EMBO J 27, 2977–2987 (2008); published online 19 November 2008
EMBO J 27, 2988–2997 (2008); published online 19 November 2008
The formation of protein disulphide bonds in newly synthesised secretory proteins within the endoplasmic reticulum (ER) depends on an electron transfer system in which oxidising equivalents are passed from molecular oxygen through several carriers within the oxidase Ero1 to protein disulphide isomerase (PDI) and then to the reduced protein substrate. Recent articles published in The EMBO Journal highlight a crucial and surprising feature of the control of this process in mammalian cells; the activity of the key oxidase Ero1α is downregulated in oxidising conditions through the formation of a stable non-catalytic disulphide by a cysteine residue that participates in one of the redox active sites within the oxidase (Appenzeller-Herzog et al, 2008; Baker et al, 2008).
Our current understanding of this pathway, mainly based on studies on the system in yeast (Gross et al, 2006), is that oxidising equivalents flow from O2 to a flavin cofactor within Ero1, to a nearby ‘inner' dithiol/disulphide active site and from there to an ‘outer' dithiol/disulphide on the surface of Ero1. Ero1 oxidises the PDI that in turn delivers oxidising equivalents to newly synthesised protein substrates. Although we do not know all the details, it is clear that this is not simply a linear pathway. First, the ER lumen contains the majority of the mammalian cell's complement of oxidised glutathione (GSSG); this is believed to be generated by an inflow of GSH from the cytosol, which is oxidised by the same pathway to maintain a high-capacity GSSG/GSH oxidising buffer system within the ER lumen (Bass et al, 2004). Secondly, the Ero1 system generates H2O2, a powerful oxidant, whose subsequent action and fate is not understood. And finally, mammalian cells contain several homologous members of the PDI family that operate in the pathway of protein maturation within the ER (Ellgaard and Ruddock, 2005).
Nevertheless, it is clear that (i) oxidising equivalents enter the ER system through Ero1 (Ero1p in yeast, whereas mammalian cells contain two forms Ero1α and Ero1β), (ii) these equivalents have several possible destinations, which may vary over time and with the cell's physiological status, including the rate at which it is synthesising proteins for export and (iii) some control of the pathway is desirable. The recent articles by Ellgaard's group (Appenzeller-Herzog et al, 2008) in Copenhagen and Bulleid's group (Baker et al, 2008) in Manchester provide insight into how this control operates.
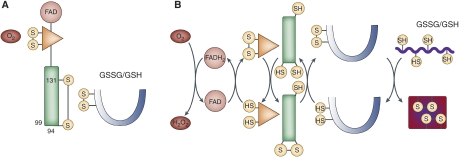
Ellgaard's group used a well-targeted transfection system to create cell lines overexpressing Ero1α (or Ero1β or mutants of Ero1α) by 20- to 30-fold; within the cells, these species were in oxidised states and resulted in greater oxidation of PDI. Yet, despite their much higher oxidase levels, the cells experienced only minimal oxidative stress, suggesting that the overall pathway is tightly regulated. Ero1α has 15 Cys residues and exists in a number of different redox states; it was known previously that these differ in their complement of ‘long-distance' disulphide bonds. The Copenhagen group isolated the form that predominates in the most oxidising conditions and used enzymic proteolysis and mass spectrometry to identify the disulphide bonds present. The crucial disulphide that characterizes this ‘most oxidised' form was found to be between Cys residues 94 and 131, which was a surprise, as Cys94 and Cys99 interact to form the ‘outer' dithiol/disulphide redox-active site. The result implied that Ero1α Cys131 and reduced PDI compete to reduce the Cys94–Cys99 active site. This was confirmed by observing the redox state of the components in response to altered levels of both PDI and Ero1α under the different conditions. It appears that Ero1α is present only in the form with a functional ‘outer active site' when there is ample reduced PDI present to compete with Cys131; otherwise, the C94-C131 bond forms. Finally, Ellgaard's group transfected cells with a mutant Ero1α lacking Cys 131 (C131A) and hence unable to form the Cys94-Cys131 disulphide; in these cells, the C94-C99 active site is always functional, and they found that the redox state in the ER of these cells was perturbed to be more highly oxidising, especially as indicated by higher levels of GSSG. They concluded that in normal cells, the formation of the Cys94–Cys131 disulphide in oxidising conditions is a regulatory mechanism that turns off Ero1α oxidase activity until there is demand for disulphide oxidation as indicated by a high level of reduced PDI. (see Figure 1).
Figure 1.
(A) In the absence of a reduced substrate, PDI and the FAD and inner disulphide redox centres of Ero1α are oxidised, the regulatory C94–C131 disulphide forms and the glutathione buffer is shifted towards GSSG. (B) In the presence of a reduced substrate, some PDI is converted to the reduced form, which reduces the regulatory disulphide; reducing equivalents flow from substrate protein to O2, all redox centres interconvert between the oxidised and reduced state and the glutathione buffer shifts towards GSH.
This work is neatly complemented by studies of the system in vitro carried out by Bulleid's group. The Manchester team expressed recombinant Ero1α and mutant versions lacking specific Cys residues; by studying the purified recombinant proteins, they determined that Cys131 and Cys94 were essential for making the ‘most oxidised' form of Ero1α, confirming the identification of this bond by the Copenhagen group. They also used recombinant Ero1α to reconstitute the Ero1α/PDI oxidative system in vitro, showing that the C131A mutant has increased oxidase activity compared with wild type and shows no lag in activity, whereas the wild type shows a lag during which the C94–C131 disulphide becomes reduced. Finally, Bulleid's group carried out a redox titration in vitro to measure the standard redox potential of the C94–C131 disulphide and found a value of −275 mV, showing that this is a strong disulphide bond, which would be reduced only when there is abundant reduced substrate present.
Taken together, the articles report a general picture in which Ero1α exists as an oxidised inactive form and is activated only when there is a ‘demand' within the ER, which is signalled by the conversion of PDI to a predominantly reduced form. As summarised by the Ellgaard team, ‘These data reveal a novel regulatory feedback system where PDI emerges as a central regulator of ER redox homeostasis.'
There are many aspects of the ER oxidative protein folding system that are still obscure: how hydrogen peroxide generated by Ero1 is detoxified, how the inward flow of GSH from the cytosol is controlled, whether and how oxidising equivalents pass to the numerous members of the PDI family other than PDI itself, what the significance is of the abundant protein-SSG mixed disulphides in the system. Nevertheless, these two articles provide a basis for understanding key aspects of the mechanism by which the flow of oxidising equivalents from oxygen to nascent secretory polypeptides is regulated.
References
- Appenzeller-Herzog C, Riemer J, Christensen B, Sørensen ES, Ellgaard L (2008) A novel disulphide switch mechanism in Ero1alpha balances ER oxidation in human cells. EMBO J 27: 2977–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KM, Chakravarthi S, Langton KP, Sheppard AM, Lu H, Bulleid NJ (2008) Low reduction potential of Ero1alpha regulatory disulphides ensures tight control of substrate oxidation. EMBO J 27: 2988–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass R, Ruddock LW, Klappa P, Freedman RB (2004) A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J Biol Chem 279: 5257–5262 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Ruddock LW (2005) The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep 6: 28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C, Fass D (2006) Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. PNAS 103: 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]