Abstract
When we detect conflicting irrelevant stimuli (e.g., nearby conversations), we often minimize distraction by increasing attention to relevant stimuli. However, dissociating the neural substrates of processes that detect conflict and processes that increase attention has proven exceptionally difficult. Using a novel cross-modal attentional cueing task in humans, we observed regional specialization for these processes in the cognitive division of the anterior cingulate cortex (ACCcd). Activity in a dorsal subregion was associated with increasing attention to relevant stimuli, correlated with behavioral measures of orienting attention to those stimuli, and resembled activity in dorsolateral prefrontal regions that are also thought to bias attention toward relevant stimuli. In contrast, activity in a rostral subregion was associated only with detecting response conflict caused by irrelevant stimuli. These findings support a 2-component model for minimizing distraction and speak to a longstanding debate over how the ACCcd contributes to cognitive control.
Keywords: cognitive control, cross-modal, fMRI, response conflict, selective attention
Introduction
Think about the last time you spoke with a friend at a crowded party. Occasionally, when you detected the volume of background conversations rising, you probably increased attention to your friend's voice in order to avoid becoming distracted. Consistent with this example, minimizing distraction is thought to depend on complementary brain systems that first detect the presence of distracting stimuli and then quickly increase attention to relevant stimuli (Carter et al. 1998; Botvinick et al. 2001; Kerns 2006). Identifying these systems has attracted much interest recently because heightened levels of distraction are associated with adverse outcomes in numerous clinical syndromes, including drug addiction (Goldstein et al. 2007), attention deficit and hyperactivity disorder (Dickstein et al. 2006), and schizophrenia (Kerns et al. 2005). However, because these systems are thought to be active at nearly the same time, dissociating them has proven to be a difficult and controversial enterprise.
At the center of this controversy lies the precise contribution to cognitive control that is made by the so-called “cognitive division” of the anterior cingulate cortex (ACCcd; the subscript “cd” refers to “cognitive division” and is used throughout the paper to distinguish the dorsal and rostral subregions of ACCcd that we investigate from dorsal and rostral regions of the ACC as a whole). Some models posit that the ACCcd increases attention to task-relevant stimuli (Posner and DiGirolamo 1998; Dreher and Berman 2002; Weissman et al. 2005). Others posit that the ACCcd signals the coactivation of competing responses (i.e., response conflict, which can be highly distracting) to the dorsolateral prefrontal cortex (DLPFC) which, in turn, resolves conflict by increasing attention to relevant stimuli (Carter et al. 1998; Botvinick et al. 2001; Kerns 2006). Still others posit a role for the ACCcd in response selection (Roelofs et al. 2006), novelty detection (Ranganath and Rainer 2003; Matsumoto et al. 2007), anticipation (Murtha et al. 1996), error monitoring (Gehring et al. 1993; Gehring and Fencsik 2001), reward assessment (Bush et al. 2002), and computing error likelihood (Brown and Braver 2005). Although numerous investigators have sought to determine which model best explains ACCcd activity (Botvinick et al. 1999; Banich et al. 2000; MacDonald et al. 2000; Weissman et al. 2004; Kerns et al. 2004), the findings have been mixed and relatively little consensus has been reached.
Given the heterogeneity of findings in the literature, some authors have suggested the existence of regional specialization in the ACCcd for distinct control processes (Bush et al. 2002; Milham and Banich 2005; Goldstein et al. 2007). In line with the regional specialization hypothesis, we recently reported evidence implicating a dorsal subregion of the ACCcd in increasing attention to relevant stimuli and a rostral subregion in detecting response conflict caused by irrelevant stimuli (Weissman et al. 2004). In our prior study, however, demands on processes that increase attention to relevant stimuli were confounded with the expected difficulty of the upcoming task, which also influences ACCcd activity (Brown and Braver 2005).
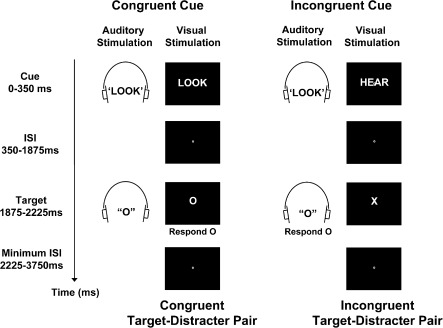
In the present study we therefore used a novel cross-modal attentional cueing task to investigate regional specialization in the ACCcd for processes that increase attention to relevant stimuli and processes that detect response conflict (Fig. 1). In each trial, participants received a visually presented cue word (“Look” or “Hear”) that instructed them to attend to and identify either the visual letter or the auditory letter of a possibly upcoming, audiovisual target–distracter letter pair. The visually presented cue word was accompanied by an irrelevant, binaurally presented auditory word that was equally likely to signal the same task as the visual word (less demanding congruent cues) or a different task (more demanding incongruent cues). After a brief interval, an audiovisual target–distracter letter pair was presented. The distracter letter was equally likely to be mapped to the same response as the target letter (less demanding congruent target–distracter pairs) or to a different response (more demanding incongruent target–distracter pairs). To isolate cue-triggered activity associated with increasing attention to relevant stimuli from target-triggered activity associated with detecting response conflict, in cue-only trials (33%) the cue was not followed by a target–distracter letter pair.
Figure 1.
Experimental task. In each trial, a visually presented cue word (“Look” or “Hear”) instructed participants to attend to and identify either the visual letter (“X” or “O”) or the auditory letter (“X” or “O”) of a possibly upcoming target–distracter letter pair. To modulate demands on cue-triggered processes that increase attention to relevant stimuli, we varied whether an irrelevant auditory word signaled the same task as the visual word (“Congruent Cue”) or a different task (“Incongruent Cue”). After a 1.875-s interval, an audiovisual target–distracter letter pair was presented. To modulate demands on target-triggered processes that detect response conflict, we varied whether the distracter letter was mapped to the same response as the target (“Congruent Target–Distracter Pair”) or to a different response (“Incongruent Target–Distracter Pair”). In cue-only trials (33%, not shown), the cue was not followed by a target–distracter letter pair.
Our hypothesis predicts that dorsal and rostral subregions of the ACCcd, respectively, should be differentially sensitive to processes that increase attention to relevant stimuli and processes that detect response conflict. Processes (and brain regions) that increase attention to relevant stimuli should be more strongly recruited by incongruent than by congruent cue-only trials. Indeed, during the processing of incongruent cues, such processes should need to work especially hard to ensure that attention is oriented to the cued modality rather than to the irrelevant modality signaled by the distracter word. Our hypothesis therefore predicts relatively strong effects of cue congruency (i.e., peak activity that is greater for incongruent than for congruent cue-only trials) in the dorsal ACCcd. On the other hand, processes (and brain regions) that detect response conflict should be more highly activated by incongruent than by congruent target–distracter pairs, because only incongruent target–distracter pairs engender response conflict. Thus, our hypothesis predicts relatively strong effects of target congruency (i.e., peak activity that is greater for incongruent than for congruent target–distracter pairs) in the rostral ACCcd.
Materials and Methods
Participants
Seventeen healthy participants (7 males and 10 females, age range, 19–36 years) took part in the study. All had normal or corrected-to-normal vision and had no history of serious neurological trauma or disorders. All except one were right handed. Before the magnetic resonance (MR) session, each participant practiced the experimental task. Participants were paid $20 per hour for their participation, which lasted approximately 2 h. Participants gave informed consent before the experiment in accordance with the University of Michigan Behavioral Sciences Institutional Review Board.
Experimental Task
An IBM-compatible PC was used to present stimuli and to record the participants’ responses. Visual stimuli were projected onto a screen at the back of the bore of the magnet that participants viewed through a mirror. Auditory stimuli were voice recordings of a female speaker (duration, 350 ms) delivered binaurally through MR-compatible headphones. Headphone volume was adjusted for each participant so that the auditory stimuli could be heard clearly over the background MR scanner noise. All stimuli were presented using Presentation software (Neurobehavioral Systems, Albany, CA). Responses were made using the index and middle fingers of the right hand and recorded with an MR-compatible response box.
In each 3.75-second trial, a visually presented cue word (“Look” or “Hear”: 3.12° x 0.86°) instructed participants to attend to and identify either the visual letter (“X,” 1.10° x 1.36°; or “O,” 1.18° x 1.38°) or the auditory letter (“X” or “O”) of a possibly upcoming, audiovisual target–distracter letter pair. The visually presented cue word (duration, 350 ms) was accompanied by an irrelevant, binaurally presented auditory word (duration, 350 ms) that was equally likely to signal the same task as the visual cue (Fig. 1, top left) or a different task (Fig. 1, top right). After a brief interval (cue-target stimulus onset asynchrony, 1875 ms), an audiovisual target–distracter letter pair was presented (duration, 350 ms). The distracter letter in the uncued modality was equally likely to be mapped to the same response as the target letter (Fig. 1, bottom left) or to a different response (Fig. 1, bottom right). Participants were instructed to press one button if the cued target letter was an X and a different button if it was an O, as quickly as possible without making mistakes, using the index and middle fingers of their right hand (stimulus-response mappings were counterbalanced across participants). The next trial began after an inter-trial-interval that lasted between 0 and 6.25 s.
We used 2 main trial types to distinguish brain activity associated with cues from activity associated with targets. To isolate activity related to cues, we included “cue-only” trials in which only the cue was presented (33% of all trials). To isolate activity related to targets, we included “cue-plus-target” trials in which a cue was followed by a target (66% of all trials). Using a mixture of cue-only and cue-plus-target trials allows one to distinguish neural activity for cues from activity for targets even in rapid event-related functional MRI (fMRI) designs (Corbetta et al. 2000).
We were also able to identify differences in activity associated with incongruent and congruent target–distracter pairs in cue-plus-target trials. Indeed, exactly the same cues appeared in 1) cue-plus-target trials containing incongruent target–distracter pairs and 2) cue-plus-target trials containing congruent target–distracter pairs. Thus, contrasting activity for these different trial types allowed us to subtract out the common cue activations, thereby isolating differences in activity between incongruent and congruent target–distracter pairs.
In all trials, the fixation dot (0.15° x 0.17°) changed color from white to red 1.875 s after cue onset (coincident with target presentation in cue-plus-target trials and to signal that no target would occur in cue-only trials). Participants were instructed to cease attending if the fixation dot turned red and a target failed to appear (cue-only trials).
Event-Related Design
In every run, there were 12 event-related trial types (4 cue-only and 8 cue-plus-target), each of which was presented 8 times in a completely randomized order. The 4 cue-only trial types consisted of the 4 possible combinations of Cue Type (look, hear) and Cue Congruency (congruent, incongruent). The 8 cue-plus-target trials consisted of the 8 possible combinations of Cue Type (look, hear), Cue Congruency (congruent, incongruent), and Target Congruency (congruent, incongruent). To optimize regression estimates of the blood oxygenation level–dependent (BOLD) responses produced by each of the 12 trial types, the intertrial interval (ITI) was varied between zero and 5 time repetitions (TRs) (0 and 6.25 s) using a nearly exponential distribution that favored short ITIs (Miezin et al. 2000).
Data Acquisition
All MRI images were collected on a 3-T GE Signa (Waukesha, WI) whole-body scanner with a standard head coil. The BOLD signal was measured with a reverse spiral imaging sequence (TR, 1250 ms; time echo [TE], 30 ms; field of view, 22 cm; 27 contiguous 4.5-mm-thick slices; in-plane resolution, 3.44 × 3.44 mm). Anatomical images were collected in-plane with the functional images using a T1-weighted gradient-echo sequence (TR, 250 ms; TE, 5.4 ms; flip angle, 90°, in-plane resolution 0.86 × 0.86 mm). Every participant completed 5 runs, each consisting of 96 trials. During each run, 395 brain volumes were collected. The first 6 functional images of each run contained no trials and were discarded.
Data Analysis
Using SPM2 (Friston 1995), the functional images were corrected for asynchronous slice acquisition and head movement, normalized to MNI (Montreal Neurological Institute) space with dimensions 3.75 mm × 3.75 mm × 4.5 mm, and spatially smoothed with a 3-dimensional Gaussian filter (8 mm at full-width half-max). Due to head movements greater than 3 mm, the final run was eliminated from 2 participants’ data, and the final 2 runs were removed from one participant's data. Next, the time series for each run was analyzed using a version of the general linear model that makes no assumptions about the shape of the BOLD response. This model, sometimes called the finite impulse response model, estimates the average stimulus-locked fMRI response for each trial type and has been used successfully in many prior studies (Shulman et al. 1999; Ollinger, Corbetta, et al. 2001; Ollinger, Schulman, et al. 2001). We estimated 14 TRs (17.5 s) of the average BOLD response for each of the 12 trial types. This resulted in 168 regressors (12 trial types × 14 time points) being entered into the design matrix. We also included 6 head movement regressors (i.e., SPM2 motion estimates) and 2 regressors for the linear trend and the y-intercept term. Parameter estimates for each run were converted to units of percent change from baseline and then averaged across runs for each participant.
Voxelwise Analyses
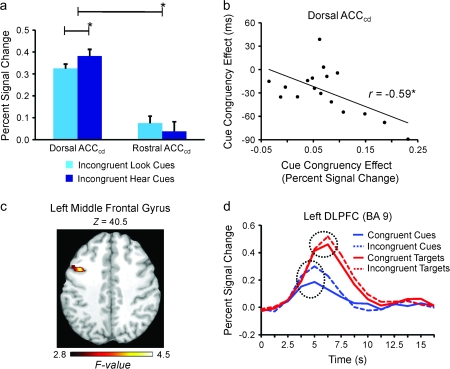
A voxelwise, repeated-measures analysis of variance (ANOVA) was conducted to test for a significant 3-way interaction between Cue Congruency, Target Congruency, and Time (0–17.5 s) in prefrontal regions (thresholded at F13, 208 = 2.8, P < 0.001 and 5 contiguous voxels). This analysis identified a region of the left DLPFC (10 voxels; MNI center of mass: x = −44, y = 10, z = 39; Brodmann area [BA] 9).
Region of Interest Analyses
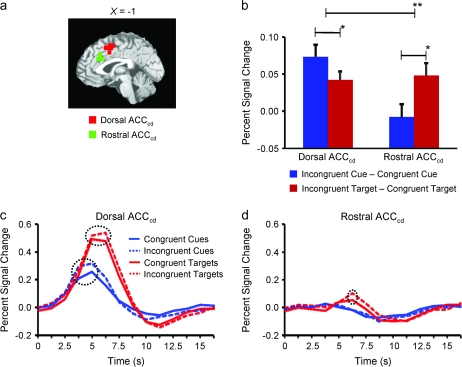
Two regions of interest from our prior study of regional specialization in the ACCcd (Weissman et al. 2004) were chosen to test whether there is regional specialization in the ACCcd for processes that increase attention to relevant stimuli and processes that detect response conflict from irrelevant stimuli: 1) a dorsal subregion of the ACCcd that included parts of the caudal ACC and presupplementary motor area (20 voxels; MNI center of mass: x = −2, y = 6, z = 52; BA 32) and 2) a rostral subregion of the ACCcd that included parts of the rostral cingulate zone (20 voxels; MNI center of mass: x = 0, y = 25, z = 29; BA 32). Region of interest (ROI) analyses were also performed for a region of the left DLPFC that was identified in a voxelwise analysis of the present data (see Voxelwise Analyses).
In all ROI analyses, we averaged the responses to particular trial types across all voxels within each ROI. Statistical tests were then performed to contrast peak activity for the different trial types. In the rostral ACCcd, inspection of the average BOLD responses revealed that peak activity in cue-only trials occurred 3.75 s after cue onset, whereas peak activity in cue-plus-target trials occurred 6.25 s after cue onset (Fig. 2d). The delay of peak activity in cue-plus-target trials is consistent with the target being presented about 2 s after the cue. In both the dorsal ACCcd and the left DLPFC (Figs 2c and 3d), inspection of the average BOLD responses revealed that peak activity in cue-only trials was distributed across 2 time points (3.75 and 5 s after cue onset) as was peak activity in cue-plus-target trials (5 and 6.25 s after cue onset). Thus, for both cue-only and cue-plus-target trials, peak activity in these regions was defined as the average amount of activity across 2 time points. We made a single exception to these definitions of peak activity when contrasting activity for incongruent hear cue-only and incongruent look cue-only trials in the dorsal ACCcd. Inspection of the average BOLD responses for these trial types revealed substantial interparticipant variability in the timing of peak activity. Therefore, for each of these trial types, we defined peak activity separately in each participant as the maximum activation at either 3.75 or 5 s after cue onset and performed statistical tests on that single time point. Given the small number of ROIs (i.e., 3), we considered P-values less than 0.05 to be significant. Moreover, because all of our hypotheses were directional all t-tests were one tailed.
Figure 2.
Regional specialization for cognitive control in the ACCcd. (a) Saggital slice indicating our rostral ACCcd subregion (green) and our dorsal ACCcd subregion (red) on the MNI-normalized brain. (b) Activity specific to cue congruency and target congruency in the dorsal ACCcd and in the rostral ACCcd. In the dorsal ACCcd, we observed significantly greater activity specific to cue congruency than to target congruency, whereas in the rostral ACCcd we observed exactly the opposite effect. (c) The average fMRI signal across time (in units of percent signal change from baseline) in the dorsal ACCcd for the various cue and target stimuli. There were significant effects of both cue congruency (i.e., greater peak activity for incongruent cues than for congruent cues) and target congruency (i.e., greater peak activity for incongruent target–distracter pairs than for congruent target–distracter pairs). (d) The average fMRI signal across time for the various cue and target stimuli in the rostral ACCcd. There was a significant effect of target congruency, but not of cue congruency. In (b), a single asterisk denotes P < 0.05, whereas 2 asterisks denote P < 0.005. Error bars represent S.E.M. Dashed circles in (c) and (d) indicate significant differences in peak activity (P < 0.05).
Figure 3.
Effects of cue type, cue congruency, and target congruency in the ACCcd and in the left DLPFC. (a) Peak activity was significantly greater for incongruent hear cue-only trials than for incongruent look cue-only trials in the dorsal ACCcd, but not in the rostral ACCcd. (b) An across-participants correlation showing that participants with larger differences in peak activity between incongruent cue-only trials and congruent cue-only trials in the dorsal ACCcd tended to respond more quickly to targets following incongruent cues than to targets following congruent cues. (c) A region of the left prefrontal cortex (24 voxels; BAs 6, 8, and 9), centered in the left DLPFC, in which cue congruency modulated activity significantly more than target congruency displayed on the MNI-normalized brain. (d) The average fMRI signal across time in the left DLPFC (10 voxels; BA 9) for the various cue and target stimuli. Dashed circles represent significant differences in peak activity (P < 0.05). In (a) and (b), a single asterisk represents P < 0.05. Error bars represent SEM.
The ROI analyses also involved correlating behavioral and neural (i.e., fMRI) measures of attention. Specifically, we correlated the behavioral cue congruency effect (i.e., the degree to which responses to targets were faster after incongruent than after congruent cues) with the neural cue congruency effect (i.e., the degree to which incongruent cue-only trials evoked greater activity than congruent cue-only trials in particular ROIs). The purpose of these correlations was to gain greater insight into the behavioral significance of the brain activations that we observed.
Results
Behavior
The behavioral data indicated that our task manipulations were highly effective. First, we observed an effect of cue congruency: participants were faster (870 ms vs. 899 ms; F1,16 = 14.5, P < 0.005) and marginally more accurate (98% vs. 97%; F1,16 = 4.2, P < 0.06) when responding to targets that followed incongruent (compared with congruent) cues. Because increasing attention to relevant stimuli facilitates identifying those stimuli (Posner 1980; Stoffer 1993), this result suggests that, in line with predictions, participants recruited processes that increase attention to relevant stimuli more strongly when they encountered incongruent cues than when they encountered congruent cues. Second, in line with prior work (Weissman et al. 2004), we observed an effect of target congruency: participants were both slower (929 ms vs. 840 ms; F1,16 = 44.6, P < 0.00001) and less accurate (96% vs. 99%; F1,16 = 34.2, P < 0.00005) when responding to incongruent (compared with congruent) target–distracter pairs. Third, the target congruency effect was smaller, F1,16 = 7.42, P < 0.02, following incongruent cues (69 ms, t16 = 4.94, P < 0.0001) than following congruent cues (109 ms, t16 = 6.63, P < 0.00001). Because increasing attention to relevant stimuli reduces interference from irrelevant stimuli (Lavie 1995; Weissman et al. 2004), this result provides further evidence that participants increased attention to relevant stimuli more strongly when they encountered incongruent cues than when they encountered congruent cues. Fourth, the target congruency effect was also smaller, F1,16 = 4.70, P < 0.05, when the cue in the immediately preceding trial was incongruent (79 ms, t16 = 4.96, P < 0.0001) than when it was congruent (116 ms, t16 = 5.59, P < 0.00005), indicating that the effect of more strongly increasing attention to relevant stimuli following an incongruent (compared with congruent) cue persisted across trials.
In addition to the main findings above, which were averaged across the visual and auditory modalities, we observed 2 differences between the visual and auditory modalities that were not crucial for testing our hypotheses. First, the cue congruency effect was larger when participants responded to visual targets (54 ms; t16 = 5.27, P < 0.00005) compared with auditory targets (2 ms; t16 = 0.17, P < 0.44), F1,16 = 17.59, P < 0.001. And, second, as in our prior study (Weissman et al. 2004) participants were both slower (915 vs. 854 ms; F1,16 = 20.6, P < 0.0005) and less accurate (97% vs. 98%; F1,16 = 5.5, P < 0.05) when they responded to auditory targets than when they responded to visual targets. No other behavioral effects were significant.
Functional MRI
Our hypothesis predicts that the dorsal ACCcd should be especially sensitive to cue congruency, whereas the rostral ACCcd should be especially sensitive to target congruency. To test this prediction, we probed activity within 2 ROIs that were identified in our prior study of regional specialization in the ACCcd (Weissman et al. 2004) (Fig. 2b): 1) a dorsal subregion of the ACCcd that included parts of the caudal ACC and presupplementary motor area (20 voxels; MNI center of mass: x = −2, y = 6, z = 52; BA 32) and 2) a rostral subregion of the ACCcd that included parts of the rostral cingulate zone (20 voxels; MNI center of mass: x = 0, y = 25, z = 29; BA 32).
In line with our hypothesis, ROI analyses on peak activity revealed a significant 3-way interaction between ACCcd Subregion (rostral, dorsal), Cue Congruency (congruent, incongruent), and Target Congruency (congruent, incongruent), F1,16 = 12.62, P < 0.005 (Fig. 2b). First, and consistent with a role in increasing attention to relevant stimuli, in the dorsal subregion the cue congruency effect was significantly larger than the target congruency effect, t16 = 1.94, P < 0.04 (Fig. 2b). Additional tests revealed significant effects of both cue congruency, t16 = 4.34, P < 0.00005, and target congruency, t16 = 3.48, P < 0.0005 (Fig. 2c), consistent with models in which resolving response conflict involves further increasing attention to relevant stimuli (Weissman et al. 2004). Second, and consistent with a role in detecting response conflict, in the rostral subregion the target congruency effect was significantly larger than the cue congruency effect, t16 = 2.12, P < 0.025 (Fig. 2b). Further tests revealed a significant target congruency effect, t16 = 2.816, P < 0.0005, in the absence of a significant cue congruency effect, t16 = 0.48, P < 0.49 (Fig. 2d). These findings support our hypothesis that a dorsal subregion of the ACCcd is especially involved in increasing attention to relevant stimuli, whereas a rostral subregion is differentially involved in detecting response conflict.
Three additional predictions stem from our hypothesis that the dorsal subregion of the ACCcd increases attention to relevant stimuli. First, as in our prior study (Weissman et al. 2004), the dorsal subregion should show greater activity in hear cue-only trials than in look cue-only trials, because only in hear cue-only trials is it necessary to switch attention from the visual cue to the auditory modality. Moreover, this effect should be most visible in incongruent cue-only trials in which attention to the visual aspect of the cue is absolutely necessary to correctly identify the upcoming task. In line with this prediction, peak activity in the dorsal subregion was significantly greater for incongruent hear cue-only than for incongruent look cue-only trials, t16 = 2.26, P < 0.02 (Fig. 3a), but did not significantly differ for congruent hear cue-only and congruent look cue-only trials, t16 < 1. Note that semantic conflict between the relevant visual aspect of the cue and the irrelevant auditory aspect was present in both incongruent hear cue-only and incongruent look cue-only trials, making it highly unlikely that the difference in activity between these trial types indexed processes that monitor for semantic conflict. Also important, a significant difference in peak activity between incongruent and congruent cue-only trials was not observed in the rostral subregion, t16 = 1.33, P > 0.10, suggesting that this subregion was not involved in increasing attention to relevant stimuli, and leading to a significant interaction between ACCcd Subregion (rostral, dorsal) and Incongruent Cue Type (Look, Hear), F1,16 = 5.34, P < 0.04 (Fig. 3a).
Second, if the dorsal subregion participates in increasing attention to relevant stimuli, then participants who show the largest cue congruency effect in the dorsal subregion should exhibit the fastest speedup in response time for targets that follow incongruent (compared with congruent) cues. In line with this prediction, an across-participants correlation indicated that the larger the effect of cue congruency on dorsal subregion peak activity in an individual participant, the faster that participant tended to respond to targets that followed incongruent (compared with congruent) cues, r15 = −0.59, P < 0.05 (Fig. 3b). This correlation was not significant in the rostral subregion, r15 = −0.37, P = 0.14, suggesting that this subregion was not involved in increasing attention to relevant stimuli.
Third, if the dorsal subregion helps to increase attention to relevant stimuli, then the pattern of activity in this region should mirror that in the left DLPFC, a region that is widely posited to focus attention on relevant stimuli (Banich et al. 2000; MacDonald et al. 2000; Miller and Cohen 2001; Weissman et al. 2004). Moreover, this effect should be most pronounced at the time of peak activation. Consistent with this prediction, a voxelwise, repeated-measures ANOVA restricted to prefrontal regions (thresholded at F13, 208 = 2.8, P < 0.001 and 5 contiguous voxels) revealed a significant 3-way interaction between Cue Congruency, Target Congruency, and Time (0–17.5 s) in the left middle frontal gyrus (24 voxels; MNI center of mass: x = −42, y = 10, z = 39; BAs 6, 8, and 9; Fig. 3c), and part of this region was located within the left DLPFC (10 voxels; MNI center of mass: x = −44, y = 10, z = 39; BA 9). Subsequent ROI analyses of the simple effects of this interaction focused on peak activity in the left DLPFC. These analyses confirmed that, as in the dorsal ACCcd subregion (but opposite to the rostral ACCcd subregion), the cue congruency effect was significantly larger than the target congruency effect, t16 = 1.81, P < 0.05. Also as in the dorsal ACCcd subregion, there were significant effects of both cue congruency, t16 = 3.16, P < 0.005, and target congruency, t16 = 2.22, P < 0.025 (Fig. 3d). Further underscoring the similar patterns of activity that we observed in the left DLPFC and in dorsal subregions of the ACCcd, the 3-way interaction between ROI (dorsal ACCcd, left DLPFC), Cue Congruency (congruent, incongruent) and Target Congruency (congruent, incongruent) was far from achieving significance, F1,16 = 1.0, P > 0.33. These findings further implicate the dorsal ACCcd in increasing attention to relevant stimuli.
Discussion
Minimizing distraction is thought to involve distinct control processes that first detect conflict caused by irrelevant stimuli and then quickly resolve such conflict by increasing attention to stimuli of interest (Carter et al. 1998; Botvinick et al. 2001; Kerns 2006). Consistent with this 2-component model, we found that rostral and dorsal subregions of the ACCcd, respectively, are differentially involved in implementing processes that detect response conflict and processes that increase attention to relevant stimuli. This finding sheds new light on the brain mechanisms that minimize distraction (Carter et al. 1998; Botvinick et al. 2001; Kerns 2006) and speaks directly to a longstanding debate over how the ACCcd contributes to cognitive control.
Several of our findings provide compelling evidence that a dorsal subregion of the ACCcd participates in increasing attention to relevant stimuli. First, activity in the dorsal subregion was significantly greater when participants shifted their attention from the visual cue to the auditory modality than when they simply maintained attention in the visual modality. This finding strongly implicates this subregion in increasing attention to relevant stimuli. Second, the cue congruency effect was significantly larger than the target congruency effect not only in the dorsal subregion of the ACCcd, but also in the left DLPFC, a region that is widely posited to increase attention to relevant stimuli (Banich et al. 2000; MacDonald et al. 2000; Miller and Cohen 2001; Weissman et al. 2004). Moreover, a significant effect of target congruency was also observed in both regions, consistent with models in which resolving conflict involves further increasing attention to relevant stimuli (Botvinick et al. 2001; Weissman et al. 2004, 2005). Given that our findings implicate both dorsal subregions of the ACCcd and the left DLPFC in implementing processes that increase attention to relevant stimuli, future work should be aimed at determining whether these regions make identical or distinct contributions to such processes. Third, in line with prior findings that increasing attention to relevant stimuli speeds response times to identify those stimuli (Posner 1980; Stoffer 1993), the more a given participant exhibited greater activity in the dorsal subregion for incongruent cue-only than for congruent cue-only trials (i.e., a cue congruency effect), the more that participant tended to respond faster to targets that followed incongruent cues than to targets that followed congruent cues. Taken in isolation, one might interpret this correlation as indicating that greater conflict detection by the dorsal subregion leads to greater recruitment of other brain regions (e.g., the DLPFC) that resolve conflict during cue processing. However, given that several of our other findings implicate the dorsal subregion in implementing attentional processes, the most parsimonious interpretation of our findings is that the dorsal subregion of the ACCcd implements attentional processes in multiple contexts, consistent with recent claims that the dorsal ACCcd is a critical component of a “core task-set system” (Dosenbach et al. 2006, 2007).
Of importance, our findings also weigh against the possibility that the cue congruency effect in the dorsal subregion of the ACCcd reflects control processes other than those that increase attention to relevant stimuli. First, the cue congruency effect is unlikely to index processes that detect pre-response (e.g., semantic) conflict (Weissman et al. 2003; van Veen and Carter 2005). Indeed, even when semantic conflict was equated during the processing of incongruent cues, switching attention from the visual to the auditory modality was associated with greater activity in the dorsal subregion than was maintaining attention in the visual modality. Second, the cue congruency effect is unlikely to reflect processes that signal an increased likelihood of making an error in an upcoming task (Brown and Braver 2005) or an increased probability of receiving a reduced reward when an error is relatively likely (Hewig et al. 2007). Specifically, activity in the dorsal subregion was greater for incongruent than for congruent cues despite the fact that behavioral performance was both faster and more accurate for targets that followed incongruent cues than for targets that followed congruent cues. Third, the cue congruency effect is unlikely to index processes underlying response selection (Roelofs et al. 2006) because no responses were made to the cue stimuli. Fourth, the cue congruency effect is unlikely to index the expectation of greater response conflict in an upcoming task (Sohn et al. 2007) because behavioral measures of response conflict were significantly smaller following incongruent cues than following congruent cues. And, fifth, the cue congruency effect is unlikely to index processes underlying anticipation (Murtha et al. 1996) or novelty detection (Ranganath and Rainer 2003; Matsumoto et al. 2007) because we held constant the nature of the task that followed incongruent and congruent cues. For all of these reasons, our findings are most compatible with a role for the dorsal subregion of the ACCcd in increasing attention to relevant stimuli (Posner and DiGirolamo 1998; Dreher and Berman 2002).
Although we have argued against an interpretation of the cue congruency effect in dorsal subregions of the ACCcd as reflecting processes that monitor for semantic conflict, one might wonder whether a visually presented cue instructing participants to shift their attention to the auditory modality is inherently associated with greater semantic conflict than a visually presented cue instructing subjects to maintain their attention in the visual modality. Such a view may appear plausible at first, but 2 pieces of data argue against it as an alternative account of our findings. First, we did not observe significantly greater activity in the dorsal ACCcd for congruent hear cue-only than for congruent look cue-only trials, even though, according to this view, congruent hear cue-only trials should be associated with greater semantic conflict than congruent look cue-only trials. Second, in a prior study (Weissman et al. 2004) we observed significantly greater activity for hear cue-only than for look cue-only trials, even though each type of cue was presented in the visual modality in half the trials and in the auditory modality in the other half, a manipulation that should have equated for these trial types the specific form of semantic conflict that is under consideration. For these reasons (and others discussed in the preceding paragraph), we would argue that the cue congruency effect that we have observed in the dorsal ACCcd is much more consistent with a role for this region in implementing attentional processes than with a role in monitoring for semantic conflict.
We have also argued that the cue congruency effect in dorsal subregions of the ACCcd is unlikely to index processes underlying response selection (Roelofs et al. 2006) because no responses were made to the cue stimuli. Nonetheless, it is important to consider whether our findings might be accounted for by a more broadly conceived response selection model (Milham and Banich 2005). In this model, dorsal subregions of the ACCcd that are activated during cue processing might link information from the currently relevant sensory stream to mechanisms that plan future responses. Some investigators have argued that support for this type of response selection model comes from findings that dorsal ACCcd activity is greater for both congruent and incongruent trials than for neutral trials in the classic Stroop task (Milham and Banich 2005). The central claim is that demands on processes that link a relevant channel of information (ink color) to response mechanisms are greater when an irrelevant channel of information (word identity) contains task-relevant information (a color-related word) than when it contains task-irrelevant information (a color-unrelated word). Clearly, this response selection model differs from the attention-based model that we favor, which posits that dorsal subregions of the ACCcd bias attention at perceptual stages of processing toward whichever stream of sensory information is currently relevant.
We now consider whether the response selection model can provide a better account of our findings than the attention-based model. According to the response selection model, cue-related activity in dorsal subregions of the ACCcd reflects processes that select the channel of information (auditory or visual) upon which a future response will be based. In this view, basing a response on the auditory channel should impose similar demands on response selection processes as basing a response on the visual channel. In both cases, information from a single sensory modality needs to be linked to response mechanisms, and there is no a priori reason to hypothesize that this link should be more difficult to make for one sensory modality than for another. For example, the number of irrelevant channels that contain task-relevant information during cue processing (i.e., one auditory channel) is the same regardless of whether participants are cued to direct their attention toward the auditory or toward the visual sensory modality. Contrary to the response selection view, however, we observed greater activity in dorsal subregions of the ACCcd for hear cue-only than for look cue-only trials. As we discussed earlier, this finding is highly consistent with our view that the dorsal ACCcd implements attentional processes. Indeed, demands on attentional processes should have been greater when participants were cued to shift attention away from the visual modality and toward the auditory modality (hear cue-only trials) than when they were cued to maintain attention in the visual modality (look cue-only trials). The response selection model is not about increasing attention to relevant stimuli at perceptual stages of processing, but rather about presetting or biasing response-related aspects of selection. Therefore, our finding that the dorsal ACCcd is more highly activated for hear cue-only than for look cue-only trials appears to be better explained by the attention model than by the response selection model.
Our conclusion that the dorsal subregion of the ACCcd increases attention to relevant stimuli raises an important question about how we should interpret previous findings implicating these regions in various aspects of performance monitoring, such as conflict monitoring, error monitoring, and reward assessment (Ridderinkhof et al. 2004). In our view, the present data suggest that dorsal ACCcd activity attributed to performance monitoring in some prior studies may actually have reflected attentional processes. Specifically, as in the present study, dorsal ACCcd activity that varied with demands on performance monitoring processes might also have varied with demands on cue-triggered attentional processes, even when demands on performance monitoring processes were minimal. Such a result would be highly consistent with a role for the dorsal ACCcd in implementing attentional processes that are recruited not only during cue processing to orient attention, but also during target processing to resolve response conflict by further increasing attention to relevant stimuli (Weissman et al. 2004). Unfortunately, only a handful of previous investigators have used experimental designs in which attentional and performance monitoring processes can be distinguished from one another as in the present study (MacDonald et al. 2000; Weissman et al. 2004, 2005). Thus, additional studies are needed to determine whether, and to what degree, dorsal ACCcd activity that is frequently associated with various performance monitoring processes (e.g., error monitoring, reward assessment, etc.) may actually reflect attentional processes.
The present findings also weigh against the possibility that the target congruency effect in the rostral subregion of the ACCcd reflects control processes other than those that detect response conflict. First, if these regions detected pre-response (e.g., semantic) conflict (Weissman et al. 2003; van Veen and Carter 2005), then we should have observed greater activity for incongruent than for congruent cue-only trials. Incongruent cue-only trials were high in semantic conflict because the 2 words (“Look” and “Hear”) had different meanings, whereas congruent cue-only trials were low in semantic conflict because the same word (e.g., “Look”) was presented twice. However, we observed no such effect. Second, if these regions signaled either when an error was relatively likely in an upcoming task (Brown and Braver 2005) or when an upcoming task was more likely to be less rewarding because an error was relatively likely (Hewig et al. 2007), then we should have observed greater activity for congruent than for incongruent cue-only trials. Indeed, as we mentioned earlier, behavioral performance was worse for targets that followed congruent cues than for targets that followed incongruent cues. However, once again we observed no such effect. Thus, our findings are most compatible with a role for the rostral subregion of the ACCcd in detecting response conflict.
More broadly, the present results add to a growing body of work indicating regional specialization of function in the ACC (Bush et al. 2002; Somerville et al. 2006; Goldstein et al. 2007). A major finding of this work has been that relatively dorsal and caudal ACC regions (i.e., the so-called “cognitive” division of the ACC) participate in implementing cognitive processes, whereas relatively ventral and rostral ACC regions (i.e., the so-called “emotional” division of the ACC) contribute to emotional processes. The present findings of regional specialization completely within the cognitive division of the ACC indicate regional specialization on a much finer spatial scale than have many prior studies, consistent with recent data indicating that rostral and dorsal subregions of the ACCcd exhibit different patterns of functional connectivity with other brain regions when participants are not actively performing a cognitive task (Margulies et al. 2007). As such, our findings suggest that brain imaging techniques offering relatively high degrees of spatial resolution may be useful for mapping the complete spatial topography of cognitive and emotional processes in the ACC. Such techniques have already been applied successfully to study regional specialization within the visual system. For example, recent findings from “high-resolution” fMRI suggest that regions of the visual cortex that are specialized for processing faces can, in fact, be subdivided into smaller regions that are specialized for processing different types of objects (Grill-Spector et al. 2006). Future high-resolution studies may therefore be helpful for obtaining a more fine-grained characterization of regional specialization in the ACC for various cognitive control processes.
Although the present findings of regional specialization in the ACCcd provide novel support for 2-component models of minimizing distraction, they also have some limitations. Most important, they do not reveal the relative timing with which different brain regions become activated during the process of minimizing distraction. For instance, brain regions that detect response conflict should become activated before brain regions that increase attention to relevant stimuli. Given the sluggishness of the hemodynamic signal that is measured with fMRI, brain imaging techniques offering higher temporal resolution will likely be necessary to test such important predictions.
In conclusion, our findings support a 2-component model of minimizing distraction from irrelevant stimuli (Carter et al. 1998; Botvinick et al. 2001; Kerns 2006). Moreover, they speak to a longstanding controversy over the role of the ACCcd in cognitive control by showing that, rather than performing a single cognitive control process as some models posit (Botvinick et al. 2001; Carter et al. 1998; Kerns 2006), the ACCcd implements multiple control processes. Future studies characterizing the spatial topography and relative timing of control processes in the ACC may enhance our understanding of behavior in neurologically intact populations and in numerous clinical syndromes that are characterized by disruptions of cognitive control, including drug addiction (Goldstein et al. 2007), attention deficit and hyperactivity disorder (Dickstein et al. 2006), and schizophrenia (Kerns et al. 2005).
Funding
National Institutes of Health grant (1R03DA021345-01) to D.H.W.
Acknowledgments
We wish to thank Rebecca J. Compton and Cindy Lustig for helpful comments on an earlier version of this manuscript. Conflict of Interest: None declared.
References
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Barad V, Gullett D, et al. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Brain Res Cogn Brain Res. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Bull. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Berman KF. Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci USA. 2002;99:14595–14600. doi: 10.1073/pnas.222193299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nat Neurosci. 2006;9:1177–1185. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- Hewig J, Trippe R, Hecht H, Coles MGH, Holroyd CB, Miltner WHR. Decision-making in blackjack: an electrophysiological analysis. Cereb Cortex. 2007;17:865–877. doi: 10.1093/cercor/bhk040. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMR1 study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger A, Carter CS. Anterior cingulate conflict monitoring and adjustment in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual Load as a Necessary Condition for Selective Attention. J Exp Psychol Hum Percept Perform. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Tanaka K. Effects of novelty on activity of lateral and medial prefrontal neurons. Neurosci Res. 2007;57:268–276. doi: 10.1016/j.neures.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A. Anticipation causes increased blood flow to the anterior cingulate cortex. Hum Brain Mapp. 1996;4:103–112. doi: 10.1002/(SICI)1097-0193(1996)4:2<103::AID-HBM2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI I. The method. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. Cambridge (MA): MIT Press; 1998. pp. 401–423. [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuiss S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roelofs A, van Turennout M, Coles MGH. Anterior cingulate cortex activity can be independent of response conflict in Stroop-like tasks. Proc Natl Acad Sci USA. 2006;103:13884–13889. doi: 10.1073/pnas.0606265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M-H, Albert MV, Jung K, Carter CS, Anderson JR. Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:10330–10334. doi: 10.1073/pnas.0703225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Stoffer TH. The time-course of attentional zooming—a comparison of voluntary and involuntary allocation of attention to the levels of compound stimuli. Psychol Res. 1993;56:14–25. doi: 10.1007/BF00572129. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage. 2005;27:497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19:1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. J Neurosci. 2004;24:10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]