (sometimes referred to as “subsequent forgetting” effects; Otten and Rugg 2001c; Daselaar et al. 2004) were reliable at P ≤ 0.001 (2-tailed), and did not differ according to group at P ≤ 0.05 (one-tailed). The outcomes of these analyses are illustrated in Figure 1A and listed in Table 5. Subsequent memory effects were evident in a large expanse of left inferior prefrontal cortex, along with a smaller right prefrontal region, as well as other cortical regions including left fusiform and lateral parietal cortex. As can be seen in Figure 1B, an additional cluster was revealed at a reduced threshold (P ≤ 0.005 one-tailed) in the vicinity of the left anterior hippocampus (peak co-ordinates −27, −9, −21; Z = 3.15, P ≤ 0.001, cluster size 14). This cluster included 7 voxels that survived small volume correction (P ≤ 0.05) for a 10 mm sphere centered on the peak of the common hippocampal effect (co-ordinates −30, −15, −30) reported by Morcom et al. (2003).
Figure 1.
Common subsequent memory effects in young and older adults: (A) Subsequent memory effects (P ≤ 0.0005) rendered onto the 3-dimensional single-subject MNI reference brain; (B) left hippocampal subsequent memory effect (yellow ellipse; P ≤ 0.005) superimposed on a coronal (y = −9) slice of the single-subject MNI template; and (C) reversed subsequent memory effects (P ≤ 0.0005 superimposed on sagittal (x = 12) and coronal (y = −72) slices of the single-subject MNI template. All effects were exclusively masked with the bidirectional interaction between age and subsequent memory/forgetting effect (P ≤ 0.1).
Table 5.
Peak voxel of regions showing common subsequent memory effect and reversed effect in young and older adults exclusively masked with the bidirectional interaction between age and subsequent memory/forgetting effects
| Analysis | Location |
Peak z | N | Region | BA | ||
| x | y | z | |||||
| Subsequent memory effect | −45 | 18 | 24 | 6.81 | 1295 | Inferior frontal gyrus | BA45 |
| −3 | 24 | 57 | 3.96 | 92 | Superior frontal gyrus | BA8 | |
| −51 | −33 | −6 | 3.51 | 5 | Middle temporal gyrus | BA21 | |
| −51 | −48 | −24 | 5.02 | 74 | Fusiform | BA37 | |
| −6 | −57 | 0 | 3.62 | 11 | Lingual gyrus | BA18 | |
| −30 | −84 | 36 | 4.04 | 147 | Superior occipital gyrus | BA19 | |
| 57 | 27 | 15 | 3.92 | 72 | Inferior frontal gyrus | BA45 | |
| 15 | 0 | 24 | 3.81 | 33 | Caudate | ||
| 39 | −21 | 15 | 3.58 | 5 | Insula | BA13 | |
| 30 | −42 | 18 | 3.55 | 11 | White matter | ||
| Reversed subsequent memory effect | 54 | −54 | 39 | 3.62 | 25 | Inferior parietal lobule | BA40 |
| 12 | −63 | 39 | 4.95 | 250 | Precuneus | BA7 | |
The reverse contrast (forgotten > remembered) revealed reversed subsequent memory effects in an extensive region of bilateral medial parietal cortex, as well as in right lateral parietal cortex (Fig. 1C and Table 5).
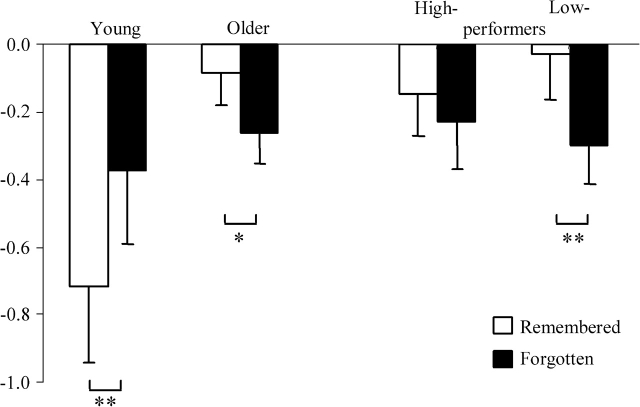
Group × Subsequent Memory Interaction
Interaction contrasts (each side thresholded at P ≤ 0.0005, with cluster extent of 5) were employed to detect between-group differences in the magnitude of subsequent memory effects. No voxels were identified where effects were greater for young than for old subjects. In the case of the reverse contrast, a cluster of 5 voxels was identified in posterior cingulate cortex (peak co-ordinates −12, −48, 27; Z = 3.73). Within-group contrasts of the peak parameter estimates illustrated in Figure 2 revealed that in the young group activity elicited by remembered items was reliably lower than that for forgotten items (t(15) = 3.26, P ≤ 0.01, 2-tailed), whereas the older group demonstrated an effect in the opposite direction (t(31) = 2.42, P ≤ 0.05, 2-tailed). Further analysis revealed that neither the high- nor the low-performing subgroup demonstrated significant reversed subsequent memory effects in this region. A reliable effect in the opposite direction was found in the latter subgroup only (t(15) ≤ 1 and t(15) = 3.16, P ≤ 0.01, 2-tailed, in high- and low-performers, respectively).
Figure 2.
Parameter estimates of the peak voxel in posterior cingulate cortex (co-ordinates −12, −48, 27) showing age-related difference in reversed subsequent memory effect (*P ≤ 0.05; **P ≤ 0.01).
Lateralization of Prefrontal Subsequent Memory Effects
Following Morcom et al. (2003), between-group differences in the lateralization of prefrontal subsequent memory effects were assessed using a voxel-of-interest approach. For each subject, parameter estimates representing the activity elicited by remembered and forgotten study items were extracted from 5 voxels in the left prefrontal cortex and from homotopic voxels in the right hemisphere. The voxels in the left hemisphere were located at local peaks evident in the main effect of subsequent memory that was revealed by a voxel-wise, mixed-design 2-way ANOVA with young, high-performing and low-performing groups as a between subjects factor. Thus, these voxels were selected in a manner that was unbiased with respect to the 3 groups. The identified voxels sampled activity from the full extent of the left prefrontal region demonstrating subsequent memory effects (co-ordinates: −51, 33, −3; −48, 30, 6; −36, 30, −15; −51, 24, 15; −45, 15, 27).
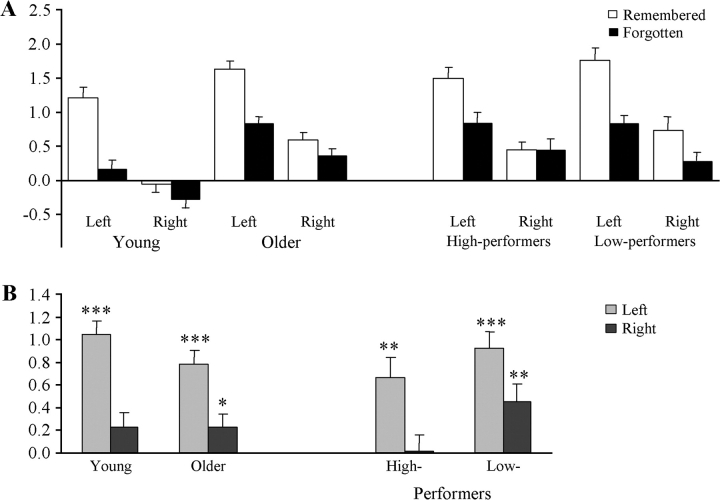
Mean parameter estimates for activity elicited by later remembered and forgotten items from left and right hemispheres, collapsed across the voxels of interest, are illustrated in Figure 3A for the young and older subjects, and separately for the low- and high-performing older subgroups (see Fig. 3B for depiction of parameter estimates for the subsequent memory effects). To determine whether the present results replicated those of Morcom et al. (2003), an ANOVA was conducted on the parameter estimates employing the factors of age group (young vs. older), subsequent memory (remembered vs. forgotten), hemisphere, and voxel of interest. The results of the ANOVA are summarized in Table 6, where it can be seen that among the significant effects was a Group × Subsequent Memory × Hemisphere interaction. As is evident from Figure 3B, the interaction reflects the tendency for subsequent memory effects to be more strongly lateralized in the younger subjects, replicating the findings of Morcom et al. (2003). The reliable main effect of group, indicative of greater item-related activity in the older subjects with respect to baseline, was also reported in the former study.
Figure 3.
Lateralization of subsequent memory effects in young, older adults, and high- and low-performing older adults: (A) averaged parameter estimates for remembered and forgotten items and (B) subsequent memory effects (Remembered minus Forgotten) collapsed across the 5 prefrontal voxels of interest (see text; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
Table 6.
Outcome of ANOVAs on young adults versus older adults, high-performing and low-performing older adults, for peak voxels in left prefrontal region showing common subsequent memory effects and their homotopic voxels in right prefrontal region
| Analysis | Effect | df | F | P |
| Young versus older adults | Group | 1,46 | 19.63 | ≤0.001 |
| Subsequent memory | 1,46 | 43.23 | ≤0.001 | |
| Hemisphere | 1,46 | 92.01 | ≤0.001 | |
| Voxel | 3.1,143.3 | 9.86 | ≤0.001 | |
| Subsequent memory × Hemisphere | 1,46 | 115.89 | ≤0.001 | |
| Subsequent memory × Voxel | 2.9,131.4 | 4.60 | ≤0.01 | |
| Hemisphere × Voxel | 3.2,145.1 | 6.77 | ≤0.001 | |
| Group × Subsequent memory × Hemisphere | 1,46 | 4.26 | ≤0.05 | |
| Subsequent memory × Hemisphere × Voxel | 2.7,126.0 | 3.14 | ≤0.05 | |
| Young versus high-performing older adults | Group | 1,30 | 13.3 | ≤0.001 |
| Subsequent memory | 1,30 | 25.10 | ≤0.001 | |
| Hemisphere | 1,30 | 80.54 | ≤0.001 | |
| Voxel | 3.0,88.6 | 6.81 | ≤0.001 | |
| Subsequent memory × Hemisphere | 1,30 | 89.07 | ≤0.001 | |
| Hemisphere × Voxel | 3.2,97.2 | 5.55 | ≤0.001 | |
| Subsequent memory × Hemisphere × Voxel | 3.4,101.5 | 3.40 | ≤0.05 | |
| Young versus low-performing older adults | Group | 1,30 | 16.99 | ≤0.001 |
| Subsequent memory | 1,30 | 49.01 | ≤0.001 | |
| Hemisphere | 1,30 | 63.94 | ≤0.001 | |
| Voxel | 3.0,89.3 | 8.36 | ≤0.001 | |
| Subsequent memory × Hemisphere | 1,30 | 114.83 | ≤0.001 | |
| Subsequent memory × Voxel | 3.0,91.2 | 4.65 | ≤0.01 | |
| Hemisphere × Voxel | 3.0,90.7 | 4.78 | ≤0.01 | |
| Group × Subsequent memory × Hemisphere | 1,30 | 8.42 | ≤0.01 |
Additional ANOVAs were conducted to contrast the prefrontal subsequent memory effects in the young subjects with each of the 2 older subgroups. The outcomes of these ANOVAs are also summarized in Table 6. As is evident from the table, the Group × Subsequent memory × Hemisphere interaction was significant for the contrast between the young and the low-performing, but not the high-performing, subgroup. Further within-group analyses separately conducted on each hemisphere (employing the factors of subsequent memory and voxel of interest) revealed that whereas left prefrontal subsequent memory effects were reliable in all 3 groups (F1,15 = 70.73, 13.26, and 40.96 for young, high-performers, and low-performers respectively; all P values ≤ 0.005), right prefrontal effects were reliable in the low-performing older subgroup only (F1,15 = 8.36; P ≤ 0.01). (A reviewer queried whether these findings might be a reflection of selecting voxels in the right hemisphere which, although homotopic with those exhibiting strong across-group left prefrontal subsequent memory effects, do not themselves demonstrate reliable across-group subsequent memory effects. Accordingly, we extracted parameter estimates for the peak of the right prefrontal cluster where such effects were independently reliable according to the threshold applied to our whole brain, voxel-wise analysis [see Table 5]. Even for this voxel, the selection of which is biased in favor of finding significant within-group effects, subsequent memory effects were reliable in the low-performing subgroup but not the high-performing subgroup [t(15) = 3.15, P ≤ 0.005 (1-tailed) and t(15) = 1.62, P > 0.06, respectively]. The subsequent memory effect at this voxel was also reliable in the young group [t(15) = 2.55, P ≤ 0.025].).
In addition to the group-wise analyses described above, we computed across-subject correlations between the magnitudes of left and right prefrontal subsequent memory effects, and of the difference between them, and measures of performance on the experimental task and neuropsychological tests. No significant correlations were observed across either the entire 48 subjects, or the older group alone.
Discussion
In agreement with Morcom et al. (2003), the present study revealed age-invariant subsequent memory effects (greater activity elicited by later remembered than later forgotten study items) in a large expanse of left inferior prefrontal cortex, along with several other cortical regions and the left anterior hippocampal formation. Again replicating the previous study, a voxel-of-interest analysis revealed that prefrontal subsequent memory effects were distributed more bilaterally in older than in young subjects. Crucially, this age-related difference in the lateral asymmetry of encoding-related activity was carried by subjects who performed relatively poorly on the later memory test. In addition to an analysis of subsequent memory effects, the present study also identified an extensive region of medial parietal cortex that exhibited age-invariant reversed subsequent memory effects (greater activity for subsequently forgotten than subsequently remembered items). Inferior to this region, however, was a small region where reversed effects were evident solely in the young subjects, and where poorly performing older subjects demonstrated an effect in the opposite direction. Below, we expand upon these and other findings, focusing on their relevance for an understanding of the functional significance of age-related changes in the neural correlates of successful episodic encoding.
Neuropsychological Performance
Relative to the young group, the older subjects showed the typical pattern of age-related impairment on the neuropsychological test battery, performing poorly on tests of long-term memory and speeded cognition. This pattern of performance differed somewhat between older subjects who performed relatively well on the experimental memory task and those who performed less well (see below). Whereas both older subgroups demonstrated lower scores on the speeded tests and on one test of long-term memory (delayed paragraph recall), only the low-performing subjects were significantly impaired on the other long-term memory tests. Thus, segregating the older subjects according to their performance on the experimental task also segregated them according to long-term memory function more generally.
Behavioral Performance
Performance on the study task was well matched between the 2 age groups, although the older subjects tended to respond more slowly than the young group. A subsequent memory analysis of study RT revealed a small but reliable trend for later remembered (confidently recognized) items to be responded to more slowly than items that were later forgotten (that is, items that were missed or recognized with low confidence). This finding may reflect a benefit to encoding from relatively prolonged study processing, although it should be noted that while similar effects have been reported in some previous studies (e.g., Wagner et al. 1998; Morcom et al. 2003; Uncapher and Rugg 2005b), other studies have reported opposite (e.g. Otten and Rugg 2001a, 2001b) or null (e.g., Otten et al. 2001; Uncapher and Rugg 2005a) findings. Regardless of the explanation for the present subsequent memory RT effect, it did not differ in magnitude between the 2 age groups (or between the 2 older subgroups) and hence does not compromise the interpretation of age-related differences in fMRI subsequent memory effects.
The pattern of recognition performance closely resembled the pattern reported by Morcom et al. (2003). Young subjects out-performed older subjects, and this difference was carried entirely by items attracting confident judgments. When the older group was subdivided by a median split on confident recognition performance, however, the high-performing subgroup performed at a level equivalent to that of the young group. Therefore, any differences in fMRI subsequent memory effects between these 2 groups cannot be attributed to the confounding effects of performance. The same cannot be said in the case of the low-performing older subgroup, an issue to which we will return below.
fMRI Findings
Two key aims of the present study were to replicate previous findings of an age-related decrease in the lateralization of prefrontal subsequent memory effects (Morcom et al. 2003), and to investigate the relation between degree of lateralization and memory performance. We were successful in the first of these aims: as in Morcom et al. (2003), a voxel-of-interest analysis revealed that subsequent memory effects in inferior lateral prefrontal cortex were distributed more bilaterally in the older than the younger group (see Fig. 3B). With respect to the second aim, the results were again clear: decreased asymmetry in prefrontal subsequent memory effects was most evident in those older subjects who performed relatively poorly (below the 50th percentile) on the experimental task (see Fig. 3B). Moreover, this was the only group of subjects in whom right prefrontal subsequent memory effects at voxels homotopic with left prefrontal maxima were statistically significant.
These findings indicate that high verbal episodic memory performance in older individuals does not require engagement of right prefrontal cortex during encoding (see Miller et al. 2008 for analogous results). Thus, the findings do not support the “compensation” hypothesis of age-related right hemisphere recruitment as it is often articulated (e.g., Cabeza et al. 2002; Dolcos et al. 2002; Rosen et al. 2002; Reuter-Lorenz and Lustig 2005). As was noted in the Introduction, this does not necessarily mean that right prefrontal over-recruitment during encoding fails to benefit memory performance. Rather, as was also suggested by Miller et al. (2008) and Persson et al. (2006), over-recruitment may be an adaptive response that supports processing within cognitive domains in which functional competence is beginning to decline. Findings from studies investigating neural correlates of memory encoding and retrieval in individuals suffering from mild cognitive impairment (MCI) or at genetic risk for Alzheimer's disease (APOE-e4 carriers) offer some support for this proposal. In several of these studies (Bookheimer et al. 2000; Dickerson et al. 2004; Bondi et al. 2005; Dickerson et al. 2005; Celone et al. 2006; see Sperling 2007 for review) such individuals demonstrated enhanced encoding- or retrieval-related activity relative to age-matched controls (but see Johnson et al. 2006; Lind, Ingvar, et al. 2006; Lind, Persson, et al. 2006; Trivedi et al. 2006 for reports of reduced activity in APOE-e4 carriers). These findings have been interpreted as evidence of neural compensation in the face of incipient pathology (Dickerson et al. 2004, 2005). As was proposed by Duverne et al. (2007), over-recruitment may in fact be a compensatory response to relatively modest cognitive decline regardless of whether the decline arises from early neurodegenerative disease or as a consequence of “normal” aging. To the extent this proposal is correct, it raises the important question of when, relative to the onset of such decline, compensatory over-recruitment first becomes evident. The answer to this question will likely require recourse to longitudinal rather than cross-sectional experimental designs.
If the foregoing account is correct, the question arises as to the identity of the cognitive processes that were “compensated” by right prefrontal subsequent memory effects in our low-performing older subgroup. Because the study task was incidental, it is highly unlikely that these processes include any associated specifically with intentional memory encoding. Rather, the relevant processes were likely among those engaged by the online demands of the study task. This placed demands on such functions as retrieval, selection and evaluation of semantic information, all of which are thought to depend on left inferior prefrontal cortex (Badre and Wagner 2007). By this account, the right prefrontal subsequent memory effects evident in our low-performing older subjects reflected the recruitment of right prefrontal regions in service of task demands that were not fully met by left prefrontal cortex. Because there were no detectable differences in study performance between the high- and low-performing subgroups, right prefrontal recruitment appears to have been sufficient to allow the low-performing subjects to adequately meet the demands of this relatively easy study task. Clearly, however, it did not fully compensate for functional decline in regions supporting mnemonic processing, as evidenced by the relatively poor performance of these subjects on both the experimental task and standardized memory tests. Moreover, the finding that letter and category fluency performance—both of which depend upon lateral prefrontal cortex (Baldo and Shimamura 1998)—were compromised in the low- relative to the high-performing subgroup suggests that right prefrontal recruitment did not fully compensate for the reduction in left prefrontal support of online processing.
A very different account of the right prefrontal subsequent memory effects evident in our low-performing subjects proposes that the effects reflect changes in cortical function or organization that are deleterious for memory encoding. By this account, right prefrontal over-recruitment is an example of cortical dedifferentiation (perhaps mediated by a breakdown in transcallosal inhibition; Buckner and Logan 2002; Logan et al. 2002), the consequence of which is a decrease in computational “signal-to-noise” and hence in processing efficiency. Thus, right frontal subsequent memory effects are a consequence of a breakdown in mechanisms that, in young individuals and older people who are aging relatively successfully, ensure that processing demands are met by optimal levels of cortical recruitment. This scenario receives support from the finding of Persson et al. (2006) of a negative correlation between structural integrity of the anterior corpus callosum (as indexed by diffusion tensor imaging) and task-related activity in right ventrolateral prefrontal cortex.
Adjudicating between the “compensation” and “dedifferentiation” accounts of right prefrontal over-recruitment will not be easy and, indeed, may not be possible using only correlative methods such as MRI. More decisive evidence might come from the employment of transcranial magnetic stimulation (TMS) to selectively disrupt prefrontal function in each hemisphere (e.g., Rossi et al. 2004). If right prefrontal subsequent memory effects are adaptive, TMS to this region should disrupt encoding in those subjects in whom the effects are evident to a greater extent than in subjects in whom the effects are absent.
As already noted, in both age groups reversed subsequent memory effects (lower activity for remembered than forgotten items) were evident in an extensive region of medial parietal cortex, along with a smaller right lateral parietal region (Fig. 1C). Reversed effects have previously been reported in this region in studies of young subjects (e.g., Otten and Rugg 2001c; Daselaar et al. 2004), as well as in other regions comprising what is sometimes termed the “default-mode network” (regions where task engagement is associated with deactivation relative to a resting or baseline condition; see Buckner and Vincent 2007; Morcom and Fletcher 2007; Raichle and Snyder 2007 for recent reviews). The functional significance of reversed subsequent memory effects is currently unclear, although there is some agreement that they likely reflect the benefit to encoding deriving from a reallocation of cognitive resources from task-independent processes such as environmental monitoring to the study item (Otten and Rugg 2001c; Wagner et al. 2001; Daselaar et al. 2004). Evidently, in the present sample of older adults, a substantial part of the medial parietal cortex had retained the capacity to support such item-elicited resource reallocation (cf. Miller et al. 2008).
Inferior to the region demonstrating these common reversed subsequent memory effects was a posterior cingulate region—a region also held to belong to the default-mode network (Gusnard et al. 2001)—where reversed effects were evident in young subjects only and where, in the case of the low-performing subjects, there was a full cross-over interaction. It is unclear whether the posterior cingulate supports the same functions as the more extensive medial parietal region that exhibited the common reversed effects discussed above, or whether it should be regarded as functionally distinct. Never the less, the present findings for the posterior cingulate add to the results from 4 prior studies of encoding where reversed subsequent memory effects were also reported to be greater for young than older subjects (Morcom et al. 2003; Gutchess et al. 2005; Kukolja et al. 2007; Miller et al. 2008). In each of these prior studies, the age effects were also localized to regions implicated in the default-mode network: medial prefrontal cortex in the cases of Gutchess et al. (2005) and Kukolja et al. (2007), lateral prefrontal cortex in Morcom et al. (2003) and, similar to the present findings, medial parietal/posterior cingulate cortex in Miller et al. (2008). Although the factors responsible for the differential localization of these effects across the different studies remains to be established, at a more general level the consistency of the findings is striking. Their relevance for the understanding of age-related changes in memory performance is presently unclear, however (see also Persson et al. 2007). Miller et al. (2008) reported that age-related attenuation of medial parietal reversed subsequent memory effects was most evident in subjects who performed relatively poorly on the later memory test. In the present study, there was no evidence that mere attenuation of the effects (albeit in only a small subset of voxels evidencing the effect in the young subjects) impacted subsequent memory performance. Rather, poor performance in older subjects was associated not with the absence of a reversed effect, but with the presence of a reliable subsequent memory effect. The significance of this finding is obscure, although it could perhaps be interpreted as a further example of over-recruitment. In any case, it suggests that the failure to show reversed subsequent memory effects in a given region does not necessarily indicate that the region is insensitive to encoding success (see Morcom et al., 2003 for similar findings). The importance of gaining a fuller appreciation of the functional significance of age-related changes in reversed subsequent memory effects in the default-mode network is highlighted not only by the consistency of these findings across studies (see above), but also by reports that age-related reduction or reversal in task-related default-mode network activity is exaggerated in subjects with MCI or Alzheimer's disease (Lustig et al. 2003; Petrella, Prince, et al. 2007; Petrella, Wang, et al. 2007).
Finally, a caveat is in order regarding interpretation of the subsequent memory contrasts between the young subjects and the low-performing older subgroup. Because group and task performance were confounded in these contrasts (as is often the case is functional neuroimaging studies of cognitive aging), we cannot completely rule out the possibility that the resulting effects reflect the disparate performance levels of the 2 groups rather than more stable differences in patterns of cortical recruitment (see Morcom et al. 2003 for discussion of the various ways differential performance might influence subsequent memory effects). The findings of Morcom et al. (2003), who demonstrated that age-related differences in asymmetry of prefrontal subsequent memory effects are independent of performance level, strongly suggest that the present findings do not reflect a performance confound. Nonetheless, it will be important in future research to assess prefrontal subsequent memory effects in high- and low-performing older subjects across multiple levels of task difficulty (cf. Morcom et al. 2003).
Concluding Comments
The present findings are consistent with previous studies that reported age-related reductions in the lateralization of prefrontal cortex activity associated with successful memory encoding. In a significant extension to these prior studies, the present analyses indicate that age-related reduction in asymmetry of prefrontal subsequent memory effects is restricted to older adults with relatively poor memory performance. Thus, preservation of memory performance with increasing age does not depend upon bilateral prefrontal recruitment. The mechanisms responsible for the enhancement of right prefrontal subsequent memory effects in poorly performing older adults, and the functional significance of this enhancement, remain to be understood.
Funding
National Institute of Aging (5P50AG16573).
Acknowledgments
We thank the staff of the UCI Research Imaging Center for their assistance with data collection, Brian Minton for his help in recruiting the last older adults included in the sample of subjects, and our experimental subjects for their participation. We also acknowledge the helpful comments of 2 anonymous referees on an earlier version of this paper. Conflict of Interest: None declared.
References
- Andrade A, Paradis A-L, Rouquette S, Poline J-B. Ambiguous results in functional neuroimaging data analysis due to covariate correlation. Neuroimage. 1999;10:483–486. doi: 10.1006/nimg.1999.0479. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal love lesions. Neuropsychology. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Birren JE. Age changes in speed of behavior: its central nature and physiological correlates. In: Welford AT, Birren JE, editors. Behavior, aging, and the nervous system. Springfield (IL): Thomas; 1965. pp. 191–216. [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Logan GD. Frontal contributions to episodic memory encoding in the young and elderly. In: Parker AE, Wilding EL, Bussey T, editors. The cognitive neuroscience of memory encoding and retrieval. Philadelphia (PA): Psychology Press; 2002. pp. 59–81. [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasiticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in old adults: the HAROLD Model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J Cogn Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Cogn Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerella J. Information processing rates in the elderly. Psychol Bull. 1985;98:67–83. [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RKS, Evans AC. Brainweb: online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:425. [Google Scholar]
- Craik FIM. Age differences in human memory. In: Birren JE, Schaie KW, editors. Handbook of the psychology. New York: Van Nostrand Reinhold; 1977. pp. 384–420. [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: the role of attentional resources. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York: Plenum Press; 1982. pp. 191–211. [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale (NJ): Erlbaum; 1992. pp. 51–110. [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Deep processing activates the medial temporal lobe in young but not in old adults. Neurobiol Aging. 2003a;24:1005–1011. doi: 10.1016/s0197-4580(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003b;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Californian verbal learning test. 2nd ed. San Antonio (TX): the Psychological Corporation; 2000. [Google Scholar]
- Dennis NA, Kim H, Cabeza R. Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia. 2007;45:3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.022. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Pacurar A, Moscovitch M, Grady C. Neural correlates of auditory recognition under full and divided attention in younger and older adults. Neuropsychologia. 2006;44:2452–2464. doi: 10.1016/j.neuropsychologia.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging; a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Grady C, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Shapiro MB, Haxby JV. Age-related reduction in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modeling and receiver operating characteristic curves. Psychol Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychol Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger A, Ferris SH, Golomb J, Mittleman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12:168–169. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Wilms M, Mirzazade S, Fink GR. Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annu Rev Psychol. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Lind J, Ingvar M, Persson J, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Parietal cortex activation predicts memory decline in apolipoprotein E-epsilon4 carriers. Neuroreport. 2006;17:1683–1686. doi: 10.1097/01.wnr.0000239954.60695.c6. [DOI] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Backman L, Nilsson LG, Petersson KM, et al. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain. 2006;129:1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale J, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, Depeau K, Diamond E, Dickerson BC, Rentz DM, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0706818105. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Chein JM, Talmi D, Cohn M. Learning and memory. In: Baars BJ, Gage NM, editors. Cognition, brain, and consciousness. San Diego (CA): Academic Press; 2007. pp. 254–290. [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Nilsson LG. Memory function in normal aging. Acta Neurol Scan. 2003;179:7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Electrophysiological correlates of memory encoding are task-dependent. Brain Res Cogn Brain Res. 2001a;12:11–18. doi: 10.1016/s0926-6410(01)00015-5. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex. 2001b;11:1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001c;11:1150–1160. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Wang L, Hellegers C, Doraiswamy PM. Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS One. 2007;2:e1104. doi: 10.1371/journal.pone.0001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JR, Wang L, Krishnan S, Slavin MJ, Prince SE, Tran TT, Doraiswamy PM. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245:224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, AMr Martin, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Raz N. Neuroanatomy of aging brain: evidence from structural fMRI. In: Bigler ED, editor. Neuroimaging 2: clinical implications. New York: Academic Press; 1996. pp. 153–182. [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Mahwah (NJ): Lawrence Erlbaum Associates; 2000. pp. 1–90. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann N Y Acad Sci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JDE. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24:7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. A theory of cognitive aging. Amsterdam (The Netherlands): Elsevier Science; 1985. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory changes: a PET study. Neuroreport. 1996;7:1165–1169. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- Schiavetto A, Kohler S, Grady CL, Winocur G, Moscovitch M. Neural correlates of memory for object identity and object location: effects of aging. Neuropsychologia. 2002;40:1428–1442. doi: 10.1016/s0028-3932(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med. 2006;4:1–14. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Effects of divided attention on fMRI correlates of memory encoding. J Cogn Neurosci. 2005a;17:1923–1935. doi: 10.1162/089892905775008616. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Encoding and the durability of episodic memory: a functional magnetic resonance imaging study. J Neurosci. 2005b;25:7260–7267. doi: 10.1523/JNEUROSCI.1641-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen FM, Nijhuis FA, Tisserand DJ, Backes WH, Jolles J. Effects of aging on recognition of intentionally and incidentally stored words: an fMRI study. Neuropsychologia. 2006;44:2477–2486. doi: 10.1016/j.neuropsychologia.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale MA, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. San Antonio (TX): the Psychological Corporation; 2001. [Google Scholar]