Abstract
When leukocytes adhere to endothelial cells and are exposed to fluid shear stresses, they often retract pseudopods and reduce their attachment. Leukocytes use CD18 for membrane adhesion, but the kinetics of such integrin adhesion molecules under fluid shear is unknown. We examine on neutrophils with confocal microscopy of single adherent cells and flow cytometry of cell suspensions the CD18 expression under fluid shear after labeling with fluorescent antibodies. Fluid shear causes reduction of CD18-associated immunofluorescence of extracellular epitopes, especially in areas of the membrane exposed to elevated levels of shear (1.5 dyne/cm2 maximum shear stress; 1 dyne = 10 mN). CD18 was also translocated over the leukocyte surface from regions of higher shear to lower shear and into the membrane contact areas with the substrate. We obtained no evidence for cytoplasmic internalization of CD18. Fluid shear (5 dyne/cm2) in a suspension of human leukocytes resulted in cleavage of the extracellular domain but not against a cytoplasmic domain of CD18. Chelation of extracellular Ca2+ abolished the down-regulation of CD18. Cysteine protease inhibitors and a selective inhibitor for cathepsin B, but no blockade of other cysteine proteases such as cathepsin L and calpain, aminopeptidases, elastase, or metalloproteinases, suppressed shear-induced CD18 down-regulation. The evidence suggests that physiological levels of fluid shear cause release of cysteine protease(s) including cathepsin B, leading to cleavage of the extracellular domain of CD18 molecules and possible membrane detachment.
Endothelial cells and cells in the circulation such as platelets and leukocytes may have evolved mechanisms by which the activities of adhesion molecules on these cells are influenced by fluid shear stress. For instance, vascular membrane-adhesion molecules such as intercellular adhesion molecule-1 on endothelial cells can be regulated by physiological fluid shear stresses at the transcriptional level (1). P-selectin on platelets can be up-regulated by fluid shear (2), and so can CD29 on leukocytes (3).
During inflammation, up-regulation of CD18 expression and pseudopod formation are key requirements for neutrophil spreading, locomotion, and migration on the vascular wall and into adjacent tissue (4, 5). Fresh leukocytes subjected to fluid shear stresses at physiological levels rapidly retract pseudopods by depolymerization of F-actin and control the biomechanical properties of cytoplasm (6). The process serves to attenuate the development of inflammation (5). The retraction of pseudopods suggests that molecular membrane-adhesion mechanisms also may be influenced directly by fluid shear. Fluid shear serves to reduce neutrophil-to-neutrophil adhesion (7). However, no direct documentation of the fate of membrane-adhesion sites under the influence of fluid shear is available.
We provide here evidence by labeling with several fluorescent Abs against extracellular and intracellular domains of CD18 that fluid shear stress serves to directly regulate the nature of the CD18 expression on neutrophils.
Methods
The experiments were designed to examine CD18 expression on individual neutrophils adhering to a glass surface and subject to fluid flow from a single pipette as well as on neutrophils in a suspension of whole blood subjected to steady shear flow in a cone-and-plate device. Rat and human leukocytes were examined. Because centrifugation may interfere with shear response (3), either 1 × g sedimentation or when possible whole blood without separation of leukocytes was used to observe the shear response.
Reagents. Rabbit polyclonal Ab (pAb) against human CD18 that recognizes the cytoplasmic domain of the CD18 molecule was kindly provided by Mark H. Ginsberg (The Scripps Research Institute, La Jolla, CA) (8). FITC-labeled mouse mAbs against human CD18, MEM48 (Research Diagnostics, Flanders, NJ) and 6.7 (Pharmingen) and FITC-labeled mouse mAb against rat CD18, WT.3 (Pharmingen), recognize the extracellular domain of CD18 (9, 10). Purified human cathepsin B was purchased from Calbiochem. Protease inhibitors amastatin (100 μM), bestatin (130 μM), chymostatin (100 μM), E-64 (28 μM), pepstatin (1 μM), and phosphoramidon (0.6 mM) were purchased from Roche Molecular Biochemicals. Other agents were purchased from Sigma unless indicated otherwise. The concentrations were 10 nM platelet-activating factor (PAF), 10 nM fMet-Leu-Phe, 40 mM EDTA, 5 mM 1,10-phenanthroline, 100 μM elastatinal, 100 μM E-64d, 50 μM CA074 Me (Biomol, Plymouth Meeting, PA), 30 μM calpeptin (Calbiochem), 50 μM leupeptin (Calbiochem), 50 μM GM6001 (Chemicon), 2 mM PMSF, and 50 μM tumor necrosis factor α protease inhibitor-1 (Biomol). The inhibitors, inflammatory mediators, and saline controls were applied in each group 30 min after blood collection.
Animals. The femoral veins and arteries of 40 mature male Wistar rats (280-330 g) were cannulated after general anesthesia (sodium pentobarbital, 50 mg/kg). The animals were placed on a heating pad, covered with a blanket, and maintained at 37°C. All protocols were reviewed and approved by the University of California at San Diego Animal Subjects Committee.
Observation of Single Adherent Neutrophils on a Glass Surface. Fresh leukocytes were collected from volunteers with ammonium heparin (30 units/ml). After 1 × g sedimentation at room temperature for ≈30 min, the supernatant mixture of platelets and leukocytes and sporadic red cells was resuspended in Plasma-Lyte (Baxter Health Care, Mundelein, IL; 1:10 dilution) with 2.5 mM Ca2+. Two hundred microliters of the cell suspension including 10 nM PAF was deposited on an uncoated cover glass, and, after 15 min, nonadherent cells were rinsed gently off the glass with Plasma-Lyte. Adherent cells were incubated with FITC-labeled mAb MEM48 (1/10 dilution) in the presence of PAF at room temperature for 10 min. Afterward, the glass was washed gently with Plasma-Lyte, and the adherent leukocytes were observed with an inverted laser confocal microscope (Bio-Rad MRC 1024 confocal system) with an ×60 objective (Nikon; numerical aperture = 1.4, oil immersion). FITC fluorescent intensity as well as bright-field transmission images of each cell were recorded and digitally analyzed (IMAGE 1.63, National Institutes of Health public domain software). Because the Ab blocks the CD18 adhesion site, most leukocytes become close to spherical within ≈60 min of its application. The experiments therefore were completed 30 min after application of the Ab, and only activated cells with pseudopods were chosen for analysis.
Micropipettes with 4- to 6-μm internal tip diameters were fabricated by using a pipette puller (Sutter Instruments, Novato, CA) filled with Plasma-Lyte and connected to a reservoir with adjustable hydrodynamic pressure. To apply fluid flow over the neutrophil surface, each micropipette was positioned adjacent to the cell. The micropipette angle was adjusted to 30° relative to the glass surface, and the horizontal distance between cell surface and pipette tip was kept at 8 μm (5, 6).
The magnitude of the fluid stress on the surface of the neutrophils was computed numerically from the Stokes approximation of the equation of motion for a Newtonian fluid based on cell size, pipette tip diameter, pipette angle, the distance between pipette tip and cell surface, and maximum velocity out of the pipette (6). This computation has shown that flow from the micropipette tip leads to a nonuniform distribution of fluid shear stress over the surface of the neutrophil, with higher levels of shear stress on the side of the cell closer to the pipette tip and lower levels downstream and away from the pipette. All neutrophils were exposed to similar shear stresses of the order of ≈1.5 dyne/cm2 (1 dyne = 10 mN) (at the upstream position of the cell) for 2 min.
CD18 Expression on Leukocytes in Sheared Whole-Blood Suspension. Because observation of CD18 by immunofluorescence with confocal microscopy is limited to individual cells, we examined average cell population levels also with flow cytometry in which larger number of cells are examined. For this purpose, the cells remained suspended in whole blood and were subject to stochastic interactions with other blood cells in the shear field of a cone-and-plate device (3, 5).
Arterial or venous blood (0.6 ml) in ammonium heparin (30 units/ml) was collected from the rats or human volunteers, respectively. Fresh whole blood was divided into two aliquots: one aliquot (0.3 ml) was subjected in the cone-and-plate device to fluid shear stress (5.0 dyne/cm2 for 10 min) 50 min after blood collection; the other aliquot remained unsheared (3, 5). Immediately after shear stress application, the blood was fixed with 0.4% paraformaldehyde. Unsheared control samples (0.3 ml) were also fixed at the same time and in the same fashion. All procedures were completed 60 min after blood collection.
Flow cytometry. After fixation, 5.25 μl/ml FITC-labeled mAbs (WT.3 for rat blood and MEM48 or 6.7 for human blood) or FITC-labeled IgG isotypes as control were added to each blood sample for 30 min at room temperature. Erythrocytes in the blood were removed by fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson) followed by FACS buffer washes with centrifugation (400 × g for 5 min). The cells were resuspended in FACS buffer, and CD18 expression on the cells was determined with a flow cytometer (FACS analyzer, Becton Dickinson).
Confocal microscopy. Confocal microscopy shows that incubation with FITC-labeled Abs against the extracellular domain of CD18 leads to labeling only on the cell surface (data not shown) such that flow cytometry yields a measure of the CD18 expression on the surface of the cell only. This same method, however, cannot be used with a pAb against the cytoplasmic domain of CD18, because Ab labeling inside the cell is insufficient for detection. Therefore, we incubated the cells with the pAb after permeabilization with FACS lysing solution. The FITC intensity on the cytoplasmic domain of the plasma membrane was recorded with confocal microscopy. Flow-cytometric analysis (which detects all fluorescence) is not appropriate for measurement of CD18 molecules on the cytoplasmic side of the membrane only, because after permeabilization the pAb becomes located not only on the cytoplasmic side of the membrane but also in granules (data not shown). The specificity of the pAb was confirmed by using Chinese hamster ovary cells, which do not express CD18 molecules.
After fixation, erythrocytes in the blood were removed by FACS lysing solution (Becton Dickinson) followed by FACS buffer washes with centrifugation. Added to each group (in 100 μl of FACS buffer) for 30 min at room temperature was the pAb (1 μl/ml) that recognizes cytoplasmic domain of CD18. After washing, the cells in 100 μl of FACS buffer was incubated with 10 μl/ml FITC-labeled anti-rabbit IgG for 30 min followed by washing. The cells then were resuspended in 200 μl of FACS buffer, and FITC fluorescent intensity as well as bright-field transmission images of each cell were recorded with a laser confocal microscope, and the intensity of plasma membrane of neutrophils was analyzed digitally by using NIH IMAGE. The cells incubated with FITC-labeled anti-rabbit IgG without pAb incubation were used as controls.
Human cathepsin B application. Human blood collected from volunteers with ammonium heparin (5 ml) was centrifuged at 400 × g for 5 min. Leukocyte buffer coat was incubated in 100 μlof200 mM sodium acetate containing 8 mM DTT, 2.5 mM CaCl2, and 2.7 mM l-cysteine (pH 6.5) with and without 0.3 units/ml purified human cathepsin B for 30 min followed by fixation with 0.4% paraformaldehyde. After immunolabeling with mAb MEM48 as described above, CD18 expression on neutrophils was determined with a flow cytometer and expressed in terms of fluorescent units.
Data Analysis. Measurements are expressed as mean ± standard deviation. Differences between groups were analyzed by ANOVA and Fisher's protected least-significant difference test. A probability of P < 0.05 was considered significant.
Results
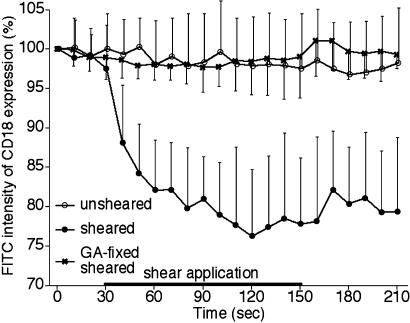
CD18 Expression on Activated Adherent Leukocytes. Overall CD18 expression on single neutrophils recorded by confocal microscopy. Without fluid shear, the total integrated florescent intensity along the perimeter of individual adherent neutrophils, labeled with the FITC-labeled CD18 mAb, MEM48 (Fig. 1), was almost constant over an observation period of ≈15 min. On sheared neutrophils the FITC intensity due to labeled CD18 was reduced significantly as early as 30 sec after fluid shear application (1.5 dyne/cm2 at the proximal side of the cell) (Fig. 1). The CD18 down-regulation was not due to a shear-induced loss of the fluorescence of the tagged mAb, because fixation of the cells with 0.5% glutaraldehyde before immunolabeling and shear application led to a constant CD18 expression regardless of the time of exposure to shear (Fig. 1).
Fig. 1.
Time course of total membrane CD18 expression on human neutrophils adhering to a glass surface as measured by confocal microscopy. The intensity values are normalized with their values at 0 sec in each of the following groups: unsheared (open circles), sheared cells (filled circles), and sheared cells fixed with glutaraldehyde (GA) before shearing (sheared glutaraldehyde-fixed cells,  ). In the sheared-cell group, fluid shear (≈1.5 dyne/cm2 at the upstream position of the cell) was applied from 60 to 180 sec. n = 8 cells in each group. Values in the sheared group are significantly lower than that in the unsheared group from 40 to 210 sec (P < 0.0001), whereas there are no significant differences in values between the unsheared group and the sheared glutaraldehyde-fixed group at any time point.
). In the sheared-cell group, fluid shear (≈1.5 dyne/cm2 at the upstream position of the cell) was applied from 60 to 180 sec. n = 8 cells in each group. Values in the sheared group are significantly lower than that in the unsheared group from 40 to 210 sec (P < 0.0001), whereas there are no significant differences in values between the unsheared group and the sheared glutaraldehyde-fixed group at any time point.
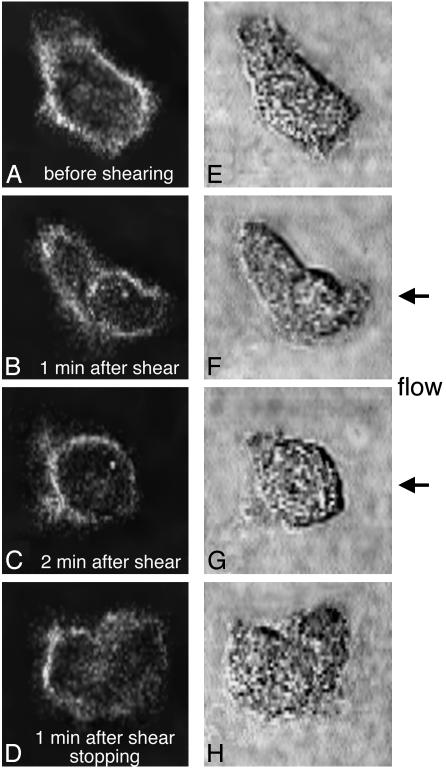
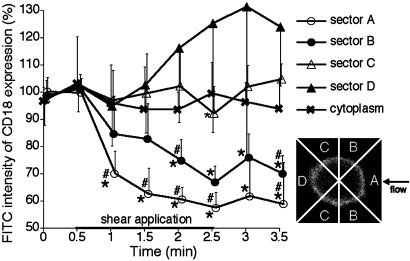
CD18 distribution during fluid shear. When the CD18 distribution on the membrane was observed with confocal microscopy on neutrophils migrating on a glass surface and fluorescently labeled with the same mAb, MEM48 (Fig. 2), a relatively uniform distribution of CD18 was observed in the absence of fluid shear (Fig. 2 A). In contrast, during fluid flow CD18 was reduced in regions with higher shear stress (close to the pipette) compared with regions of lower shear stress (away from the pipette). FITC-labeled CD18 was redistributed over the neutrophil surface from regions of higher fluid shear stresses toward lower stresses at the same time at which pseudopods retracted (Fig. 2). This was demonstrated directly by fluorescence measurements in four sectors (A-D, Fig. 3). The CD18 was shifted from the higher shear stress region close to the pipette (sector A) toward the downstream region further away from the pipette (sector D). In sectors A and B, a reduction in the CD18-associated FITC intensity was detected within 30 sec after shear application. However, although FITC intensity in sector A close to the pipette was decreased continuously during shear application, an upward shift of FITC intensity in sector B on the cell was detected ≈60 sec after shear application. Moreover, CD18-associated FITC intensity in sector D away from the pipette was increased continuously 60 sec after shear application. Even 60 sec after cessation of shear application, FITC intensity in sector D was still significantly higher than that in sectors A and B, although the cells had started pseudopod projection (Figs. 2 D and H, and 3). In the cytoplasm no significant shift of the FITC intensity was encountered during fluid shear (Fig. 3). The CD18 distribution by fluid shear was reversible, and a uniform distribution of CD18 was recovered at least 10 min after termination of the shear application (data not shown).
Fig. 2.
Confocal images of CD18-associated FITC fluorescence (A-D) and bright-field pseudopod formation (E-H) of a human neutrophil on a glass surface before (A and E) and 60 sec after (B and F) and 120 sec after (C and G) shear stress application (1.5 dyne/cm2 at the upstream position of the cell) and 60 sec after termination of the shear stress application (D and H). The fluid flow was produced from a pipette positioned on the right side of the cell ≈8 μm just outside the field of view shown.
Fig. 3.
Time course of the fraction of CD18 expression on confocal image of human neutrophils on a glass surface in response to fluid shear. The average value of intensities over the membrane of the neutrophils during the unsheared period (0 and 0.5 min) serve to normalize the values in each group. The average FITC intensity on the surface membrane [divided into sectors A (open circles), close to the micropipette, B (filled circles), C (open triangles), and D (filled triangles) on the cell perimeter (as shown in the Inset)] and in the cytoplasm ( ) was measured. n = 8 cells in each group. #, P < 0.05 vs. sector C; *, P < 0.05 vs. sector D.
) was measured. n = 8 cells in each group. #, P < 0.05 vs. sector C; *, P < 0.05 vs. sector D.
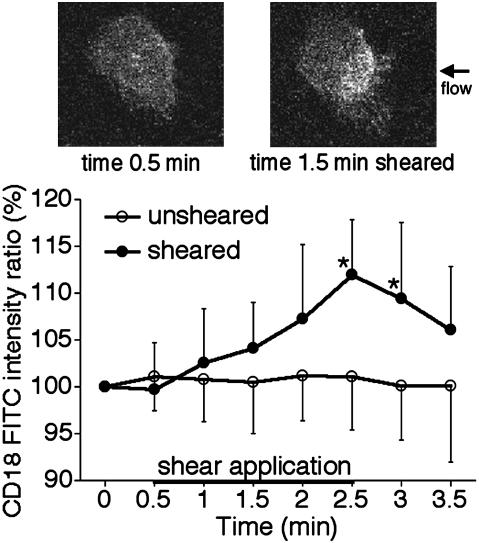
CD18 expression in the contact area with glass surface. In unsheared neutrophils, the average FITC intensity associated with CD18 in the glass contact area was relatively constant. In contrast, a significant rise of CD18 labeling in the glass contact area was observed during fluid shear (Fig. 4). The increase in FITC intensity associated with CD18 was observed first in regions close to the pipette (Fig. 4), and the high-intensity area gradually spread in regions away from the pipette. In some cells the fluorescence in the contact area became homogeneously high 2 min after shear application. After termination of shear application, the tendency of a decrease in FITC intensity associated with CD18 was observed (Fig. 4), suggesting that CD18 was redistributed as the shear stopped.
Fig. 4.
Time course of CD18 expression in the contact area with a glass surface on confocal images of human neutrophils: unsheared cells (open circles); sheared cells (filled circles). In the sheared group, a fluid shear (1.5 dyne/cm2 at the upstream position of the cell) was applied from 0.5 to 2.5 min. n = 8 cells in each group. *, P = 0.0011 (at 2.5 min) and 0.0187 (at 3.0 min) vs. the unsheared group. The micrographs show confocal images of CD18-associated FITC fluorescence on the contact area at 0.5 min (Upper Left) and 1.5 min (Upper Right). The entire area shown is in contact with the glass.
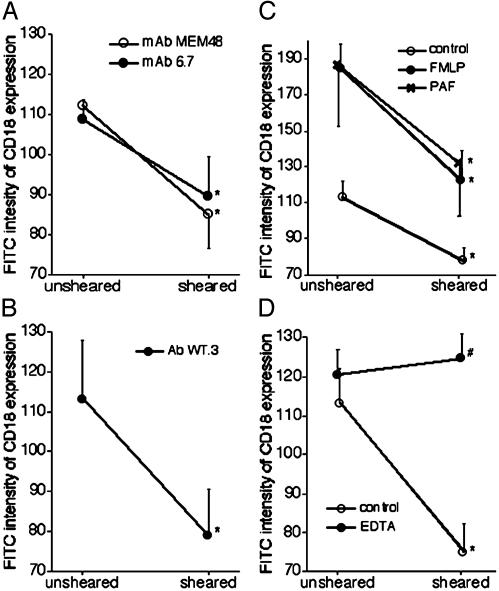
CD18 Down-Regulation on Neutrophils in Whole-Blood Suspension Measured by Flow Cytometry. The average fluorescent intensity on rat and human neutrophils after shear in the cone-and-plate device was significantly lower than on unsheared cells. This trend was observed after immunolabeling with FITC-labeled mAbs against different extracellular domains of CD18, WT.3, MEM48, and 6.7 (from 113.2 ± 14.7 to 78.9 ± 11.9, 103.2 ± 9.7 to 74.2 ± 9.1, and 103.1 ± 3.7 to 97.0 ± 12.7 fluorescent intensity units, respectively; Fig. 5 A and B). The response is consistent with the observation on the adherent neutrophils described above. Down-regulation of CD18 in response to shear stress was also observed in the presence of 10 nM PAF and fMet-Leu-Phe (Fig. 5C). In contrast, the calcium chelator EDTA completely inhibited CD18 down-regulation by fluid shear (Fig. 5D).
Fig. 5.
Effect of fluid shear application (5 dyne/cm2) on CD18 expression of circulating human (A) and rat (B-D) neutrophils measured with flow cytometry. (A) Human neutrophils labeled by mAbs MEM48 (open circles) and 6.7 (filled circles). (B) Rat neutrophils labeled by mAb WT.3. (C) Control (open circles), 10 nM fMet-Leu-Phe (FMLP, filled circles), or 10 nM PAF ( ). (D) Control (open circles) and EDTA (filled circles). n = 6 in each group. *, P < 0.05 vs. unsheared cells in each group; #, P < 0.001 vs. control sheared cells.
). (D) Control (open circles) and EDTA (filled circles). n = 6 in each group. *, P < 0.05 vs. unsheared cells in each group; #, P < 0.001 vs. control sheared cells.
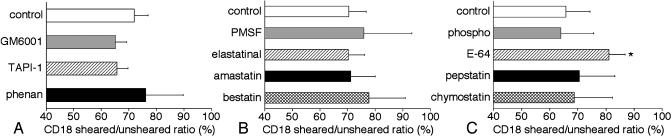
Proteases and the CD18 Down-Regulation by Fluid Shear. Effect of protease inhibitors on rat neutrophils. The inhibitors for metalloproteinases (MMPs) including a disintegrin and MMPs (ADAMs), tumor necrosis factor α protease inhibitor-1 and GM6001, and the zinc chelator 1,10-phenanthroline did not significantly inhibit CD18 down-regulation on rat neutrophils by fluid shear (Fig. 6A). The serine protease inhibitors, PMSF, and the aminopeptidase inhibitors amastatin and bestatin also did not influence the CD18 expression in the presence of fluid shear (Fig. 6B). A similar lack of influence on the shear-induced CD18 down-regulation was observed with the aspartate protease inhibitor pepstatin, the chymotrypsin inhibitor chymostatin, and the metalloendopeptidase inhibitor phosphoramidon. In contrast, the cysteine protease inhibitor E-64 significantly suppressed a decrease in CD18 on rat neutrophils in response to fluid shear (Fig. 6C).
Fig. 6.
CD18 labeling ratio (sheared/unsheared) of circulating rat neutrophils measured with flow cytometry in response to fluid shear (5 dyne/cm2) with the following protease inhibitors: control, GM6001, tumor necrosis factor α protease inhibitor-1 (TAPI-1), and 1,10-phenanthroline (phenan) (A); control, PMSF, elastatinal, amastatin, and bestatin (B); and control, phosphoramidon (phospho), E-64, pepstatin, and chymostatin (C). n = 4 animals in each group. *, P = 0.023 vs. control.
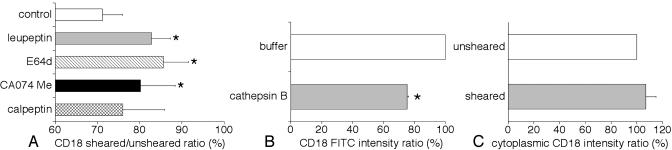
Effect of cysteine protease inhibitors on human neutrophils. The cysteine protease inhibitors E-64d and leupeptin significantly reduced the ability of fluid shear to down-regulate CD18 on human neutrophils (Fig. 7A). The selective inhibitor for cathepsin B, CA074 Me, but not the selective inhibitor for calpain and cathepsin L, calpeptin, also blocked shear-induced CD18 down-regulation (Fig. 7A).
Fig. 7.
CD18 label ratio (sheared/unsheared) on human neutrophils after shear in the cone-and-plate device. Values of FITC intensity in control unsheared cells are normalized to 100% in each group. (A) CD18 labeling ratio of circulating human neutrophils in response to fluid shear with the cysteine protease inhibitors leupeptin, E-64d, the selective inhibitor for cathepsin B, and CA074 Me and the selective inhibitor for calpain and cathepsin L, calpeptin. n = 4 blood samples in each group. *, P < 0.02 vs. control. (B) CD18 expression ratio on human neutrophils with purified human cathepsin B. n = 4 blood samples in each group. *, P < 0.001 vs. control. (C) Expression ratio of CD18 cytoplasmic domain label on circulating human neutrophils in response to fluid shear. n = 6 blood samples in each group.
Cathepsin B and CD18 membrane expression. The application of human cathepsin B (0.3 units/ml) caused a significant reduction of CD18 on human neutrophils compared with control (Fig. 7B).
The cytoplasmic domain of CD18 on human neutrophils during fluid shear. We detected no significant difference in CD18-associated FITC intensity on the plasma membrane between sheared and unsheared neutrophils immunolabeled with the pAb against cytoplasmic domain of human CD18 (Fig. 7C). These results suggest that CD18 is cleaved in the extracellular domain by cathepsin B during fluid shear.
Discussion
Regulation of CD18 Expression on Leukocytes by Fluid Shear. Although a number of mechanisms have been described that lead to up-regulation of CD18 on the cytoplasmic membrane (e.g., via stimulation by inflammatory mediators), few mechanisms are known that serve to down-regulate integrin adhesion molecules except for the action of glucocorticoids (11). The current evidence shows that fluid shear stress leads to down-regulation of the extracellular domain of membrane CD18 expression on neutrophils even in the presence of inflammatory mediators such as PAF and fMet-Leu-Phe (Figs. 1 and 5 A-C). Confocal microcopy suggests further that, in addition to down-regulation, CD18 can be redistributed by fluid shear application over the surface of the activated cell toward regions on the cell surface with lower shear stress including the contact area with a substrate (Figs. 2, 3, 4). The effect can be achieved at a level of 1.5 dyne/cm2, well within the physiological range in many parts of the circulation.
Furthermore, the redistribution of CD18 due to fluid shear suggests that the shear response to CD18 expression on leukocytes is a local membrane phenomenon. This is in line with observations of actin depolymerization, suggesting a local control of fluid stress. By use of relatively small pipettes (inner tip diameter less than ≈0.5 μm), which serves to produce a localized fluid shear stress on individual leukocytes, the shear stress-mediated pseudopod retraction is kept spatially limited to the position close to the pipette tip (12).
Mechanisms of CD18 Down-Regulation in Response to Fluid Shear. CD18 molecules (CD11b/CD18 and CD11c/CD18) are stored in intracellular pools, and on activation neutrophils increase the surface expression of CD18 molecules by fusion of the granules with the plasma membrane within minutes by several folds (13). The process is mediated by tyrosine phosphorylation (14). Internalization of CD18 molecules into the cell cytoplasm, however, is less likely involved in the down-regulation observed at the plasma membrane, because the FITC intensity in the cytoplasm of leukocytes was not increasing during shear application (Fig. 3). Instead, CD18 on the cell surface appears to be cleaved during fluid shear.
Several proteases are involved in the shedding of membrane-adhesion molecules. For example, MMPs are associated with the shedding of L-selectin on leukocytes (15). A subfamily of MMPs, the ADAMs family (16), is associated with membrane protein shedding. Serine proteases are required for proteolytic shedding of CD43 (17). Elastase plays a role in modulating β2 integrin-mediated cell adhesion as an endogenous ligand for release of adhesion during cell detachment (18). Therefore, we hypothesized that some of these proteases may be associated with cleavage of extracellular CD18 in response to fluid shear. However, protease inhibitors for MMPs, ADAMs, serine proteases, elastase, and aminopeptidases had no significant effect on shear-induced CD18 down-regulation on leukocytes (Fig. 6). In contrast, the cysteine protease inhibitor E-64 significantly suppresses CD18 down-regulation by fluid shear (Fig. 6C).
Cysteine proteases are involved in the cleavage of membrane proteins on leukocytes. Bacterial cysteine proteases, gingipains, degrade monocyte CD14 (19). Calpain can cleave integrin β cytoplasmic domains (20). Neutrophils are known to have at least four different cysteine proteases in the cytoplasm: calpain and cathepsins B, L, and H (21, 22). The current evidence indicates that the selective inhibitor for cathepsin B, CA074 Me, but not the selective inhibitor for calpain and cathepsin L, calpeptin, has the ability to reduce significantly shear-induced CD18 down-regulation (Fig. 7A), and that purified human cathepsin B could degrade CD18 on human neutrophils (Fig. 7B). Thus, fluid shear serves to cleave CD18 molecules on neutrophils by release of cysteine protease(s) including cathepsin B.
The mAbs against human CD18 used in the present study, MEM48 and 6.7, both recognize extracellular cysteine-rich domain (9). The reduction of FITC intensity was observed with either one of these two mAbs but not with an Ab against the cytoplasmic domain of CD18 (Figs. 5B and 7C). This evidence suggests that only the extracellular domains of the CD18 molecule on the plasma membrane of neutrophils may be cleaved during fluid shear.
In light of the evidence that EDTA completely inhibited the CD18 down-regulation in response to fluid shear, extracellular Ca2+ seems to be a requirement for the shear stress response in leukocytes (Fig. 5D). Because Ca2+ is not required for activation of cathepsin B, it may be a requirement for other processes during shear-induced CD18 down-regulation such as fluid shear sensing and/or release of protease(s) granules. Leukocytes have many granules containing various kinds of enzymes such as cathepsin B. The granules can move within the cell, fuse with the plasma membrane, and release their contents (degranulation). Although the exact intracellular localization of cathepsin B in leukocytes remains to be investigated, it is reported that cathepsin B colocalizes with granzyme A (23). We previously observed that extracellular Ca2+ is also needed for enhancement of cytoplasmic vesicular movement in response to shear stress (6). The vesicular degranulation induced by fluid shear may be associated with CD18 down-regulation, because cathepsin B may be released by the granule exocytosis. Therefore, we examined cathepsin B localization with immunohistochemistry before and after shear application, but the difference was too small for detection with current techniques (data not shown). Relatively few granules may be required to achieve the extracellular cleavage.
Kinetics of CD18 Down-Regulation in Response to Fluid Shear in Vivo. The moment when leukocytes enter the circulation they are exposed to fluid shear. Fluid shear serves to keep activated leukocytes in a passive state by reduction of pseudopod formation (5) and CD18 down-regulation on the surface. However, on activated adherent cells, both cleavage and redistribution of CD18 may occur, and the balance between them may depend on the extent of cell activation. In the presence of inflammatory mediators, both pseudopod formation and CD18 expression are enhanced, a process that facilitates neutrophil attachment, locomotion on the endothelium, and migration into the tissue (4, 12, 24-26). In mild inflammation, fluid shear still keeps the cells in a spherical state, leading to little contact area with the substrate or low shear sectors (5), and CD18 can be down-regulated by fluid shear (Fig. 5C). However, in more activated states, inflammatory mediators attenuate pseudopod retraction by shear, and the cells no longer keep a spherical shape (5) and instead form a contact region with the endothelium. The fluid shear stress in this region is much lower than on the membrane exposed to the blood stream (27). According to the current results, this may lead toward redistribution of CD18 molecules from the noncontact region with higher shear stresses toward the membrane contact region with lower and even zero shear stresses (Fig. 4). In the contact region, less cleavage and more redistribution of CD18 may result in an increase in CD18. This process facilitates CD18-mediated pseudopod projection in the contact region and cell migration into the tissue, because increased surface expression of CD18 is required for enhanced adherence-dependent cell locomotion (4, 25, 26). Elevation of the CD18 expression in the membrane enhances migration especially once the cells are underneath the endothelium and exposed to lower fluid shear stresses than on the luminal side of the endothelium. Thus, although cleavage of CD18 by fluid shear may have an antiinflammatory effect, CD18 redistribution caused by shear may, in contrast, enhance this aspect of the inflammatory reaction.
Acknowledgments
We thank Dr. Mark H. Ginsberg (The Scripps Research Institute, La Jolla, CA) for the Ab against the cytoplasmic domain of CD18, Chinese hamster ovary cells, and critical comments about this manuscript; Drs. Shunichi Usami and Jeff Price (Department of Bioengineering, University of California at San Diego) for valuable advice with optimal confocal microscopy; and Dr. John Frangos for the use of the cone-and-plate device. This work was supported by U.S. Public Health Service/National Institutes of Health Grant HL-43026.
Abbreviations: pAb, polyclonal Ab; PAF, platelet-activating factor; FACS, fluorescence-activated cell sorter; MMP, metalloproteinase; ADAMs, a disintegrin and MMPs.
References
- 1.Davis, P. (1995) Physiol. Rev. 75, 519-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstantinos, K., Neelamegham, S., Burns, A. R., Hentzen, E., Kansas, G. S., Snapp, K. R., Berg, E. L., Hellums, J. D., Smith, C. W., McIntire, L. V. & Simon, S. I. (1998) Circulation 98, 873-882. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda, S. & Schmid-Schönbein, G. W. (2002) J. Leukocyte Biol. 72, 133-139. [PubMed] [Google Scholar]
- 4.Hughes, B. J., Hollers, J. C., Crockett-Torabi, E. & Smith, C. W. (1992) J. Clin. Invest. 90, 1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda, S., Yasu, T., Predescu, D. N. & Schmid-Schönbein, G. W. (2000) Circ. Res. 86, E13-E18. [DOI] [PubMed] [Google Scholar]
- 6.Moazzam, F., Delano, F. A., Zweifach, B. W. & Schmid-Schönbein, G. W. (1997) Proc. Natl. Acad. Sci. USA 94, 5338-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon, S. I. & Goldsmith, H. L. (2002) Ann. Biomed. Eng. 30, 315-332. [DOI] [PubMed] [Google Scholar]
- 8.O'Toole, T. E., Katagiri, Y., Faull, R. J., Peter, K., Tamura, R., Quaranta, V., Loftus, J. C., Shattil, S. J. & Ginsberg, M. H. (1994) J. Cell. Biol. 124, 1047-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu, C., Ferzly, M., Takagi, J. & Springer, T. A. (2001) J. Immunol. 166, 5629-5637. [DOI] [PubMed] [Google Scholar]
- 10.Tamatani, T., Kotani, M. & Miyasaka, M. (1991) Eur. J. Immunol. 21, 627-633. [DOI] [PubMed] [Google Scholar]
- 11.Cronstein, B. N., Kimmel, S. C., Levin, R. I., Martiniuk, F. & Weissmann, G. (1992) Proc. Natl. Acad. Sci. USA 98, 9991-9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda, S. & Schmid-Schönbein, G. W. (2003) in Molecular Basis for Microcirculatory Disorders, eds. Schmid-Schönbein, G. W. & Granger, N. D. (Springer, Paris), pp. 161-170.
- 13.Arnaout, A. M. (1990) Blood 75, 1037-1050. [PubMed] [Google Scholar]
- 14.Naccache, P. H., Jean, N., Liao, N. W., Bator, J. M., McColl, S. R. & Kubes, P. (1994) Blood 84, 616-624. [PubMed] [Google Scholar]
- 15.Walcheck, B., Kahn, J., Fischer, J. M., Wang, B. B., Fisk, R. S., Payan, D. G., Feehan, C., Betageri, R., Darlak, K., Spatola, A. F. & Kishimoto, T. K. (1996) Nature 380, 720-723. [DOI] [PubMed] [Google Scholar]
- 16.Loechel, F., Fox, J. W., Murphy, G., Albrechtsen, R. & Wewer, U. M. (2000) Biochem. Biophys. Res. Comm. 278, 511-515. [DOI] [PubMed] [Google Scholar]
- 17.Carney, D. F., Jagels, M. A., Hugli, T. E., Sands, H. & Rubin, H. (1998) J. Leukocyte Biol. 63, 75-82. [DOI] [PubMed] [Google Scholar]
- 18.Cai, T.-W. & Wright, S. D. (1996) J. Exp. Med. 184, 1213-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugawara, S., Nemoto, E., Tada, H., Miyake, K., Imamura, T. & Takada, H. (2000) J. Immunol. 165, 411-418. [DOI] [PubMed] [Google Scholar]
- 20.Pfaff, M., Du, X. & Ginsberg, M. H. (1999) FEBS Lett. 460, 17-22. [DOI] [PubMed] [Google Scholar]
- 21.Bando, Y., Kominami, E. & Katunuma, N. (1986) J. Biochem. 100, 35-42. [DOI] [PubMed] [Google Scholar]
- 22.Pontremoli, S. & Melloni, E. (1989) Revis. Biol. Celular. 20, 161-177. [PubMed] [Google Scholar]
- 23.Griffiths, G. M. & Isaaz, S. (1993) J. Cell Biol. 120, 885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderwood, D. A., Shattil, S. J. & Ginsberg, M. H. (2000) J. Biol. Chem. 275, 22607-22610. [DOI] [PubMed] [Google Scholar]
- 25.Vedder, N. B. & Harlan, J. M. (1998) J. Clin. Invest. 81, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleifenbaum, B., Moser, R., Patarroyo, M. & Fehr, J. (1989) J. Immunol. 142, 3537-3545. [PubMed] [Google Scholar]
- 27.Sugihara-Seki, M. & Schmid-Schönbein, G. W. (2003) J. Biomechanic. Eng. 125, 628-638. [DOI] [PubMed] [Google Scholar]