Abstract
Treatment-related toxicities such as mucositis and infections are both more frequent and more severe in children with Down syndrome (DS) and acute lymphoblastic leukemia (ALL) compared to non-DS ALL. Altered methotrexate pharmacodynamics play a role, but severe toxicities also occur in treatment courses that lack methotrexate. We hypothesized that this might be attributable to heightened cytotoxic effects of other ALL chemotherapeutic agents on DS versus non-DS host tissues. Panels of DS and non-DS lymphoblastoid cell lines (LCLs) and primary fibroblast cell lines were treated with asparaginase, dexamethasone, doxorubicin, mafosfamide and vincristine. LCL survival was assessed using the MTT assay, and fibroblast proliferation using the clonogenic survival assay. No significant differences were observed between DS and non-DS cell lines using either assay. Both DS and non-DS cell lines were resistant to dexamethasone at the maximal concentrations tested, and did not differ significantly in sensitivity to the other drugs studied. Thus, heightened in vitro cytotoxicity does not appear to account for the increased treatment-related toxicities observed in patients with DS ALL.
Keywords: Down syndrome, acute lymphoblastic leukemia, MTT assay, clonogenic survival assay
1. Introduction
Children with Down syndrome (DS) have a 10- to 20-fold higher risk of developing acute lymphoblastic leukemia (ALL) and constitute 3% of pediatric ALL cases. There is mixed data as to whether patients with DS have inferior survival compared to patients with non-DS ALL, but studies consistently demonstrate that DS ALL patients exhibit more frequent and severe treatment-related toxicities, particularly mucositis and infections (1). Increased methotrexate-induced mucositis is a well-recognized occurrence in patients with DS ALL, likely due to increased dosage of the reduced folate carrier gene (RFC1) on chromosome 21 causing enhanced intracellular methotrexate transport (2). However, mucositis and infectious complications are also more frequent in DS ALL patients in treatment phases which lack methotrexate. The basis for the differential toxicity in DS patients of the chemotherapeutic agents employed in these phases is uncertain. Of note, patients with DS and acute myeloid leukemia (AML), who are treated with many chemotherapeutic agents of the same classes used in treatment of ALL, demonstrate a similar disproportionate incidence of toxicities compared to non-DS children with AML (reviewed in (3)).
We hypothesized that increased treatment-related toxicity in DS ALL may occur due to disproportionate cytotoxic effects of chemotherapy on nonleukemic DS host cells. To investigate, we exposed DS and non-DS lymphoblastoid cell lines (LCLs) and fibroblast cell lines to chemotherapeutic agents in vitro and compared their survival and proliferation. Both LCLs and fibroblasts were studied in order to represent the cell lineages involved in infectious complications and mucositis, respectively. The chemotherapeutic agents tested were those commonly employed in ALL induction and intensification phases, when most treatment-related toxicity occurs: asparaginase, dexamethasone, doxorubicin, vincristine, and mafosfamide (the active drug generated in vivo by hepatic activation of the prodrug cyclophosphamide). The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay (4) was used in LCLs to measure viability following drug exposure. The clonogenic survival assay was used in the fibroblast cell lines to assess proliferation, since a cytostatic effect on fibroblasts would not affect viability and hence not be detectable by the MTT assay.
2. Materials and methods
This study was performed in accord with a protocol approved by the Baylor College of Medicine Institutional Review Board.
2.1. Cell lines
Lymphoblastoid cell lines AG09802, AG16945, AG09387, and GM05398, and untransformed fibroblast cell lines AG06922, AG05397, GM00498, and GM05659 were purchased from Coriell Cell Repositories (Camden, NJ). Additional LCLs (DS4, DS5, DS6, DS8, NoDS1, NoDS2, and NoDS3) were kindly provided by Dr. John Belmont. ALL cell lines RS4, Reh and HSB2 were obtained from American Type Culture Collection (ATCC Rockville, MD). LCLs were cultured in RPMI 1640 medium (Gibco, Grand Island, NJ) supplemented with 10% bovine growth serum (Hyclone, Logan, UT) and 1% antibiotic/antimycotic (Gibco, Grand Island, NJ), and incubated at 37°C in 5% CO2. For the RS4 cell line only, medium was also supplemented with 0.45% sucrose. Fibroblast cells were grown in minimal essential medium supplemented with L-glutamine 200mM (Gibco, Grand Island, NJ). All LCLs and fibroblasts used were at less than 22 passages. The same bovine growth serum lot was used in all experiments to avoid differences in culture conditions.
2.2. Chemotherapeutic agents
Dexamethasone, doxorubicin and vincristine were obtained from Sigma-Aldrich (St. Louis, MO), asparaginase from Merck (West Point, PA) and mafosfamide L-lysine from Baxter Oncology (Halle, Germany). Mafosfamide and asparaginase stock solutions were prepared in phosphate buffered saline (PBS); doxorubicin and vincristine in water; and dexamethasone in methanol (CAS No. 67-56-1).
2.3. MTT Assay
Cell viability was assessed using the MTT colorimetric assay as previously described (5). Briefly, DS and non-DS LCLs as well as positive control ALL cell lines were seeded at between 0.5–2 × 105 cells/ml in 96-well plates and cultured for 24 hours, followed by incubation with drug for 72 hours. Cells were exposed to drug at the following concentration ranges: asparaginase at 3.6 × 10−5 to 10 IU/ml; dexamethasone at 1 × 10−5 to 100 uM; doxorubicin at 0.61 to 10,000 nM; mafosfamide at 3.6 × 10−4 to 100 uM; and vincristine at 5.6 ×10−6 to 2.1 uM. Absorbance was measured by microplate spectrophotometer reader (Anthos Analytical, Durham, NC) at 550 nm. Two to four independent replicates were performed for each experiment. Manta 2.0 (Dazdaq Solutions, United Kingdom) was used to convert raw absorbance to percentage survival values. Cell survival data was graphed using KaleidaGraph 4.0 (Synergy Software, Reading, PA). The IC50 value for each drug, or the inhibitory concentration required to reduce cell number by 50%, was determined by visual inspection of the resultant survival graphs.
2.4. Clonogenic survival assay
Antiproliferative effects on DS and non-DS fibroblast cell lines were assessed by clonogenic survival assay, as previously described (6). Cells were grown to 70% confluence and then seeded in 10 cm tissue culture plates at densities varying from 300–1800 cells/ml. All plates were seeded in duplicate. After incubation for 24 hours, cells were exposed to varying concentrations of drug: asparaginase at 0.002–20 IU/ml; dexamethasone at 0.08–100,000 nM; doxorubicin at 0.8–10,000 nM; mafosfamide at 10–100,000 nM; and vincristine at 0.5–200 nM. Cells were incubated with doxorubicin and mafosfamide for 24 hours, and with asparaginase, dexamethasone, and vincristine for 48 hours. Plates were incubated until colonies of 50 or more cells were observed, approximately 11–14 days. Plating efficiency and survival fraction were calculated according to Munshi et al. (7) and then graphed using KaleidaGraph 4.0. The LD10, the drug concentration lethal to all but 10% of cells, was determined for each drug by visual inspection of the resultant graphs.
3. Results
We exposed DS and non-DS cell lines to common ALL chemotherapeutic agents over a 106-fold range of concentrations for LCLs and 104-fold range for primary fibroblasts. Duration of drug exposure was 24 hours for drugs with a half-life less than 24 hours (doxorubicin and mafosfamide), and 48 hours for drugs with a half-life greater than 24 hours (asparaginase, dexamethasone, and vincristine). For the MTT assay, replicates of six wells were used for each drug concentration and two replicate 96-well plates were performed for each experiment. Two or three independent experiments were performed for each drug in the MTT assay experiments. For the clonogenic survival assay, one experiment consisting of two replicates was performed for each drug. Data showed relatively low variability across experimental replicates (Figures 1 and 2).
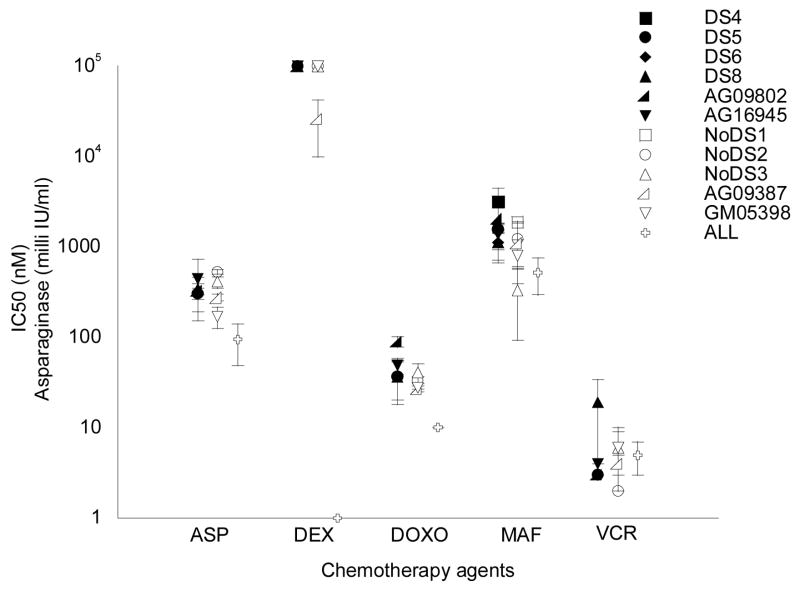
Figure 1.
Summary of IC50 values for DS and non-DS lymphoblastoid cell lines (LCLs) using the MTT assay. Black circles indicate DS LCLs; open circles represent non-DS LCLs; and open crosses represent control ALL cell lines. Each point represents the mean IC50 calculated from two or three independent experiments. Error bars indicate the standard error of the mean. The control ALL cell lines were RS4 for dexamethasone; Reh for vincristine; and HSB2 for asparaginase, doxorubicin and mafosfamide.
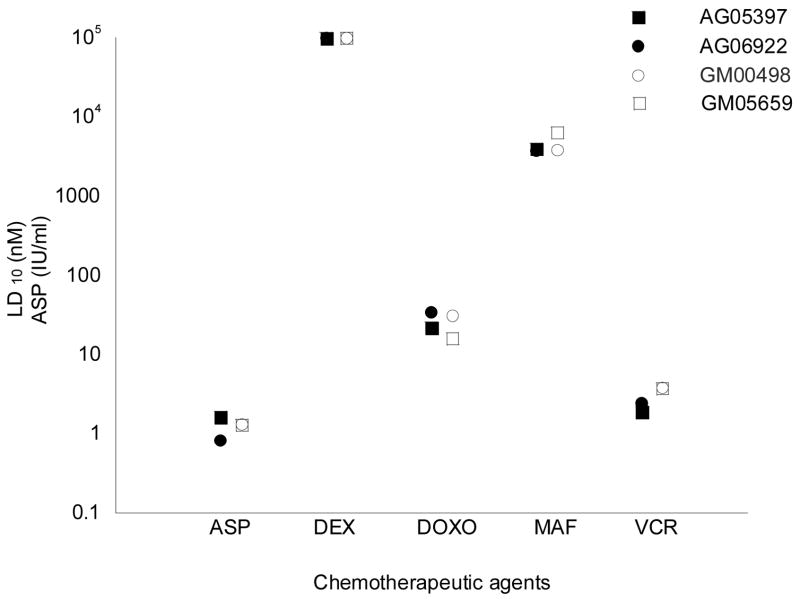
Figure 2.
Summary of LD10 values for DS and non-DS fibroblast cell lines using the clonogenic survival assay. Black circles indicate DS cell lines; open circles represent non-DS cell lines. Each point represents the mean LD10 calculated from two experiments. Error bars are not displayed since the standard error of the mean was less than the size of the circle icon representing each point.
DS and non-DS LCLs showed no significant differences in IC50 values (the inhibitory concentration required to reduce cell number by 50%) for asparaginase, doxorubicin, mafosfamide, or vincristine using the MTT assay (Figure 1). LCLs of both genotypes were resistant to dexamethasone at the highest concentration tested (100 uM). Control ALL cell lines demonstrated IC50 values that were equivalent (for vincristine) or lower (for all other drugs) than the IC50 values of the nonleukemic DS and non-DS LCLs, which was expected since leukemic cell lines generally demonstrate greater chemosensitivity than nonleukemic LCLs.
DS and non-DS primary fibroblast cell lines similarly demonstrated no significant differences in LD10 values (the drug concentration that resulted in 10% colony-forming activity) for asparaginase, doxorubicin, mafosfamide, or vincristine using the clonogenic survival assay (Figure 2). Both DS and non-DS fibroblast cell lines were resistant to dexamethasone at the highest concentration tested.
4. Discussion
We report no significant differences in cytotoxicity between DS and non-DS LCLs and primary fibroblasts exposed to common ALL chemotherapeutic agents. These results do not support the hypothesis that increased treatment-related toxicity in DS ALL occurs due to disproportionate cytotoxic effects on host somatic cells. While two prior studies have reported on the chemosensitivity of DS leukemic blasts (8, 9), only one examined the chemosensitivity of nonleukemic DS versus non-DS cells (8), which is the relevant cell population to examine in studying the basis for increased host toxicity. Zwaan et al. examined primary peripheral blood mononuclear cells, and found no significant difference in sensitivity to vincristine and daunorubicin, and resistance at all concentrations tested for prednisolone and asparaginase, as well as several other chemotherapeutic agents which were not examined in the present study since they are not employed in front-line ALL induction and intensification regimens (8). Our findings confirm the lack of significant differences in chemosensitivity between DS and non-DS nonleukemic cells, and extend this finding to fibroblast as well as lymphoid cells. IC50 values were generally higher for nonleukemic versus leukemic cell lines in the present study, although unlike in Zwaan et al., drug resistance was not observed except for dexamethasone, likely due to the fact that LCLs are typically more chemosensitive in vitro than primary lymphoblasts. The finding of dexamethasone resistance is not surprising since steroids do not exert traditional cytotoxic effects.
The increased treatment-related toxicities observed in patients with DS ALL may be attributable to one or more alternative factors which were not addressed in the present study: differences in drug pharmacokinetics; differential effects of particular chemotherapy combinations; and/or intrinsic differences in the DS host immune system and mucosal barrier (reviewed in (10)), which occur throughout the genome (11) and are largely independent of the chemotherapeutic agents administered. It is possible that specific DS tissues which were not tested in the present study display differential toxicity; however, lymphoblasts and fibroblasts are the only normal tissues from which permanent cell lines are available for study. It is also possible that susceptibility to treatment-related toxicities shows substantial variation among individuals with DS, and testing of cell lines derived from specific DS ALL patients known to have experienced severe toxicities could demonstrate effects which were missed in the present study. In vitro studies cannot adequately address all of these complex factors. Additional clinical and translational studies are necessary to tease out the factors responsible for increased toxicity in DS ALL and aid in attempts to maximize therapeutic potential while minimizing excess toxicity.
Acknowledgments
Mariela Valle was supported by a National Institutes of Health Training Grant (Grant GM069234), and Karen Rabin by a National Institutes of Health Pediatric Oncology Clinical Research Training Grant (Grant CA90433-06) and by the Children’s Cancer Research Fund. We gratefully acknowledge Drs. John Belmont, Stacey Berg, Susan Blaney, and Terzah Horton for provision of reagents and access to equipment; and the Baylor College of Medicine SMART PREP program faculty and scholars for their comments and support.
Footnotes
The authors declare no conflict of interest.
Contributions
M.V. performed experiments and participated in data analysis and writing of the manuscript. S.P. participated in experimental design, data analysis, and writing of the manuscript. K.R. participated in experimental conception and design, data analysis, and writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitlock JA. Down syndrome and acute lymphoblastic leukaemia. Br J Haematol. 2006 Dec;135(5):595–602. doi: 10.1111/j.1365-2141.2006.06337.x. [DOI] [PubMed] [Google Scholar]
- 2.Taub JW, Ge Y. Down syndrome, drug metabolism and chromosome 21. Pediatr Blood Cancer. 2005 Jan;44(1):33–9. doi: 10.1002/pbc.20092. [DOI] [PubMed] [Google Scholar]
- 3.Gamis AS. Acute myeloid leukemia and Down syndrome evolution of modern therapy--state of the art review. Pediatr Blood Cancer. 2005 Jan;44(1):13–20. doi: 10.1002/pbc.20207. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 5.Horton TM, Gannavarapu A, Blaney SM, D’Argenio DZ, Plon SE, Berg SL. Bortezomib interactions with chemotherapy agents in acute leukemia in vitro. Cancer Chemother Pharmacol. 2006 Jul;58(1):13–23. doi: 10.1007/s00280-005-0135-z. [DOI] [PubMed] [Google Scholar]
- 6.Hisama FM, Chen YH, Meyn MS, Oshima J, Weissman SM. WRN or telomerase constructs reverse 4-nitroquinoline 1-oxide sensitivity in transformed Werner syndrome fibroblasts. Cancer Res. 2000 May 1;60(9):2372–6. [PubMed] [Google Scholar]
- 7.Munshi A, Hobbs M, Meyn RE. Clonogenic cell survival assay. In: Blumenthal RD, editor. Methods in molecular medicine. Totowa, N.J: Humana Press; 2005. pp. 21–8. [DOI] [PubMed] [Google Scholar]
- 8.Zwaan CM, Kaspers GJ, Pieters R, Hahlen K, Janka-Schaub GE, Van Zantwijk CH, et al. Different drug sensitivity profiles of acute myeloid and lymphoblastic leukemia and normal peripheral blood mononuclear cells in children with and without Down syndrome. Blood. 2002 Jan 1;99(1):245–51. doi: 10.1182/blood.v99.1.245. [DOI] [PubMed] [Google Scholar]
- 9.Frost BM, Gustafsson G, Larsson R, Nygren P, Lonnerholm G. Cellular cytotoxic drug sensitivity in children with acute leukemia and Down’s syndrome: an explanation to differences in clinical outcome? Leukemia. 2000 May;14(5):943–4. doi: 10.1038/sj.leu.2401753. [DOI] [PubMed] [Google Scholar]
- 10.Ugazio AG, Maccario R, Notarangelo LD, Burgio GR. Immunology of Down syndrome: a review. Am J Med Genet Suppl. 1990;7:204–12. doi: 10.1002/ajmg.1320370742. [DOI] [PubMed] [Google Scholar]
- 11.Sommer CA, Pavarino-Bertelli EC, Goloni-Bertollo EM, Henrique-Silva F. Identification of dysregulated genes in lymphocytes from children with Down syndrome. Genome. 2008 Jan;51(1):19–29. doi: 10.1139/g07-100. [DOI] [PubMed] [Google Scholar]