Summary
Individuals differ widely in cortisol output over the day and cortisol reactivity to challenge, both of which are relevant to disease risk. There is limited evidence concerning the heritability of these differences, so we evaluated the heritability of cortisol levels in the afternoon and cortisol reactivity using a twin design. The study involved 80 monozygotic (MZ) and 70 dizygotic (DZ) same-sex twin pairs aged 11.2 years on average. Salivary cortisol was measured in the afternoon at home before and after playing a computer game. Ratings of excitement and upset were also obtained, and objective task performance was assessed. Salivary cortisol levels averaged 4.08 (S.D. 2.3) nmol/l at pretask baseline, and declined on average over the session to 3.45 (1.9) nmol/l immediately after the tasks and 2.87 (1.6) nmol/l 10 min later. There were, however, marked individual differences, with cortisol reactivity (difference between pretask baseline and post-task 1) ranging from +4.53 to −6.23 nmol/l. Intra-class correlations for all the cortisol parameters were substantially greater for MZ (range 0.41–0.57) than for DZ (0.11–0.29) twin pairs. Quantitative genetic modelling confirmed significant heritability for pretask baseline cortisol (58%), the two post-task values (60 and 56%), and cortisol reactivity (44%). The study lacked power for assessing sex differences. Subjective reports of excitement were also somewhat heritable, but there was little covariation of cortisol and subjective responses, so genetic influences on covariation could not be tested. These findings indicate that individual differences in children’s cortisol levels recorded before tasks and cortisol reactivity to behavioural challenges are influenced by genetic factors.
Keywords: Cortisol, Genetics, Reactivity, Twins, Children
1. Introduction
There are marked individual differences both in cortisol levels over the day and cortisol responses to behavioural challenge. These differences are thought to be relevant to a range of pathologies, including depression, abdominal adiposity, cognitive impairment in old age, hypertension, autoimmune disease and resistance to infection (Bjorntorp, 2001; Herbert et al., 2006; McEwen, 2007). Individual differences are determined by a range of factors including the perinatal environment (Meaney, 2001; Phillips, 2007), early childhood adversity (Heim et al., 2000), and psychosocial factors such as stress exposure, social support and psychological traits (Miller et al., 2007). Polymorphisms of genes regulating glucocorticoid and mineralocorticoid receptor function are associated with cortisol responsivity (Wüst et al., 2004; DeRijk et al., 2006). Nevertheless, the contribution of genetic factors to cortisol variation in the population may vary with factors such as timing of assessments and whether cortisol is measured under resting conditions or in response to challenge. Bartels et al. (2003a) reported a meta-analysis of five twin studies in which the heritability of ‘basal’ cortisol was put at 62%, but their analysis conflated measures taken under resting conditions in the morning and the cortisol awakening response (CAR), the increase in cortisol that typically occurs over the first 30–45 min after waking. Wüst et al. (2000) observed significant heritability of the CAR but not cortisol over the remainder of the day in a study of 52 monozygotic (MZ) and 52 dizygotic (DZ) twin pairs. This pattern was replicated in a larger study of 199 MZ and 272 DZ adult twin pairs, in which the CAR showed round 30% heritability, with no significant effects for values recorded later in the day (Kupper et al., 2005). A mixed pattern was recorded in a study of 180 pairs of 12-year-old twins, with significant heritability for samples taken early in the day and at noon, but not in the evening (Bartels et al., 2003b). A report from the Wisconsin twin project showed no heritability for samples taken in the afternoon in younger children (average age 8.64 years) (Schreiber et al., 2006). These findings suggest that cortisol levels soon after waking and early in the day are heritable, while resting cortisol levels later in the day are not. Studies of the heritability of cortisol reactivity to behavioural challenge have been inconsistent (Kirschbaum et al., 1992). However, Federenko et al. (2004) demonstrated that heritability of cortisol reactions to a standard stress battery increased with repeated exposure, so may depend on the context of task presentation. A study of 19-month-old twins has suggested that cortisol reactivity to unfamiliar situations was more heritable in infants who had not experienced familial adversity than in those with risk factors such as low birth weight, low socioeconomic status (SES), and maternal hostile behaviours (Ouellet-Morin et al., 2008).
The majority of studies of cortisol over the day have relied on participants being provided with sampling devices and collecting saliva samples at predetermined times without supervision. This may result in additional error, since respondents will be in diverse situations, and not all samples are reliably taken at the required times (Kudielka et al., 2003). Since cortisol levels vary over the day, and the twins may not collect their samples at the same times, heritability could be underestimated. In the present study, we tested a sample of young twins in their own homes under standardised conditions, with simultaneous cortisol measures from each member of the pair obtained by the research team. In addition to measuring baseline levels, we sampled cortisol after the administration of a computer game. The game was not intended to provoke stress, but to act as a behavioural challenge that would elicit individual differences in cortisol responsivity. Computer games have been widely used in young children to stimulate physiological responses, and the cardiovascular responses to computer games have been shown to relate to future risk of high blood pressure (Treiber et al., 2001). The challenging nature of the game was assessed by taking ratings of excitement, and we checked whether or not distress was elicited by obtaining ratings of upset. These subjective measures also provided an opportunity to assess covariation in endocrine and subjective responses. Recent molecular genetic studies have suggested that polymorphisms in enzymes regulating monoamine neurotransmitter pathways may affect both endocrine and subjective responses to psychological stress (Jabbi et al., 2007). We reasoned that if cortisol is heritable, this could be due either to genetic influences on primary mechanisms within the hypothalamic-pituitary-adrenocortical (HPA) axis, or to shared genetic influences on emotional and cortisol responses. We therefore assessed the heritability of subjective responses, and evaluated the covariation of cortisol and subjective responses. If there was significant covariation, we planned to carry out bivariate modelling to determine the extent to which genetic and environmental factors accounted this covariation.
2. Method
2.1. Participants
Participants in this study were part of the Twins’ Early Development Study (TEDS), a population-based cohort of twins born in the UK in 1994, 1995, and 1996. The TEDS cohort is reasonably representative of population demographics, as described elsewhere (Oliver and Plomin, 2007). Zygosity was assessed through a parent questionnaire of physical similarity, which has been shown to be over 95% accurate when compared with DNA testing (Price et al., 2000). Where zygosity was unclear from the questionnaire, DNA testing was conducted. The subsample tested in this investigation was part of the follow-up in a substudy primarily concerned with genetic and environmental determinants of childhood adiposity and eating behaviours (Wardle et al., 2008). The sample consisted of same-sex twins, so that the DZ could be matched with MZ twin pairs. 173 families with same-sex twins were visited at home for this study and cortisol data were collected from 150 pairs, consisting of 30 MZ male (MZM), 50 MZ female (MZF), 30 DZ male (DZM) and 40 DZ female (DZF) pairs. There were no differences in the characteristics of participants who did and did not provide cortisol samples. This study was approved by the research ethics committees of Kings College London and University College London, and every child’s parents provided written informed consent.
2.2. Procedure
Families were visited at home by trained researchers. Most sessions (71%) were held after 1530 h, but 29% took place at mid-day, starting between 1200 and 1330 h. None of the children had eaten within 3 h of the cortisol assessment. Children’s heights were measured to the nearest 1.0 mm with a portable stadiometer and weights to the nearest 0.1 kg using a digital Tanita scale. Body fat was measured using a Maltron Body Fat Analyser. The children were then told that they would play a computer game but that before playing saliva sample would be collected. Saliva samples were collected with Salivettes (Sarstedt, Leicester) with each cotton roll held in the mouth for two minutes. The pretask baseline saliva sample was collected, and each twin rated how excited and upset they felt by ticking one of a set of five cartoon faces with varying sizes of smiles, corresponding to a five-point scale where 1 = not at all, and 5 = very much. The rules of the computer game were explained and they played for 10 min. The game (Mutant Storm™) is a cooperative, arcade-type shooting game, involving a series of levels of increasing difficulty. Each participant had five ‘lives’ in each game, and when these had been used up, a new game was initiated. Performance of each twin pair was assessed in terms of the average number of levels reached in each game, and the mean score per level. The average number of levels per game was skewed, so was log transformed before analysis.
A second saliva sample and assessment of excitement and upset were taken immediately after the game (post-task 1). The children then carried out an unrelated task (the details of are not described here), and the third saliva sample (post-task 2) and set of subjective ratings were obtained 10 min after post-task 1. The session then continued with additional eating behaviour tasks concerned with satiety and food responsiveness. The twins and their mothers received retail vouchers in thanks for their participation. Data collection took place between March 2005 and June 2006.
Family socioeconomic background (SES) was indexed by the mother’s educational level. Families in which the mothers had no more than the minimum qualifications in the English educational system (GCSE equivalent or less) were identified as lower SES, and families in which the mothers had A-level equivalent or a university degree were identified as higher SES.
2.3. Cortisol assays
Salivettes were stored at −20 °C until the completion of the study. Cortisol was analysed using a high sensitivity chemiluminscence assay (IBL-Hamburg, Hamburg, Germany) at the Technical University Dresden (Germany). Inter- and intra-assay coefficients of variance (CVs) were <8%. Missing values resulted in 77-79 of the 80 MZ pairs and 64-67 of the 70 DZ pairs having data analysed for each sample time, as detailed in Table 2.
Table 2.
Intra-class twin correlations for cortisol measures
| Monozygotic |
Dizygotic |
|||
|---|---|---|---|---|
| ICC (95% CI) | N | ICC (95% CI) | N | |
| Pretask baseline | 0.57 (0.40; 0.70) | 79 | 0.29 (0.06; 0.50) | 67 |
| Post-task 1 | 0.54 (0.37; 0.68) | 77 | 0.26 (0.02; 0.47) | 65 |
| Post-task 2 | 0.46 (0.26; 0.62) | 77 | 0.31 (0.07; 0.51) | 65 |
| Reactivity | 0.41 (0.20; 0.58) | 77 | 0.11 (−0.14; 0.35) | 64 |
ICC = intra-class correlation with 95% confidence intervals; N = number of twin pairs.
2.4. Statistical analysis
Twin designs permit genetic and environmental influences on phenotyes to be investigated. MZ twins are genetically identical, whereas nonidentical (DZ) twins share on average 50% of segregating genes. The extent that a phenotypic trait is influenced by genetics is reflected in the greater within-pair resemblance in MZ than DZ twins (Plomin et al., 2008). Comparisons of twin intra-class correlations permit the estimation of genetic effects, shared environment effects (effects that make participants from the same family more similar), and non-shared environment effects (effects that make participants from the same family different).
The background characteristics of the male and female MZ and DZ twins were compared using analysis of variance for continuous variables and χ2 tests for categorical variables. The distribution of cortisol values was inspected, and transformation was not required. Cortisol profiles over the session were analysed using repeated measures analysis of variance with zygosity as the between-person factor, and sample as the within-person factor. As power analyses indicated that the power to detect sex differences in heritability was low, male and female data were combined for both zygosities. We tested whether cortisol responses were different when tested at mid-day or in the afternoon by including time of day as an additional between-person factor. The Greenhouse-Geisser correction was applied when the sphericity assumption was violated, but uncorrected degrees of freedom are presented in the Results. Cortisol reactivity was computed as the change between pretask baseline and post-task 1. Similar methods were used to analyse subjective responses.
We computed within-pair correlations in MZ and DZ twin pairs to give an estimate of the contribution of inherited genetic differences to phenotypic variation in pretask baseline, post-task 1, and post-task-2 cortisol, and in cortisol reactivity. Quantitative genetic model fitting was also applied (Plomin et al., 2008), in which observable variation is decomposed into additive genetic components, and shared and non-shared environmental components. We used Mx software (Version 1.7.03) for structural equation modelling (Neale et al., 2002) with raw data to test the fit of the models to the data and obtain confidence intervals for estimates of genetic and environmental effects. Heritability analysis was performed using age-sex standardized residuals for pretask baseline, post-task 1, post-task 2, and for cortisol reactivity. The results are presented in terms of additive genetic variance (a2), shared environmental variance (c2) and non-shared environmental variance including error (e2), all with 95% confidence intervals (CI). In addition to the ACE model, we tested the fit of more parsimonious AE, CE and E models. The fit of these models was compared with the ACE model using the likelihood ratio test. We estimated that with this sample size, we had adequate power to detect >50% additive genetic (A) effects, but that power for detecting shared environment components was limited. The shared environment estimates should therefore be treated with caution.
Similar methods were used to model genetic and environmental influences on subjective experience during the study. Additionally, we planned to assess genetic influences on the covariation of cortisol and subjective responses with bivariate models using Cholesky decomposition. However, since the covariation between cortisol and subjective responses was small and inconsistent, these analyses are not presented.
3. Results
The characteristics of the study sample are summarised in Table 1. Participants were aged 11.2 years on average, and the majority were of white European origin. Around one third of the families were categorised as higher SES based on the mother’s educational qualifications. There were no gender or zygosity differences, and no significant interactions between gender and zygosity on any of the variables listed in Table 1.
Table 1.
Characteristics of participants
| Monozygotic twin pairs |
Dizygotic twin pairs |
|||
|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |
| Twin pairs (n) | 30 | 50 | 30 | 40 |
| Age (years) | 10.9 ± 0.69 | 11.2 ± 0.49 | 11.3 ± 0.43 | 11.2 ± 0.49 |
| Ethnicity: white | 93.2% | 90% | 89.8% | 97.4% |
| Maternal education: higher | 42.4% | 31.3% | 37.3% | 29.3% |
| Body weight (kg) | 40.0 ± 8.0 | 40.8 ± 10.4 | 41.6 ± 10.2 | 41.8 ± 12.0 |
| Height (m) | 1.45 ± 0.07 | 1.46 ± 0.08 | 1.48 ± 0.06 | 1.45 ± 0.08 |
3.1. Cortisol responses
Repeated measures analysis of variance of cortisol showed a main effect of sample time (F(2,658) = 103.7, p < 0.001), with no differences between MZ and DZ twins. Cortisol levels declined between pretask baseline and post-task samples, with mean values of 4.08 ± 2.3 nmol/l at baseline, 3.45 ± 1.9 nmol/l at post-task 1 and 2.87 ± 1.6 nmol/l at post-task 2. Cortisol was higher when testing took place at mid-day compared with the afternoon (F(1,280) = 5.04, p = 0.026; overall means 3.96 ± 1.9 and 3.25 ± 1.7 nmol/l), but cortisol reactivity did not vary with time of starting the session, and there were no interactions between time and zygosity.
There were marked variations in cortisol reactivity, with differences between pretask baseline and post-task 1 ranging from +4.53 to −6.23 nmol/l and 24% of participants showing an increase in cortisol between baseline and post-task 1. There was also a main effect of maternal education in cortisol level (F(1,280) = 5.57, p < 0.001), with children from higher SES families having higher cortisol throughout the protocol (means 3.81 ± 2.00 vs. 3.29 ± 1.78 nmol/l). However, there was no association between SES and zygosity in cortisol levels or cortisol responses.
The twin correlations for the four-cortisol parameters are shown in Table 2. MZ correlations greatly exceeded those of the DZ twins, suggesting a strong genetic influence. Doubling the difference between the MZ and the DZ correlations to estimate heritability indicated substantial genetic influence on cortisol, ranging from 30% for post-task 2 to 60% for reactivity. These effects were confirmed in the model fitting (Table 3). Heritability estimates were strong for all four parameters, ranging from 44% (cortisol reactivity) to 60% (post-task 1). The remaining variance was attributable to non-shared environment and error, since there were no significant shared environment effects. The ACE model was a better fit than the CE or E models, but did not differ from the AE model (Δχ2 = 0.00, Δdf = 1, p = 1.00 for each model). When heritability estimates were calculated only for participants who began the study in the afternoon, the findings were similar to those presented in Table 3.
Table 3.
Cortisol heritability analyses using Mx (ACE models)
| Model fitting results (parameter estimates and confidence intervals) |
|||
|---|---|---|---|
| Additive genetic effect (a2) | Shared environment effect (c2) | Non-shared environment effect (e2) | |
| Pretask baseline | 0.58 (0.13; 0.70) | 0.00 (0.00; 0.37) | 0.42 (0.30; 0.58) |
| Post-task 1 | 0.60 (0.43; 0.72) | 0.00 (0.00; 0.24) | 0.40 (0.28; 0.59) |
| Post-task 2 | 0.56 (0.38; 0.69) | 0.00 (0.00; 0.33) | 0.44 (0.31; 0.64) |
| Reactivity | 0.44 (0.12; 0.62) | 0.00 (0.00; 0.20) | 0.56 (0.38; 0.79) |
Variance with 95% confidence intervals.
3.2. Subjective responses
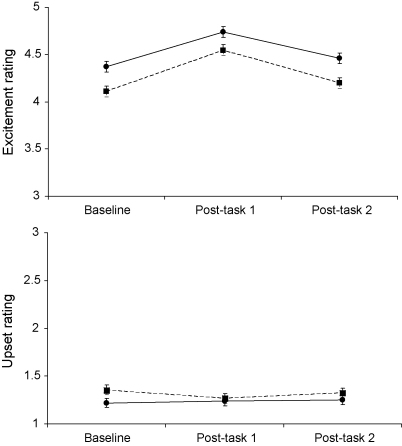
Analysis of excitement ratings showed main effects of zygosity (F(1,283) = 17.17, p < 0.001) and sample (F(2, 566) = 42.61, p < 0.001), but no interaction between zygosity and sample. As can be seen in Fig. 1, excitement ratings were high on average, but increased between baseline and post-task 1, declining back towards baseline on post-task 2. Overall, excitement ratings were greater in MZ than DZ twins. By contrast, ratings of upset were very low, and did not change consistently over trials (Fig. 1, lower panel). Between 75 and 81% of participants gave the minimum upset rating at each time point. There were no differences between MZ and DZ twins in ratings of upset. In the absence of consistent responses to tasks in ratings of upset, genetic modelling was carried out only on excitement ratings.
Fig. 1.
Mean ratings of excitement (upper panel) and upset (lower panel) at baseline, post-task 1 and post-task 2 samples. MZ twins are shown with solid lines, and DZ twins with dashed lines. Error bars are S.E.M.
Table 4 summarizes the intra-class twin correlations for excitement ratings. These yielded heritability estimates ranging from 41% for ratings at post-task 1 to 64% for baseline ratings. In the model-fitting analysis (Table 5), significant heritability effects emerged for baseline (53%), post-task 1 (46%) and post-task 2 (63%), but not for reactivity. The remaining variance was attributable to the non-shared environmental and error, with no shared environment effects. The ACE and AE models showed a similar fit (p = 1.00 for each model), but both were better fits than the CE or E models (0.023 < p < 0.001).
Table 4.
Intra-class twin correlations for excitement ratings
| Monozygotic |
Dizygotic |
|||
|---|---|---|---|---|
| ICC (95% CI) | N | ICC (95% CI) | N | |
| Pretask baseline | 0.53 (0.36; 0.67) | 80 | 0.21 (−0.01; 0.42) | 74 |
| Post-task 1 | 0.37 (0.17; 0.55) | 80 | 0.16 (−0.07; 0.38) | 72 |
| Post-task 2 | 0.53 (0.35; 0.67) | 79 | 0.31 (0.09; 0.51) | 73 |
| Reactivity | 0.34 (0.13; 0.52) | 80 | 0.04 (−0.18; 0.27) | 72 |
ICC = intra-class correlation with 95% confidence intervals; N = number of twin pairs.
Table 5.
Excitement heritability analyses using Mx (ACE models)
| Model fitting results (parameter estimates and confidence intervals) |
|||
|---|---|---|---|
| Additive genetic effect (a2) | Shared environment effect (c2) | Non-shared environment effect (e2) | |
| Pretask baseline | 0.53 (0.09; 0.66) | 0.00 (0.00; 0.37) | 0.47 (0.34; 0.63) |
| Post-task 1 | 0.46 (0.09; 0.62) | 0.00 (0.00; 0.24) | 0.54 (0.38; 0.75) |
| Post-task 2 | 0.63 (0.23; 0.74) | 0.00 (0.00; 0.31) | 0.37 (0.26; 0.53) |
| Reactivity | 0.31 (0.00; 0.50) | 0.00 (0.00; 0.22) | 0.69 (0.50; 0.91) |
Variance with 95% confidence intervals.
3.3. Covariation between cortisol and subjective responses
The within-person associations between cortisol and excitement ratings were weak at all stages of the study. As can be seen in Table 6, the only significant association was a negative relationship between cortisol and excitement at post-task 2 in MZ twins. Formal modelling of the overlap in genetic contributions to cortisol and excitement is therefore not presented.
Table 6.
Bivariate analyses of subjective and cortisol responses
| Within-twin cortisol–excitement correlations |
||
|---|---|---|
| MZ twins | DZ twins | |
| Pretask baseline | −0.07 | −0.12 |
| Post-task 1 | 0.10 | 0.02 |
| Post-task 2 | −0.17* | −0.02 |
| Reactivity | −0.09 | 0.07 |
p < 0.05.
3.4. Task performance
Overall, participants completed an average of 4.43 (S.D. 1.6) levels per game, and their mean score per game was 49.9 ± 9.0. There were no differences in game performance between MZ and DZ twins. In MZ but not DZ twins, cortisol at baseline, post-task 1 and post-task 2 was positively correlated with the number of levels completed (r = 0.20–0.25, p < 0.05).
4. Discussion
The results of this study show there is a substantial genetic contribution (56–60%) to cortisol levels recorded before and after behavioural tasks administered between mid-day and late afternoon in 11-year-old twins. Cortisol reactivity to behavioural challenge was also heritable (44%). Subjective excitement was also moderately heritable, but we found no evidence for associations between subjective responses and cortisol responses. Cortisol was positively associated with one measure of objective behavioural performance in MZ but not DZ twins.
Our results are apparently at odds with several studies of adults that have not shown significant heritability of cortisol samples collected between 1300 and 1900 h (Wüst et al., 2000; Kupper et al., 2005). In children, significant heritability has been observed for samples taken at 1230 h, but not later in the afternoon (Bartels et al., 2003b; Schreiber et al., 2006). The explanation may lie in differences in study protocol. In our study, members of the twin pair were tested at the same time of day, in a standardised setting in which both children were engaged in similar activities. Saliva samples in previous studies have not been taken under controlled conditions, and there were no objective checks that the twins took the samples at the same time. Cortisol variance is related both to activities and time of day, and this may have obscured genetic effects.
Our protocol did not elicit increases in cortisol in response to tasks on average, rather a progressive decline in mean levels. Testing took place in the afternoon, when cortisol levels fall because of the diurnal rhythm in output. A very strong stimulus may be required to counter this pattern of change and generate an absolute rise in cortisol. A similar finding of average decreases in cortisol following challenging behavioural tasks has been recorded in other studies, because of the influence of diurnal effects (e.g. Kunz-Ebrecht et al., 2003). Additionally, the children in this study were quite excited at the time the baseline samples were collected; they were being specially visited at home by a team of researchers, and had already undergone body fat measurements, with the fitting of impedance electrodes. Their cortisol levels may therefore have been somewhat elevated at baseline, accentuating the decline over time, and the pretask baseline cortisol values cannot be regarded as basal levels. However, participants were not stressed at baseline, since ratings of upset were very low; indeed, more than 75% of participants gave the minimum score on the upset rating at baseline. Despite the mean decrease over time, there were wide individual differences in cortisol reactivity, and excitement increased significantly in response to the task. We chose not to use a more stressful tasks such as the Trier Social Stress Test for children (TSST) which would have generated mean increases (Buske-Kirschbaum et al., 2003), because the challenge was part of a larger assessment, and we did not wish to upset children or their parents. Exciting video games have been used extensively to elicit psychophysiological reactivity (Treiber et al., 1993), and individual differences in response have been shown to predict clinically relevant differences in disease risk (Treiber et al., 2003).
The fact that conditions were only moderately challenging may account in part for discrepancies with previous studies showing low heritability of cortisol reactivity to challenge (Kirschbaum et al., 1992). The result is compatible with Federenko et al.’s (2004) observations in adults, which showed that heritability of cortisol responses to the TSST were not significant on the first administration, but only emerged with repeated stress testing. On these later sessions, anxiety responses were attenuated, since participants were familiar with the challenges. Our testing may have resembled the familiar, low anxiety context in which heritability of cortisol reactivity emerged in Federenko’s study.
We measured subjective responses alongside cortisol, unlike previous twin studies. Cortisol is typically strongly influenced by subjective experiences of distress, excitement, and other affective states (Dickerson and Kemeny, 2004). Positive affective states such as excitement are partly heritable (Lykken and Tellegen, 1996; Boardman et al., 2008). It is conceivable therefore that heritability of cortisol could be related to the inheritance of emotional responses. Eisenberger et al. (2007) have recently shown that variations in cortisol reactivity are associated with activity both in brain regions involved in neuroendocrine regulation and those underpinning social emotional regulatory processes.
In this study, the subjective response data indicated that the primary experience of the task was excitement rather than negative affect. As can be seen in Fig. 1, excitement ratings were high throughout the session, while ratings of upset were very low. Floor effects were evident for the ratings of upset, with a large proportion of children responding at the lowest point throughout the study. It is interesting that significant heritability effects were observed for baseline and post-task 2 ratings of excitement, but not for post-task 1 (Table 5). One reason may be that excitement responses to the task were limited by the maximum point on the scale, compromising the range of individual differences.
The association between cortisol responses and subjective ratings of excitement was weak, but this is not uncommon in acute challenge studies in adults (Feldman et al., 1999). Several previous studies have shown that the magnitude of physiological reactions is not reliably predicted by subjective responses, since these are loosely coupled, parallel response systems (Steptoe, 2007). Therefore we did not formally test genetic influences on covariation between subjective and cortisol responses. These findings suggest that genetic influences primarily acted at the HPA level. A number of glucocorticoid receptor gene variants have been identified that affect cortisol levels (Wüst et al., 2004; DeRijk et al., 2006), while modifications in the central nervous system expression of genes regulating HPA axis function may also be relevant (Alfonso et al., 2005).
The analyses presented here showed no influence of shared environment either on cortisol or excitement responses, despite the fact that the twins were living in the same home and attending the same school (Tables 3 and 5). Moreover, unlike previous studies, our study should be even more likely to identify shared environment effects because the twins were tested at the same time in a standardised setting in which both of them were engaged in similar activities. This finding implies that cortisol-relevant experiences are child-specific rather than shared by children living in the same family, even though most environmental theories about stress assume that the relevant environmental influences, such as parenting, would be shared by two children living in the same family. However, in view of the sample size, these findings concerning shared environment effects should be viewed cautiously.
This study had a number of limitations. The sample size was relatively small for assessing genetic influences, although comparable with several other studies of cortisol. In particular, our study was not powered to assess sex differences in heritability or sex limitation effects. Data were collected over a single session, and repeated testing might produce different results (Federenko et al., 2004). Repeated testing might have resulted in a reduction in anticipatory responses, making estimates of pretask baseline levels more robust. Since the task was only moderately challenging, average absolute increases were not elicited. Participants were all tested in their own homes, but variation in home environments means that the setting in which assessments took place differed between twin pairs. Nonetheless, these results add to the evidence for genetic influences on salivary cortisol, suggesting that both levels and reactivity of cortisol are moderately heritable.
Role of the funding source
The funders of this research had no involvement with the design, execution or analysis of this study, or with the drafting or approval of this article.
Conflict of interest
None.
Acknowledgements
This research was supported by the Biotechnology and Biological Sciences Research Council (31/D19086), Cancer Research UK, Medical Research Council (G0500079) and the British Heart Foundation (RG/2000002). We would like to thank the parents and children who participated in this study, the researchers who carried out home visits, and Clemens Kirschbaum for assaying the cortisol samples.
References
- Alfonso J., Frasch A.C., Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev. Neurosci. 2005;16:43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- Bartels M., Van den Berg M., Sluyter F., Boomsma D.I., de Geus E.J. Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28:121–137. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Bartels M., de Geus E.J., Kirschbaum C., Sluyter F., Boomsma D.I. Heritability of daytime cortisol levels in children. Behav. Genet. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes. Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- Boardman J.D., Blalock C.L., Button T.M. Sex differences in the heritability of resilience. Twin Res. Human Genet. 2008;11:12–27. doi: 10.1375/twin.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A., von Auer K., Krieger S., Weis S., Rauh W., Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosom. Med. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- DeRijk R.H., Wust S., Meijer O.C., Zennaro M.C., Federenko I.S., Hellhammer D.H., Giacchetti G., Vreugdenhil E., Zitman F.G., de Kloet E.R. A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J. Clin. Endocrinol. Metab. 2006;91:5083–5089. doi: 10.1210/jc.2006-0915. [DOI] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Taylor S.E., Gable S.L., Hilmert C.J., Lieberman M.D. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federenko I.S., Nagamine M., Hellhammer D.H., Wadhwa P.D., Wust S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. J. Clin. Endocrinol. Metab. 2004;89:6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- Feldman P.J., Cohen S., Lepore S.J., Matthews K.A., Kamarck T.W., Marsland A.L. Negative emotions and acute physiological responses to stress. Ann. Behav. Med. 1999;21:216–222. doi: 10.1007/BF02884836. [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Heit S., Graham Y.P., Wilcox M., Bonsall R., Miller A.H., Nemeroff C.B. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J. Am. Med. Assoc. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Herbert J., Goodyer I.M., Grossman A.B., Hastings M.H., de Kloet E.R., Lightman S.L., Lupien S.J., Roozendaal B., Seckl J.R. Do corticosteroids damage the brain? J. Neuroendocrinol. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Jabbi M., Kema I.P., van der Pompe G., te Meerman G.J., Ormel J., den Boer J.A. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr. Genet. 2007;17:183–193. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Wust S., Faig H.G., Hellhammer D.H. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J. Clin. Endocrinol. Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Broderick J.E., Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom. Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht S.R., Mohamed-Ali V., Feldman P.J., Kirschbaum C., Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav. Immun. 2003;17:373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Kupper N., de Geus E.J., van den Berg M., Kirschbaum C., Boomsma D.I., Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lykken D., Tellegen A. Happiness is a stochastic phenomenon. Psychol. Sci. 1996;7:186–189. [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Meaney M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Zhou E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Neale, M.C., Boker, S.M., Xie, G., Maes, H.M., 2002. Mx: Statistical modeling, 6th Edition, Box 980126 VCU, Richmond VA 23298: Dept of Psychiatry.
- Oliver B.R., Plomin R. Twins’ Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Res. Human Genet. 2007;10:96–105. doi: 10.1375/twin.10.1.96. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I., Boivin M., Dionne G., Lupien S.J., Arsenault L., Barr R.G., Perusse D., Tremblay R.E. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch. Gen. Psychiatry. 2008;65:211–218. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- Phillips D.I. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J. Intern. Med. 2007;261:453–460. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Plomin R., DeFries J.C., McClearn G.E., McGuffin P. 5th ed. Worth; New York: 2008. Behavioral Genetics. [Google Scholar]
- Price T.S., Freeman B., Craig I., Petrill S.A., Ebersole L., Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Res. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Schreiber J.E., Shirtcliff E., Van Hulle C., Lemery-Chalfant K., Klein M.H., Kalin N.H., Essex M.J., Goldsmith H.H. Environmental influences on family similarity in afternoon cortisol levels: twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A. Psychophysiological contributions to behavioral medicine and psychosomatics. In: Cacioppo J.T., Tassinary L.G., Bernston G., editors. The Handbook of Psychophysiology. 3rd ed. Cambridge University Press; New York: 2007. pp. 723–751. [Google Scholar]
- Treiber F.A., Kamarck T., Schneiderman N., Sheffield D., Kapuku G., Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom. Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Treiber F.A., McCaffrey F., Musante L., Rhodes T., Davis H., Strong W.B., Levy M. Ethnicity, family history of hypertension and patterns of hemodynamic reactivity in boys. Psychosom. Med. 1993;55:70–77. doi: 10.1097/00006842-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Treiber F.A., Musante L., Kapuku G., Davis C., Litaker M., Davis H. Cardiovascular (CV) responsivity and recovery to acute stress and future CV functioning in youth with family histories of CV disease: a 4-year longitudinal study. Int. J. Psychophysiol. 2001;41:65–74. doi: 10.1016/s0167-8760(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Wardle J., Carnell S., Haworth C.M., Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am. J. Clin. Nutr. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- Wüst S., Federenko I., Hellhammer D.H., Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wüst S., Van Rossum E.F., Federenko I.S., Koper J.W., Kumsta R., Hellhammer D.H. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J. Clin. Endocrinol. Metab. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]