Abstract
Plants dissipate excess excitation energy as heat by non-photochemical quenching (NPQ). NPQ has been thought to resemble in vitro aggregation quenching of the major antenna complex, light harvesting complex of photosystem II (LHC-II). Both processes are widely believed to involve a conformational change that creates a quenching centre of two neighbouring pigments within the complex. Using recombinant LHC-II lacking the pigments implicated in quenching, we show that they have no particular role. Single crystals of LHC-II emit strong, orientation-dependent fluorescence with an emission maximum at 680 nm. The average lifetime of the main 680 nm crystal emission at 100 K is 1.31 ns, but only 0.39 ns for LHC-II aggregates under identical conditions. The strong emission and comparatively long fluorescence lifetimes of single LHC-II crystals indicate that the complex is unquenched, and that therefore the crystal structure shows the active, energy-transmitting state of LHC-II. We conclude that quenching of excitation energy in the light-harvesting antenna is due to the molecular interaction with external pigments in vitro or other pigment–protein complexes such as PsbS in vivo, and does not require a conformational change within the complex.
Keywords: chlorophyll, fluorescence, light harvesting, non-photochemical quenching, photosynthesis
Introduction
Under normal light conditions, plants convert the absorbed solar energy into biologically useful chemical energy by photosynthesis. However, under high-light conditions, the excess excitation energy is dissipated in the form of heat by non-photochemical quenching (NPQ), without the risk of causing damage to the photosynthetic apparatus. The major light harvesting complex of photosystem II (LHC-II) participates in energy-dependent quenching (qE), the most significant component of NPQ (Ruban and Horton, 1994). The main characteristics of qE are a drop in chlorophyll (Chl) fluorescence yield and average fluorescence lifetime at ambient temperature, and an increase in 700 nm fluorescence at liquid nitrogen temperature (77 K), as observed in isolated thylakoids (Ruban et al, 1991; Gilmore et al, 1995) or plant leaves (Ruban and Horton, 1994).
The spectroscopic signature of unquenched LHC-II in detergent solution is strong fluorescence at 680 nm with long corresponding lifetimes (Ide et al, 1987; Vasil'ev et al, 1997; Moya et al, 2001; Palacios et al, 2002). When LHC-II forms aggregates, either by cation-induced precipitation (Burke et al, 1978) or detergent removal, both the fluorescence yield and lifetime drop by an order of magnitude, whereas the 700 nm fluorescence at 77 K increases (Mullineaux et al, 1993; Ruban et al, 1995; Vasil'ev et al, 1997), similar to qE. For these reasons, in vitro aggregation quenching has long been used as a model for qE in vivo, although their molecular mechanisms are most likely to be different. Quenching in LHC-II or the minor LHCs is widely assumed to be caused by a conformational change that switches the complex from the active, energy-transmitting state to a quenched, dissipative state (Moya et al, 2001; Pascal et al, 2005; Ruban et al, 2007; Yan et al, 2007; Ahn et al, 2008). As yet there is no conclusive evidence for such a conformational change.
Using mutants of LHC-II that lack the Chl pairs or carotenoids proposed to be involved in quenching (Pascal et al, 2005), we show that these pigments have no special role in energy dissipation. The reported change in neoxanthin (Neo) conformation associated with qE (Pascal et al, 2005) does not require an internal rearrangement of the rigid LHC-II structure. Calculations indicate that all LHC-II trimers in the crystals are excitonically coupled. The crystals used for determining the structure of the pea complex (Standfuss et al, 2005) exhibit strong low-temperature fluorescence with an emission maximum at 680 nm and lifetimes that are very much longer than those for quenched aggregates. Therefore, the structure of the pea complex in our crystals is that of the unquenched, energy-transmitting LHC-II. As all available structures of LHC-II are the same, they all show the unquenched state. qE is probably the result of a close interaction of LHC-II with another pigment protein complex, most likely PsbS, that does not involve a conformational change within the antenna complex. In LHC-II aggregates, this process is mimicked by close interactions of pigments on the outer perimeter of the LHCs.
Results and discussion
With one exception, LHC-II pigments are rigid
The X-ray structures of spinach and pea LHC-II were determined independently from crystals grown either at pH 5.4 (Standfuss et al, 2005) or 7.5 (Liu et al, 2004). The question arises whether these structures show the same or different states, and whether they correspond to a quenched or unquenched conformation of LHC-II. If the two structures were to represent different states, significant differences in the arrangement of the LHC-II pigments would be expected. The r.m.s.d. between the Cα coordinates of spinach and pea LHC-II polypeptide is 0.35 Å. If the coordinates of the chlorin rings are compared, the r.m.s.d. is only 0.25 Å (Figure 1). The structure of pea LHC-II in two-dimensional membrane crystals (Kühlbrandt et al, 1994), although at lower resolution, does not show any significant differences to the X-ray structure of the pea complex (Standfuss et al, 2005). The available structures of LHC-II from two different plant species, and especially the position and orientation of the Chl molecules attached to the LHC-II monomer, are thus identical within experimental error and therefore show the same state of the complex.
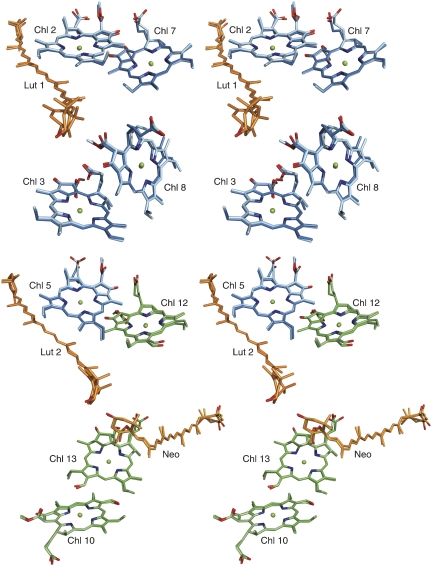
Figure 1.
Stereo diagrams of four Chl pairs and closest carotenoids in spinach (lighter colours; PDB code 1RWT) and pea (darker colours; PDB code 2BHW) LHC-II. Phytyl chains have been omitted for clarity. Cyan, Chl a; green, Chl b; orange, lutein and neoxanthin.
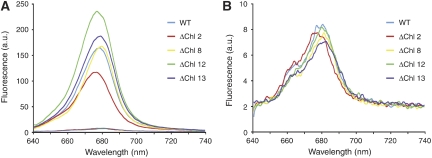
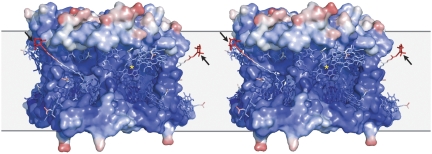
Resonance Raman spectroscopy suggested that the LHC-II-bound carotenoid Neo is twisted in 3D crystals, aggregates and leaves under qE conditions, compared with its conformation in the detergent-solubilized complex (Pascal et al, 2005; Ruban et al, 2007). The Neo twist has been suggested to have a pivotal function in switching the complex from the unquenched to the quenched state (Pascal et al, 2005). We prepared LHC-II mutants (Rogl and Kühlbrandt, 1999) (Figure 1; Supplementary Table SI) lacking pigments that have been implemented in the quenching mechanism (Pascal et al, 2005) and tested their fluorescence properties upon aggregation. In each case, the 680 nm fluorescence yield at room temperature dropped by at least an order of magnitude (Figure 2), as in native LHC-II, demonstrating that these pigments are not required for aggregation quenching. The ΔChl 13 mutant was largely deficient in Neo and violaxanthin (Vio) (Supplementary Table SII), demonstrating that neither carotenoid has a specific role in this process. Examining the crystallographic temperature (B) factor, which reflects the relative flexibility of the structure, we found that the hydrophobic core of LHC-II containing the Chls, luteins (Lut) and Vio is the most rigid part of the complex (Figure 3). The only part with a significant degree of flexibility is the portion of Neo that protrudes into the surrounding lipid or detergent. The Neo conformation is therefore most likely to be sensitive to contacts between LHC-II trimers in aggregates, crystals or in the thylakoid membrane. This would account for any twist observed by resonance Raman spectroscopy, which thus seems to be a diagnostic of close intermolecular contacts, rather than a cause of quenching in LHC-II.
Figure 2.
Room temperature fluorescence emission spectra of refolded LHC-II mutants each lacking a specific Chl-binding site (see Supplementary Table SI) and refolded wild-type complex. (A) Refolded complexes in detergent solution (solid lines) and in the aggregated state on the same scale (dashed lines, at the bottom). The spectra of individual Chl variants have not been normalized. (B) Same spectra of refolded complexes in the aggregated state as in (A) on an enlarged scale.
Figure 3.
Stereo diagram of the temperature factor distribution in the structure of spinach LHC-II (PDB code 1RWT). The colour gradient ranges from blue (lower temperature factors, rigid) to red (higher temperature factors, flexible). The only part of the structure with a high temperature factor, indicating significant flexibility, is the protruding part of Neo (arrows). The asterisk indicates Chl 2. Detergent, lipids and phytyl chains have been omitted for clarity. The approximate position of the lipid bilayer is indicated in grey.
Fluorescence emission of single crystals
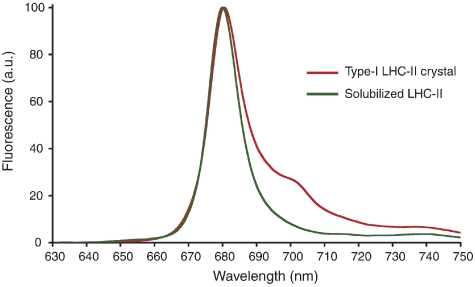
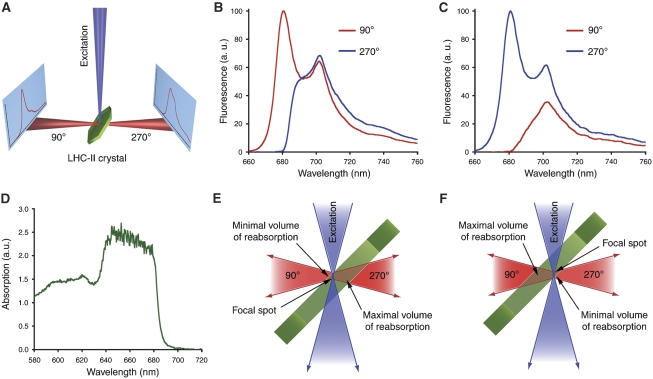
We recorded low-temperature (100 K) fluorescence emission spectra of single 3D crystals of pea LHC-II (Figure 4). As each crystal was rotated around an axis perpendicular to the incident laser beam, the emission peak at 680 nm varied from being predominant, as in detergent-solubilized trimers (Figure 4), to virtually absent (Supplementary Figure S1). The intensity of the red-shifted emission peak at ∼700 nm showed a similar, roughly orthogonal variation. With the beam focused on the crystal surface, the fluorescence recorded on the same side (90° position in Figure 5A) showed a strong peak at 680 nm. When the detector was placed on the opposite side (270° position in Figure 5A), or the crystal was rotated by 180°, emission at 680 nm was weak or absent (Figure 5B and E), and the broad 700-nm band was observed instead. The converse effect was found when the laser was focused in the crystal interior near its opposite surface (Figure 5C and F). An absorption spectrum of the same crystal (Figure 5D) indicated virtually total absorption between ∼640 and 678 nm, due to the extremely high Chl concentration of 170 mM in crystalline LHC-II. The transmittance increased steeply between 678 and 692 nm, being negligible at 680 nm but close to 100% at 700 nm. There was no indication of light-induced radiation damage during our measurements. In fact, several crystals diffracted X-rays to better than 3 Å after being exposed to the intense laser beam for about 1 h (not shown).
Figure 4.
Fluorescence emission spectra of a type-I crystal from native pea LHC-II grown at pH 5.5 at 100 K (red) and of detergent-solubilized recombinant LHC-II recorded at 77 K (green).
Figure 5.
Self-absorption in fluorescence spectra of LHC-II crystals. (A) Schematic diagram of the experiment showing the two directions in which fluorescence spectra were collected, with the detector in two alternative positions at 90° or 270° in the plane defined by the incident laser beam and the rotation axis of the crystal. (B) Fluorescence emission spectra of a crystal in two opposite directions, as defined in (A), with the exciting laser focused on the crystal surface facing the detector at the 90° position. (C) Spectra recorded of the same crystal in two opposite directions as in (B), but with the laser focus near the surface that faces the detector at the 270° position. (D) Absorption spectrum of a LHC-II crystal at 100 K. Individual absorption bands cannot be identified due to the high Chl concentration of 150 mg/ml. The sharp drop in absorption between 680 and 700 nm is evident. (E, F) Detailed drawings of the experiments in (B, C). Depending on the position of the detector (90° or 270°), or on the position of the crystal with respect to the laser focal spot, the 680 nm emission is either reabsorbed or not.
The 10 different type-I crystals that were measured showed essentially identical emission spectra, with the same strong orientation and position dependence, as did all of a similar number of cubic crystals of pea LHC-II measured in the same way (not shown). The large unit cell and symmetry of the cubic crystals (Kühlbrandt, 1987) indicate that they were of the same type as those used by Pascal et al (2005) and Yan et al (2007). Every crystal could be oriented such that a dominant 680-nm emission band was observed. In other orientations or focal positions, the same crystals showed emission at 700 nm. Fluorescence of crystalline LHC-II therefore does not depend on crystal type, plant species or age. This is consistent with our finding that the structures of pea and spinach LHC-II are identical within experimental error (Figure 1). In this context, it is interesting to note that the fluorescence properties of the crystalline complex are unchanged at a pH where quenching occurs in vivo. Polarization effects as an explanation of the orientation dependence of the crystal spectra can be excluded, as an earlier study revealed only minor linear dichroism in LHC-II crystals as a function of crystal tilt (Kühlbrandt et al, 1988). The orientation dependence of the LHC-II emission spectrum is thus clearly due to self-absorption. In agreement with our observations, self-absorption is also apparent in the precipitated complex where the 680 nm emission is progressively removed with increasing optical path lengths (Vasil'ev et al, 1997). We conclude that the striking disappearance of the 680 nm fluorescence peak cannot be taken to signify that the crystal structure shows a quenched state of the complex, as has been claimed on the basis of similar observations (Pascal et al, 2005; Yan et al, 2007; Supplementary Figures S2 and S3).
Fluorescence lifetimes
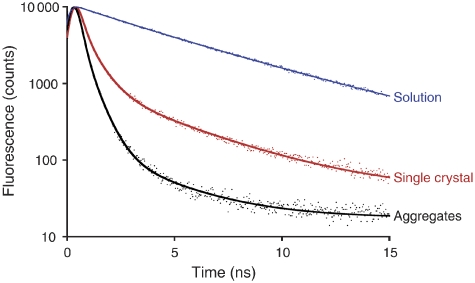
Apart from the red shift of the fluorescence maximum, earlier assertions that the crystal structure shows the quenched state (Pascal et al, 2005; Yan et al, 2007) were based on the ∼1 ns lifetime of the fluorescence emitted by LHC-II crystals. We carried out a detailed investigation of the fluorescence lifetimes of LHC-II in single crystals, aggregates and detergent solution by time-correlated single-photon counting (TCSPC), using the same experimental device for all measurements. Single crystals at 100 K indicated two main emitting species at 680 and 700 nm with average fluorescence lifetimes of 1.31 and 2.63 ns, respectively (Supplementary Figure S4; Supplementary Table SIII). The average fluorescence lifetime of the total emission was 1.98 ns. Under the same conditions, the average total fluorescence lifetime of LHC-II aggregates was 1.43 ns, but only 0.39 ns for the main 680-nm emission band (Figure 6; Supplementary Table SIII), close to the time resolution limit of our apparatus. The total emission from a frozen drop of dilute LHC-II solution at 100 K indicated two decay components (Supplementary Table SIII), with an average lifetime of 5.22 ns, in agreement with the literature (Palacios et al, 2002).
Figure 6.
TCSPC histograms of the fluorescence decays of LHC-II in dilute solution (blue), single crystals (red) and aggregates (black), with corresponding fits for the 680-nm emission band. All experiments were performed at 100 K.
The total emission of LHC-II crystals and aggregates appears overall similar (Supplementary Table SIII). This is largely due to the 700-nm region where lifetimes are long, and which is hardly affected by quenching or self-absorption. There is, however, a very clear difference in the average lifetime of the critical 680 nm emission, which is much longer than expected for a quenched state in crystalline LHC-II, but consistent with literature values for LHC-II aggregates. At room temperature, the average fluorescence lifetime of crystals dropped to ∼1 ns, in good agreement with fluorescence lifetime imaging of a spinach LHC-II crystal (Pascal et al, 2005). These lifetimes are on average 5–10 times longer than those reported recently for quenched aggregates (van Oort et al, 2007). We conclude that the strong 680 nm fluorescence and the long associated lifetimes observed in single 3D crystals are inconsistent with a quenched state, and indicate that the crystal structure of LHC-II must be that of the energy-transmitting, unquenched complex.
Energy transfer
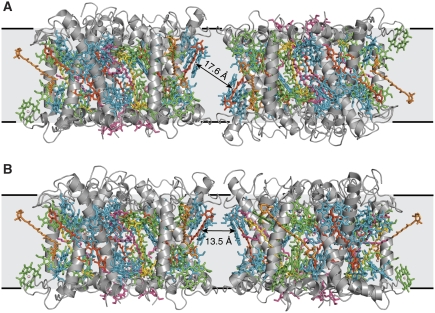
To understand energy transfer and dissipation in crystalline LHC-II, it is necessary to consider not only intramolecular Chl distances but also those between Chls in neighbouring trimers on the crystal lattice. In our type-I crystals (Standfuss et al, 2005), the closest π–π distance of an inter-trimer Chl pair (Chl 2:Chl 2′) is 14.8 Å (17.6 Å centre-to-centre; Figure 7), yielding a dipole coupling constant of 11 cm−1 (see Materials and methods). In the rhombohedral crystal form (Liu et al, 2004), the shortest π–π distance between Chl 8 and Chl 14′ is 5.4 Å, well within the range of Chl distances in the LHC-II monomer. The centre-to-centre distance is 12.3 Å, so that the dipole coupling constant is 60 cm−1 (Liu et al, 2004). Excitonic coupling of the trimers in these crystals is thus about 30% stronger than between monomers in a trimer. From these distances, we estimate Förster transfer rates between LHC-II trimers of 1065 ns−1 for rhombohedral crystals and 80 ns−1 for type-I crystals (see Materials and methods). In the thylakoid membrane, the shortest centre-to-centre distance between Chls in adjacent trimers would be ∼13.5 Å (Figure 7), yielding a transfer rate of ∼100 ns−1, which evidently allows rapid energy transfer in vivo. LHC-II trimers in both crystal forms are thus similarly or more strongly coupled than in the photosynthetic membrane, so that the energy absorbed by any one spreads rapidly to its neighbours.
Figure 7.
Interactions between neighbouring LHC-II trimers. (A) In the membrane similar to type-I crystals from pea LHC-II, neighbouring trimers are symmetrically related within each crystalline layer on a up and down manner. (B) Model for the in vivo interaction within the thylakoid membranes. The same arrangement as in the type-I crystals was used, and one of the trimers was flipped by 180° so that both trimers are pointing in the same direction as in thylakoid membranes. Additionally, we restrained the model so that the distances between atoms that are in close contact is identical in (A, B). This is necessary to avoid clashes between trimers. The centre-to-centre distance between the closest Chls in neighbouring trimers are shown. Grey, polypeptide; cyan, Chl a; green, Chl b; dark orange, lutein; light orange, neoxanthin; yellow, violaxanthin; pink, lipids.
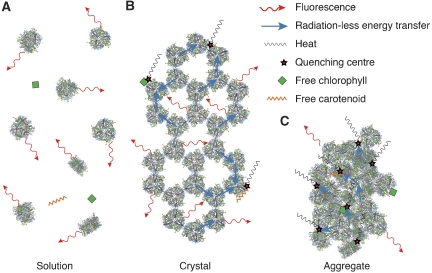
Compared with single crystals, the fluorescence lifetimes of LHC-II in detergent solution at room temperature are longer by a factor of ∼4. This is explained by the long distances between emitting trimers and the absence of nearby quenching centres in dilute solution. By contrast, LHC-II trimers on a crystal lattice are in close contact, so that the absorbed excitation energy flows rapidly from one to the other, until it is finally dissipated by randomly present quenching sites. Such quenching centres might arise, for example, through irregular molecular contacts at domain boundaries, occasional free pigments or spurious impurities (Figure 8). The shorter fluorescence lifetimes of LHC-II crystals compared with dilute solution are thus fully accounted for by excitonic coupling. The same would be true of LHC-II reconstituted into proteoliposomes, where indeed similar lifetimes have been measured (Moya et al, 2001). The state of LHC-II in single crystals thus seems to resemble its native state in the membrane.
Figure 8.
Energy transfer and dissipation by LHC-II in vitro. (A) Solubilized LHC-II trimers in detergent solution are functionally independent and re-emit absorbed light as fluorescence. (B) LHC-II crystals show similar fluorescence, although its intensity and spectral range are modified by re-absorption. Conceivably, occasional tight interaction of LHC-II trimers with one another, for example, at grain boundaries, or with contaminating free pigments can generate quenching centres, to which the energy flows through the excitonically coupled complexes. (C) Randomly compacted trimers in LHC-II aggregates generate a high density of quenching centres, resulting in reduced lifetimes and conversion of the absorbed energy into heat.
Mechanism of aggregation quenching
The irregular, tight aggregates of LHC-II (Figure 8) produced by detergent removal or salt precipitation are an artificial, non-physiological state of the complex, as are dilute detergent solutions at the other end of the scale. LHC-II aggregates are known to be quenched, with average lifetimes ranging from 0.1 to 0.6 ns, depending on the method of preparation (Vasil'ev et al, 1997; van Oort et al, 2007). We attribute these short lifetimes primarily to the formation of quenching centres through random, tight interaction of pigments exposed on the outer surface of the complex (Figure 8). This would create a large number of quenching centres that will effectively dissipate the excitation energy in aggregates (Beddard et al, 1976; Dreuw et al, 2003; van Oort et al, 2007).
Our proposed mechanism of aggregation quenching explains the well-documented fluorescence lifetime heterogeneity found in LHC-II aggregates (Barzda et al, 2001). This is especially pronounced in aggregates of LHC-II monomers (van Oort et al, 2007), in which several more Chls are exposed on the surface of the complex than in trimers. Moreover, monomer solutions inevitably contain significant amounts of free pigment, giving rise to yet more quenching centres upon aggregation. The increase in the number of quenching centres per unit volume from LHC-II in detergent solution to crystals and finally, aggregates would result in an increase in energy transfer, and easily account for the observed associated reduction in fluorescence yield and lifetimes.
Finally, our model also explains the results of an earlier study (Ruban et al, 1995), which found that the drop in fluorescence yield upon LHC-II aggregation is surprisingly reversed at 4 K. This indicates that, at very low temperature, the absorbed energy is lost as fluorescence before it can be transmitted to any quenching centres, which suggests that these must be physically distant from the emitting pigments. If emitter and quencher were located in the same monomer, as postulated by the conformational switch model, quenching should be equally effective at 4 K and at room temperature, which is clearly not the case. Although we cannot rule out the possibility that there might be other quenching mechanisms that are in themselves temperature dependent, our conclusion that controlled energy dissipation plant photosynthesis is not brought about by a conformational switch within the antenna is nevertheless sound. If the atomic structures of the quenched and unquenched states of the complex were indeed different, then LHC-II aggregates or crystals might contain a mixture of both. For crystals this can be excluded, as we show here that the chlorophyll configurations in the two independently determined X-ray structures are identical to within 0.25 Å. This would evidently not be the case if they were to represent mixed occupancies, let alone two different mixed occupancies of two different states. If the LHC-II aggregates were to harbour two different conformational states, then the recovery of the fluorescence yield at very low temperature would not be complete, as the quenched state would remain quenched at 4 K. This can also be ruled out, as detergent-solubilized LHC-II trimers and aggregates have the same fluorescence yield at 4 K (Ruban et al, 1995).
It is worth noting that a conformational switch within the antenna as a quenching mechanism could turn into a major disadvantage in vivo, as a large part of the photosynthetic apparatus might become quenched as a result of random changes in the pigment configuration of a single complex. For this reason, light-harvesting pigment–protein complexes need to be rigid, and this is indeed borne out by all known structures of such assemblies.
Implications for the mechanism of qE
A well-documented effect of NPQ is the conversion of Vio to Zea in the xanthophyll cycle. Indeed, there is some evidence that Zea itself may function as a quencher (Dreuw et al, 2003; Holt et al, 2005; Ahn et al, 2008; Avenson et al, 2008). However, recent data show that simple substitution of Zea against Vio in isolated LHC-II does not significantly influence the Chl excited state lifetime in the complex (Amarie et al, 2007; Bode et al, 2008). More recently, it has been proposed that a pH-induced conformational change in PsbS is transduced to the minor LHCs, promoting excitation energy quenching, through a Zea radical cation (Ahn et al, 2008). Although a carotenoid radical cation has been observed in isolated minor LHCs enriched in Zea (Ahn et al, 2008; Avenson et al, 2008), its role in the quenching mechanism in vivo is unclear, nor does it account for any contribution of LHC-II in qE.
We show here that a conformational switch in LHC-II can be excluded as part of the quenching mechanism. As the simple substitution of Vio by Zea in the complex does not bring about energy dissipation, the controlled interaction of pigment–protein complexes in the membrane appears to be the most probable mechanism for qE. The likely interaction partner with the LHC-II or the minor LHCs is the PsbS protein, which is required for qE in Arabidopsis (Li et al, 2000), and has been shown to interact with LHC-II and CP29 in vivo (Teardo et al, 2007). PsbS is a dimer at neutral pH that dissociates into monomers at low pH, where qE occurs (Bergantino et al, 2003), probably in response to the protonation of lumenally exposed glutamates (Li et al, 2004). Low pH also triggers the conversion of Vio into Zea in the xanthophyll cycle. The PsbS protein seems to be stable in the absence of pigments (Funk et al, 1995; Dominici et al, 2002; Bonente et al, 2008), although it has been reported to bind Zea in vitro (Aspinall-O'Dea et al, 2002), which has however been challenged (Bonente et al, 2008). Pigment binding by PsbS thus appears to be transient, and may occur only under qE conditions.
On the basis of these considerations, we propose a simple, although speculative, feedback mechanism for qE in thylakoid membranes, based on the mechanism previously suggested by Szabo et al (2005). When the lumenal pH drops as a result of high photosynthetic activity, Vio is converted into Zea, which partitions into the lipid phase of the membrane. At the same time, PsbS monomerizes. Presumably, the dimer-to-monomer transition uncovers the Zea-binding site on the hydrophobic surface of PsbS. We propose that Zea is bound in a position where, upon interaction with LHC-II or one of the minor LHCs, it is brought into van der Waals contact with a pigment exposed on the hydrophobic, membrane-embedded surface of the complex, most likely Chl 2 (or its equivalent in the minor LHCs), which is thought to be the terminal emitter in the antenna. This specific interaction would form the quenching Zea–Chl heterodimer that has been observed by femtosecond time-resolved spectroscopy of thylakoid membranes and has the very short 0.1–1 ps relaxation time expected of an efficient quencher (Holt et al, 2005). At lower light levels, the lumenal pH rises, PsbS dimerizes, the released Zea is converted back into Vio and the system reverts to normal photosynthetic activity (Supplementary Figure S5). In LHC-II aggregates, the tight interaction of LHC-II trimers with one another or with pigment impurities mimics this NPQ, although it is then significantly slower. Neither quenching process requires a conformational change within LHC-II.
Materials and methods
LHC-II isolation and crystallization
Pea LHC-II was purified and crystallized as described (Standfuss et al, 2005). Briefly, hexagonal plates were obtained by vapour diffusion at 15–22°C at 10–20% polyethyleneglycol 350 monomethyl ether, 10–20% glycerol, 50 mM MES buffer pH 5.3–5.6 and 20 mM sodium chloride. Crystals appeared after 2 weeks and grew to a final size of around 150 × 150 × 15 μm3 within 4 weeks. Thin hexagonal plates were harvested within 2–4 weeks, washed at least 2 × with a Chl-free cryoprotectant solution (polyethyleneglycol 350 monomethyl ether and glycerol added to the mother liquor at a final concentration of 20% each) and flash frozen in liquid nitrogen or immediately used for room-temperature measurements. Cubic crystals of pea LHC-II were grown as described (Kühlbrandt, 1987). Cryo-loops used for handling the crystals were thoroughly washed with ethanol and water to exclude contamination with free LHC-II or pigments.
Fluorescence measurements of crystals, solutions and aggregates of native LHC-II
Fluorescence data on LHC-II crystals were recorded at the Cryobench of the ESRF, France (Royant et al, 2007). Fluorescence lifetime measurements were performed using TCSPC. Histograms were recorded until at least 104 photons had accumulated on the channel of highest intensity. A 440-nm laser with a ∼15-μm focal spot was used for excitation. A total of ∼20 crystals were measured. Precise positioning of the excitation spot on the crystal, and of crystal orientation with respect to the incident laser beam was achieved with a computer-operated goniometer head. The wavelength dependence of the fluorescence lifetimes was analysed by placing band-pass filters with 10-nm bandwidth in front of the detector, sampling the 670–750 nm range in 10-nm intervals. For room temperature measurements, a 0.5 μl droplet of cryoprotectant containing one single crystal was sandwiched between two glass cover slips, and sealed by silicone grease to prevent evaporation, taking care not to damage the crystal. TCSPC data were analysed using either FluoTimeFit (Royant et al, 2007) or PeakFit (Seasoft Software Inc., San Jose, CA). To ensure that the spectra originated from the crystals and not the surrounding buffer, droplets of the cryoprotectant solution in which the crystals had been washed were checked for fluorescence, but none was detected.
Samples of detergent-solubilized LHC-II with Chl a/Chl b ratio (n/n) of 1.35 were centrifuged for 10 min at 14 000 g to remove any aggregates, immediately before measurement as described above. Chl concentration was measured according to Porra et al (1989). For low-temperature measurements, a 0.2 mm nylon loop was used to hold a small volume of the LHC-II solution, which was then frozen in the cryo stream. Ice formation was prevented by adding glycerol to a final concentration of 30%. LHC-II aggregates were prepared by diluting 5 μl of concentrated stock 100-fold with distilled water, and adding MgCl2 to a final concentration of 50 mM. The precipitate was collected by centrifugation at 14 000 g and washed twice with 50 mM MgCl2, before being resuspended in 500 μl buffer (Tris pH 7.5; 10 mM MgCl2). A cryo-loop was used to hold a small volume of the suspension for low-temperature measurements, as above.
Preparation of refolded LHC-II
Pea LHC-II variants WT, ΔChl 2 (N183A), ΔChl 8 (H212A), ΔChl 12 (E139A) and ΔChl 13 (Q131A) derived from the lhcb1*2 gene were expressed, purified and refolded with pigments extracted from spinach leaves as described (Rogl et al, 1998) with modifications (Standfuss and Kühlbrandt, 2004). As some mutants yielded only low amounts of trimers, the monomeric fraction was collected from the sucrose gradients and used for the fluorescence measurements of all variants. Pigment analysis was performed by HPLC (Gilmore and Yamamoto, 1991).
Fluorescence measurements on refolded LHC-II
Room temperature emission spectra of LHC-II variants were recorded on a Hitachi F-4500 fluorimeter with the excitation wavelength at 441 nm. For spectra of detergent-solubilized variants, sucrose density gradient fractions were diluted with 50 mM Tris, pH 7.5 containing 0.05% DDM. For spectra of the aggregated variants, the same fractions were diluted with equal amounts of detergent-free buffer with 50 mM MgCl2.
Coupling strength and Förster energy transfer rates calculations
A good estimate of the coupling strength V between individual Chls in LHC-II can be obtained from the equation (van Amerongen and van Grondelle, 2001)
 |
where α corresponds to 90 or 75 nm3 cm−1 for Chla:Chla or Chla:Chlb pairs, respectively. k is the orientation factor
with 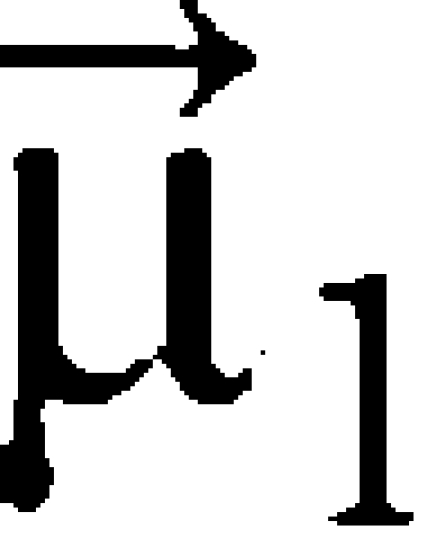 ,
, 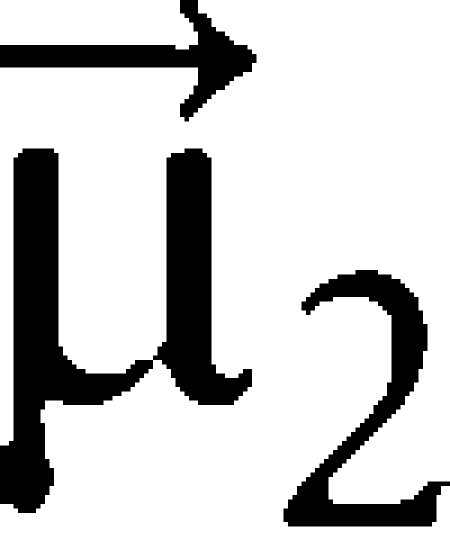 and
and 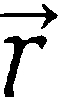 being the normalized vectors of the transition dipole moments of the individual Chls and of the centre-to-centre distance. The corresponding Förster energy transfer rates are obtained from
being the normalized vectors of the transition dipole moments of the individual Chls and of the centre-to-centre distance. The corresponding Förster energy transfer rates are obtained from
 |
where η is the refractive index (∼1.55) and β is 32.26 or 9.61 nm6 ps−1 for Chla:Chla or Chla:Chlb pairs, respectively.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Anke Terwisscha van Scheltinga for valuable discussions, Heidi Betz for technical assistance and Philippe Carpentier for help with data fitting. TB is supported by the fellowship SFRH/BD/21440/2005 from Fundação para a Ciência e Tecnologia. AD and JS acknowledge funding from Sonderforschungsbereich 472.
References
- Ahn TK, Avenson TJ, Ballottari M, Cheng Y-C, Niyogi KK, Bassi R, Fleming GR (2008) Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320: 794–797 [DOI] [PubMed] [Google Scholar]
- Amarie S, Standfuss J, Barros T, Kühlbrandt W, Dreuw A, Wachtveitl J (2007) Carotenoid radical cations as a probe for the molecular mechanism of nonphotochemical quenching in oxygenic photosynthesis. J Phys Chem B 111: 3481–3487 [DOI] [PubMed] [Google Scholar]
- Aspinall-O'Dea M, Wentworth M, Pascal A, Robert B, Ruban A, Horton P (2002) In vitro reconstitution of the activated zeaxanthin state associated with energy dissipation in plants. Proc Natl Acad Sci USA 99: 16331–16335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR (2008) Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem 283: 3550–3558 [DOI] [PubMed] [Google Scholar]
- Barzda V, de Grauw CJ, Vroom J, Kleima FJ, van Grondelle R, van Amerongen H, Gerritsen HC (2001) Fluorescence lifetime heterogeneity in aggregates of LHCII revealed by time-resolved microscopy. Biophys J 81: 538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddard GS, Carlin SE, Porter G (1976) Concentration quenching of chlorophyll fluorescence in bilayer lipid vesicles and liposomes. Chem Phys Lett 43: 27–32 [Google Scholar]
- Bergantino E, Segalla A, Brunetta A, Teardo E, Rigoni F, Giacometti GM, Szabo I (2003) Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc Natl Acad Sci USA 100: 15265–15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode S, Quentmeier CC, Liao P-N, Barros T, Walla PJ (2008) Xanthophyll-cycle dependence of the energy transfer between carotenoid dark states and chlorophylls in NPQ mutants of living plants and in LHC II. Chem Phys Lett 450: 379–385 [Google Scholar]
- Bonente G, Howes BD, Caffarri S, Smulevich G, Bassi R (2008) Interactions between the photosystem II subunit PsbS and xanthophylls studied in vivo and in vitro. J Biol Chem 283: 8434–8445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JJ, Ditto CL, Arntzen CJ (1978) Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys 187: 252–263 [DOI] [PubMed] [Google Scholar]
- Dominici P, Caffarri S, Armenante F, Ceoldo S, Crimi M, Bassi R (2002) Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J Biol Chem 277: 22750–22758 [DOI] [PubMed] [Google Scholar]
- Dreuw A, Fleming GR, Head-Gordon M (2003) Chlorophyll fluorescence quenching by xanthophylls. Phys Chem Chem Phys 5: 3247–3256 [Google Scholar]
- Funk C, Adamska I, Green BR, Andersson B, Renger G (1995) The nuclear-encoded chlorophyll-binding photosystem II-S protein is stable in the absence of pigments. J Biol Chem 270: 30141–30147 [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Hazlett TL, Govindjee (1995) Xanthophyll cycle-dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence lifetime. Proc Natl Acad Sci USA 92: 2273–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY (1991) Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J Chromatogr A 543: 137–145 [Google Scholar]
- Holt NE, Zigmantas D, Valkunas L, Li XP, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307: 433–436 [DOI] [PubMed] [Google Scholar]
- Ide JP, Klug DR, Kühlbrandt W, Giorgi LB, Porter G (1987) The state of detergent solubilized light-harvesting chlorophyll-a/B protein complex as monitored by picosecond time-resolved fluorescence and circular-dichroism. Biochim Biophys Acta 893: 349–364 [Google Scholar]
- Kühlbrandt W (1987) Three-dimensional crystals of the light-harvesting chlorophyll a/b protein complex from pea chloroplasts. J Mol Biol 194: 757–762 [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W, Becker A, Mäntele W (1988) Chlorophyll dichroism of three-dimensional crystals of the light-harvesting chlorophyll a/b–protein complex. FEBS Lett 226: 275–279 [Google Scholar]
- Kühlbrandt W, Wang DN, Fujiyoshi Y (1994) Atomic model of plant light-harvesting complex by electron crystallography. Nature 367: 614–621 [DOI] [PubMed] [Google Scholar]
- Li X-P, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279: 22866–22874 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428: 287–292 [DOI] [PubMed] [Google Scholar]
- Moya I, Silvestri M, Vallon O, Cinque G, Bassi R (2001) Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. Biochemistry 40: 12552–12561 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW, Pascal AA, Horton P, Holzwarth AR (1993) Excitation-energy quenching in aggregates of the LHC II chlorophyll–protein complex: a time-resolved fluorescence study. Biochim Biophys Acta 1141: 23–28 [Google Scholar]
- Palacios MA, de Weerd FL, Ihalainen JA, van Grondelle R, van Amerongen H (2002) Superradiance and exciton (de)localization in light-harvesting complex II from green plants? J Phys Chem B 106: 5782–5787 [Google Scholar]
- Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W, Ruban A (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436: 134–137 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Rogl H, Kosemund K, Kühlbrandt W, Collinson I (1998) Refolding of Escherichia coli produced membrane protein inclusion bodies immobilised by nickel chelating chromatography. FEBS Lett 432: 21–26 [DOI] [PubMed] [Google Scholar]
- Rogl H, Kühlbrandt W (1999) Mutant trimers of light-harvesting complex II exhibit altered pigment content and spectroscopic features. Biochemistry 38: 16214–16222 [DOI] [PubMed] [Google Scholar]
- Royant A, Carpentier P, Ohana J, McGeehan J, Paetzold B, Noirclerc-Savoye M, Vernede X, Adam V, Bourgeois D (2007) Advances in spectroscopic methods for biological crystals. 1. Fluorescence lifetime measurements. J Appl Cryst 40: 1105–1112 [Google Scholar]
- Ruban A, Horton P (1994) Spectroscopy of non-photochemical and photochemical quenching of chlorophyll fluorescence in leaves; evidence for a role of the light harvesting complex of photosystem II in the regulation of energy dissipation. Photosynth Res 40: 181–190 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Berera R, Ilioaia C, van Stokkum IH, Kennis JT, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450: 575–578 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Dekker JP, Horton P, van Grondelle R (1995) Temperature dependence of chlorophyll fluorescence from light-harvesting complex II of higher plants. Photochem Photobiol 61: 216–221 [Google Scholar]
- Ruban AV, Rees D, Noctor GD, Young A, Horton P (1991) Long-wavelength chlorophyll species are associated with amplification of high-energy-state excitation quenching in higher plants. Biochim Biophys Acta 1059: 355–360 [Google Scholar]
- Standfuss J, Kühlbrandt W (2004) The three isoforms of the light-harvesting complex II: spectroscopic features, trimer formation, and functional roles. J Biol Chem 279: 36884–36891 [DOI] [PubMed] [Google Scholar]
- Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kühlbrandt W (2005) Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J 24: 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Bergantino E, Giacometti GM (2005) Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation. EMBO Rep 6: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teardo E, de Laureto PP, Bergantino E, Dalla Vecchia F, Rigoni F, Szabo I, Giacometti GM (2007) Evidences for interaction of PsbS with photosynthetic complexes in maize thylakoids. Biochim Biophys Acta 1767: 703–711 [DOI] [PubMed] [Google Scholar]
- van Amerongen H, van Grondelle R (2001) Understanding the energy transfer function of LHCII, the major light-harvesting complex of green plants. J Phys Chem B 105: 604–617 [Google Scholar]
- van Oort B, van Hoek A, Ruban AV, van Amerongen H (2007) Aggregation of light-harvesting complex II leads to formation of efficient excitation energy traps in monomeric and trimeric complexes. FEBS Lett 581: 3528–3532 [DOI] [PubMed] [Google Scholar]
- Vasil'ev S, Irrgang KD, Schrotter T, Bergmann A, Eichler HJ, Renger G (1997) Quenching of chlorophyll a fluorescence in the aggregates of LHCII: steady state fluorescence and picosecond relaxation kinetics. Biochemistry 36: 7503–7512 [DOI] [PubMed] [Google Scholar]
- Yan H, Zhang P, Wang C, Liu Z, Chang W (2007) Two lutein molecules in LHCII have different conformations and functions: insights into the molecular mechanism of thermal dissipation in plants. Biochem Biophys Res Commun 355: 457–463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information