Abstract
We have identified a small library of novel substituted 9-aminoacridine derivatives that inhibit cell proliferation of pancreatic cancer cell lines by inducing apoptosis. (Goodell, J.R. et al., 2008. J. Med. Chem. 51, 179–182.). To further investigate their antiproliferative activities, we have assessed the antiproliferative activity of these acridine-based compounds against several pancreatic cancer cell lines. All four compounds used in this study inhibited the proliferation of pancreatic cancer cell lines in vitro. In addition, we have employed a xenograft tumor model and found that these compounds also inhibit the proliferation of pancreatic cancer in vivo. In light of the potential importance of the anticancer activity of these acridine-based compounds, we have conducted a series of biochemical assays to determine the effect of these compounds on human topoisomerase II. Unlike amsacrine, these compounds do not poison topoisomerase II. Similar to amsacrine, however, these compounds intercalate into DNA in a way that they would alter the apparent topology of the DNA substrate. Thus, inhibition of the relaxation activity of topoisomerase II by these compounds has been reexamined using a DNA strand passage assay. We have found that these compounds, indeed, inhibit the catalytic activity of topoisomerase II. Thus, these novel acridine-based compounds with anti-pancreatic cancer activity are catalytic inhibitors, not poisons, of human topoisomerase II.
Index words: acridine derivatives, anticancer drug, cancer, catalytic inhibitor, DNA intercalation, topoisomerase
1. Introduction
Topoisomerases are responsible for controlling the topology of DNA and play critical roles in DNA metabolism (Champoux, 2001; Wang, 2002). Their function is essential especially during DNA replication and chromosome segregation. There are two classes of topoisomerases, type I and type II (Champoux, 2001; Wang, 2002). Type I topoisomerases, such as human topoisomerase I, alter the linking number in steps of one by breaking one strand of duplex DNA, passing the other strand through the break, and then resealing the broken strand. Type II enzymes, such as the α and β forms of human topoisomerase II, alter the linking number in steps of two by breaking both DNA strands, passing another segment of duplex DNA through the break, and then resealing the broken strands. The importance of topoisomerases is underscored by the fact that they are the cellular targets of clinically important anticancer and antibacterial drugs (Li and Liu, 2001; Froelich-Ammon and Osheroff, 1995; Walker, and Nitiss, 2002; Reece and Maxwell, 1991; Levine et al., 1998; Drlica and Malik, 2003). For instance, human topoisomerase II is the target of several anticancer drugs, including etoposide, amsacrine, and doxorubicin, whereas two bacterial type II topoisomerases, DNA gyrase and topoisomerase IV, are the targets of quinolone antibacterial drugs, such as ciprofloxacin.
Topoisomerases break and rejoin DNA strands by forming a covalent linkage between the enzyme and the DNA at the site of strand breakage (Champoux, 2001; Wang, 2002). This covalent topoisomerase-DNA complex (often referred to as the ‘cleavage complex’) is normally a fleeting catalytic intermediate. The steady-state level of the cleavage complex depends on the cleavage-religation equilibrium, which is equivalent to the ratio of the cleavage and religation rates. If the equilibrium is shifted by a topoisomerase inhibitor to either stimulate strand cleavage or inhibit religation, the cleavage complex can persist on the DNA, as if the topoisomerase was trapped in a topoisomerase-drug-DNA ternary complex. This class of topoisomerase inhibitors, which includes etoposide, amsacrine, doxorubicin, and ciprofloxacin, is often referred to as ‘topoisomerase poisons’, because of their unique mode of action (Li and Liu, 2001; Froelich-Ammon and Osheroff, 1995; Walker, and Nitiss, 2002). The cytotoxicity of topoisomerase poisons is associated with the inhibition of DNA replication and the generation of double-strand breaks. Topoisomerase II poison-induced double-strand breaks cause chromosome rearrangements and are likely to be the etiology of therapy-related cancers (Larson et al., 1996; Broeker, 1996; Felix, 1998; Azarova et al., 2007). Thus, topoisomerase II poisons are inherently cytotoxic and mutagenic. Topoisomerase II poisons target both the α and β forms of human topoisomerase II (Cornarotti et al., 1996; Willmore, 1998). However, the two isoforms of human topoisomerase II are regulated differently. It is the α form of topoisomerase II that is expressed in proliferating and tumor cells (Capranico et al., 1992; Tsutsui et al., 1993; Watanabe et al., 1994; Turley et al., 1997) and serves as a proliferation marker for cancer, including several types of pancreatic cancer (Iacobuzio-Donahue et al., 2002; Cao et al., 2005).
The second class of topoisomerase-targeting anticancer drugs, termed the catalytic inhibitors, function by inhibiting DNA binding and/or the catalytic cycle of topoisomerases (Larsen et al., 2003). Unlike topoisomerase poisons, catalytic inhibitors do not generate double-strand breaks and tend to exhibit lower cytotoxicity. A major class of catalytic inhibitors of bacterial type II topoisomerases includes novobiocin and coumermycin A1, which inhibit topoisomerase activity by preventing ATP binding (Reece and Maxwell, 1991; Levine et al., 1998; Drlica and Malik, 2003). Most of these inhibitors have relatively low potency against eukaryotic topoisomerases. More potent catalytic inhibitors of human topoisomerase II include aclarubicin, an anthracycline that prevents binding of the enzyme to DNA, and merbarone, which inhibits DNA strand cleavage by the enzyme (Larsen et al., 2003; Sorensen et al., 1992). The bisdioxopiperazine family of anticancer drugs, such as ICRF-193, function as catalytic inhibitors of topoisomerase II in a unique fashion (Roca et al., 1994; Classen et al., 2003). During the course of the topoisomerase II reaction, the enzyme forms a non-covalent closed clamp around the DNA, where both DNA strands remain intact. ATP binding is required to form a closed clamp with topoisomerase II, and ATP hydrolysis induces a conformational change that leads to reopening of the clamp. Bisdioxopiperazines inhibit the reopening of the closed clamp by inhibiting ATP hydrolysis. Thus, bisdioxopiperazines inhibit human topoisomerase II activity by sequestering topoisomerase II in the closed clamp conformation on the chromosome (Roca et al., 1994; Classen et al., 2003).
Although multiple histological subtypes of pancreatic cancer have been described, the most common and deadliest form of pancreatic cancer is pancreatic ductal adenocarcinoma (Shore et al., 2003; Jemal et al., 2006, 2007). Advances in understanding the genetic alterations in pancreatic cancer, especially ductal adenocarcinoma, over the past decade have contributed to a better insight into the diverse processes leading to the development of this cancer. The fact remains, however, that pancreatic cancer claims approximately 30,000 lives in the United States every year and that five-year survival rates for patients suffering from this cancer are still less than 5% (Jaffee et al., 2002; Fernandez-Zapico et al., 2005). This is due largely to the lack of early detection and the highly aggressive nature of pancreatic cancer. Thus, there is a great need for the development of a method of early diagnosis and for better therapeutic interventions.
In our previous studies, we found that novel substituted 9-aminoacridine derivatives could inhibit cell proliferation of pancreatic cancer cell lines by inducing apoptosis (Goodell et al., 2008). We continued our investigation on the anti-pancreatic cancer activity of the acridine-based compounds. These compounds inhibited the proliferation of pancreatic cell lines in vitro. Furthermore, xenografts of SU86.86 pancreatic cancer cells demonstrated that these compounds could inhibit the proliferation of pancreatic cancer in vivo. Because of the potential importance of the anticancer activity of our acridine-based compounds, we decided to conduct biochemical studies to determine the effect of these compounds on human topoisomerase II. We found that these compounds intercalated into DNA. Because intercalation could alter the apparent topology of the DNA substrate in the standard topoisomerase assays, the effect of these compounds needed to be reexamined. A DNA strand passage assay showed that these compounds could inhibit the catalytic activity of human topoisomerase II, but they did not stimulate the formation of covalent topoisomerase II-DNA complexes. Thus, the acridine-based compounds acted as catalytic inhibitors, but not poisons, of human topoisomerase II.
2. Materials and methods
2.1. Chemicals, DNAs, and proteins
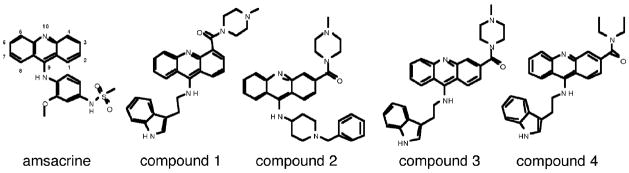
The synthesis of the 9-aminoacridine compounds has been previously described (Goodell et al., 2006) and the structures of the four acridine-based compounds used in this study are shown in Fig. 1. Amsacrine and etoposide were purchased from Sigma-Aldrich (St. Louis, MO).
Fig. 1. Structures of novel acridine-acridine based compounds with anti-pancreatic cancer activity.
Structures of the four acridine-based compounds used in this study are shown along with amsacrine.
Negatively supercoiled plasmid pBR322 DNA was purchased from New England Biolabs (Beverly, MA). Relaxed plasmid DNA was prepared by incubating negatively supercoiled pBR322 DNA with human topoisomerase I, which was purchased from Topogen (Port Orange, FL). The α form of human topoisomerase II was purchased from both Topogen and USB Corporation (Cleveland, OH).
2.2. Cell proliferation assay
Cell proliferation was measured in the presence of varying concentrations of the indicated drugs over a 72-hour time period using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for cell viability quantification. The MTT assay was carried out as described previously (Ougolkov et al., 2005).
2.3. Xenograft tumor model
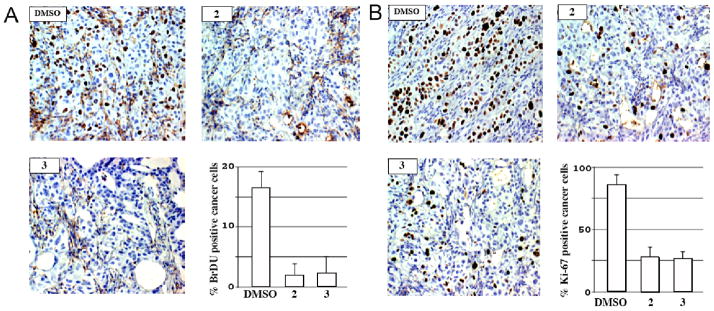
Eight-10-week-old female athymic nude mice were inoculated subcutaneously with 3 × 106 SU86.86 pancreatic cancer cells mixed with Matrigel (BD Biosciences). Two weeks after injection, established SU86.86 xenografts (tumor volume 350–400 mm3) were treated by intraperitoneal injections with diluent (DMSO), compound 2 (5 mg/kg) or compound 3 (10 mg/kg) every 12 h for 2 days and subsequently injected with Br-dU (1 mg) 3 h prior to sacrifice and tumor harvest. Tumors were subsequently removed from the mice, fixed in paraformaldehyde and paraffin-embedded. Tumor sections were analyzed for proliferating tumor cells by performing immunohistochemical staining for either BrdU or the nuclear-associated proliferation antigen, Ki-67 as described previously (Fernandez-Zapico et al., 2005). The animal studies were approved by the Institutional Animal Care and Use Committee.
2.4. DNA unwinding assay
A DNA unwinding assay (Fortune and Osheroff, 1998) was used to assess the ability of the acridine-based compounds to intercalate into plasmid DNA. This assay monitors the extent of intercalation of a drug as a function of the conversion of relaxed DNA into negatively supercoiled DNA.
Reaction mixtures (20 μl) containing 50 mM Tris-HCl (pH 7.5 at 23°C), 2.5 mM MgCl2, 0.5 mM DTT, 50 μg/ml BSA, 300 ng of relaxed plasmid DNA, and 2 units of human topoisomerase I were incubated at 37°C for 10 min in the absence or presence of the indicated concentrations of the various drugs. Reactions were terminated by extraction with phenol/chloroform/isoamyl alcohol (25:24:1, v/v) and the DNA products were analyzed by electrophoresis through vertical 1.2% agarose (BioWhittaker Molecular Applications; Rockland, ME) gels (14 × 10 × 0.3 cm) at 2 V/cm for 15 h in TAE buffer [50 mM Tris-HCl (pH 7.9 at 23°C), 40 mM sodium acetate and 1 mM EDTA]. Gels were stained with ethidium bromide and photographed using an Eagle Eye II system. (Stratagene; La Jolla, CA).
2.5. Strand passage assay
To accurately assess the effects of the acridine-based compounds on the catalytic activity of topoisomerase II, a DNA strand passage assay (Fortune et al., 1999) was used, instead of a standard relaxation assay using negatively supercoiled plasmid DNA as substrate. The DNA strand passage assay allows us to measure the catalytic activity of topoisomerase II as a function of the conversion of relaxed DNA into negatively supercoiled DNA.
Reaction mixtures (20 μl) containing 50 mM Tris-HCl (pH 8 at 23 °C), 10 mM MgCl2, 200 mM potassium glutamate, 10 mM DTT, 50 μg/ml BSA, 1 mM ATP, 300 ng of relaxed plasmid DNA, 2 units of topoisomerase II (the specific activities of the two preparations of topoisomerase II were essentially identical under these conditions), and the indicated concentrations of the various drugs were incubated at 37°C for 10 min. SDS was added to a concentration of 1% to terminate the catalytic reaction, and the reaction mixtures were further incubated at 37°C for 5 min. EDTA and proteinase K were then added to 25 mM and 100 μg/ml, respectively, and the incubation was continued for an additional 15 min at 37°C. The DNA products were then processed and analyzed as described above.
2.6. DNA cleavage assay
A DNA cleavage assay for topoisomerase II was performed according to a protocol similar to that described previously (Corbett et al., 1991; Pfeiffer and Hiasa, 2007). Topoisomerase II-catalyzed cleavage reaction mixtures (20μl) contained 50 mM Tris-HCl (pH 8 at 23 °C), 10 mM MgCl2, 10 mM DTT, 50 μg/ml BSA, 1 mM ATP, 300 ng of relaxed plasmid DNA, 6 units of topoisomerase II, and the indicated concentrations of various drugs. Reaction mixtures were incubated at 37°C for 10 min. SDS was added to a concentration of 1%, and the reaction mixtures were further incubated at 37°C for 5 min. EDTA and proteinase K were then added to 25 mM and 100 μg/ml, respectively, and the incubation was continued for an additional 60 min at 50°C. The DNA products were purified by extraction with phenol/chloroform/isoamyl alcohol (25:24:1, v/v) and then analyzed by electrophoresis through vertical 1.2% agarose gels (14 × 10 × 0.3 cm) at 2 V/cm for 15 h in TAE buffer that contained 0.5 μg/ml ethidium bromide. After destaining in water, gels were photographed and quantified using an Eagle Eye II system.
3. Results
3.1. The acridine-based compounds exhibit an anti-pancreatic cancer activity in vivo
We assessed the antiproliferative activity of the acridine-based compounds using a panel of pancreatic cancer cell lines. All four compounds exhibited antiproliferative activities (in the low μM level) against all cell lines used in this study (Table 1). Although there was a variation in the response of the various pancreatic cancer cell lines to these compounds, compound 2 was the most effective among the four compounds. We found the IC50 values of amsacrine against HupT3, Panc04.03, and SU86.86 to be 7.4 μM, 34.1 μM, and 48.3 μM, respectively. Thus, our compounds appeared to be more effective than amsacrine.
Table 1.
Inhibition of pancreatic cancer cell lines by the acridine-based compounds.a
| Compound |
||||
|---|---|---|---|---|
| Cell Line | 1 | 2 | 3 | 4 |
| BXPC-3 | 14±4 | 7±1 | 12±1 | 13±1 |
| HupT3 | 25±3 | 10±2 | 11±1 | 11±3 |
| MiaPaCa2 | 7±3 | 1±0.04 | 4±1 | 7±2 |
| Panc04.03 | 12±3 | 6±1 | 9±2 | 10±2 |
| SU86.86 | 11±1 | 6±1 | 9±1 | 12±2 |
IC50’s (μM) were determined from the MTT assay, which was performed in triplicate.
The above data showed that the acridine-based compounds affected pancreatic cancer cell proliferation in vitro. However, whether these compounds could affect tumor cell proliferation in vivo was not tested. We, therefore, established subcutaneous flank xenografts of SU86.86 pancreatic cancer cells in female athymic nude mice and examined the effect of compound 2 and compound 3 on tumor cell proliferation in vivo. Tumor sections were analyzed for proliferating tumor cells by performing immunohistochemical staining for either BrdU or the nuclear-associated proliferation antigen, Ki-67 (Fig. 2). Consistent with the results of the MTT assay (Table 1), administration of either compound 2 or compound 3 resulted in a significant loss of BrdU+ and Ki-67+ tumor cells. These results demonstrated that compound 2 and compound 3 could inhibit the proliferation of pancreatic cancer in vivo. However, only about one half the concentration of compound 2 relative to compound 3 was required to obtain similar antiproliferative effects (Fig. 2), suggesting that compound 2 was more effective than compound 3. Thus, our acridine-based compounds exhibited an anti-pancreatic cancer activity both in vitro and in vivo.
Fig. 2. In vivo inhibition of pancreatic cancer cell proliferation.
Representative sections from SU86.86 xenografts treated with diluent (DMSO), compound 2, or compound 3 and stained for either BrdU (Panel A) or Ki-67 (Panel B). One hundred tumor cells were counted per microscopic field. The percentage of positively stained cells from 5 randomly selected fields is graphically depicted. 2, compound 2; 3, compound 3.
3.2. The acridine-based compounds can intercalate into DNA
If these compounds are intercalative drugs, similar to amsacrine, the apparent inhibition of human topoisomerase II we initially observed in the standard relaxation assay (Goodell et al., 2006) may not be a direct effect on enzyme catalysis. Instead, these compounds may interfere with the DNA relaxation reaction by altering the apparent topological state of the negatively supercoiled DNA substrate. Thus, we conducted a series of biochemical assays to determine the abilities of our acridine-based compounds to intercalate into DNA and inhibit the catalytic activity of topoisomerase II.
The ethidium bromide displacement assay showed the DNA binding affinities of the acridine-based compounds (Goodell et al., 2006). The C50 values, the concentration of a compound required to reduce the enhancement in fluorescence by 50%, of compounds 1, 2, 3, and 4 were 26 μM, 2 μM, 11 μM, and 60 μM, respectively. These results showed that all four compounds could bind to DNA with different affinities: compound 2 and compound 4 exhibited the highest and the lowest DNA binding affinities, respectively.
The measurement of the DNA binding affinity does not reveal the effect of the intercalation on the topological state of the DNA molecules. Thus, we employed a DNA unwinding assay using relaxed plasmid DNA and human topoisomerase I. An intercalative agent induces constrained negative supercoils and compensatory unconstrained positive supercoils in covalently closed circular DNA. Treatment of an intercalated plasmid with human topoisomerase I removes the unconstrained positive supercoils. Subsequent extraction of the intercalative agent allows the constrained negative supercoils to be redistributed throughout the DNA molecule and converts the relaxed DNA into negatively supercoiled DNA (Fortune and Osheroff, 1998). Thus, the DNA unwinding assay is designed to directly measure the extent of the drug-induced negative supercoils.
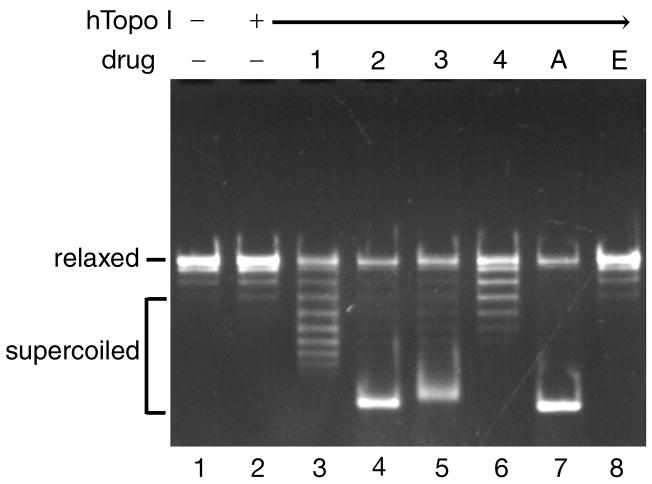
We performed the DNA unwinding in the presence of various concentrations of the acridine-based compounds. Amsacrine and etoposide were used as controls. Representative results of such assays in the presence of 100 μM drugs are shown in Fig. 3. In the presence of compound 2, compound 3, or amsacrine, relaxed plasmid DNA was converted into fully supercoiled DNA (Fig. 3, lanes 4, 5, and 7, respectively). In contrast, only limited numbers of negative supercoils were introduced when either compound 1 or compound 4 was used (Fig. 3, lanes 3 and 6, respectively). As expected, the topology of DNA did not change in the presence of etoposide, a non-intercalative topoisomerase II poison (Fig. 3, lane 8). These results showed that all four acridine-based compounds could intercalate into DNA and induce different levels of constrained negative supercoils. It should be noted that the DNA binding affinities of the four compounds (compound 2> compound 3> compound 1> compound 4) correlated well with the levels of negative supercoils induced by these compounds. A strand passage assay for human topoisomerase I showed that the acridine-based compounds did not affect the catalytic activity of topoisomerase I (data not shown).
Fig. 3. The acridine-based compounds intercalate into DNA.
The DNA unwinding reaction mixtures contained 0.3 μg of relaxed plasmid DNA, 2 units of human topoisomerase I, and 100 μM of the various drugs. Two independent experiments for each assay exhibited essentially identical results; representative results are shown. Drugs are denoted as follows: 1, compound 1; 2, compound 2; 3, compound 3; 4, compound 4; A, amsacrine; and E, etoposide.
3.3. The acridine-based compounds inhibit the catalytic activity of human topoisomerase II
As discussed above, intercalation alters the apparent topology of the plasmid DNA and could make the standard relaxation assay problematic. In the standard relaxation assay using negatively supercoiled DNA as the substrate, the plasmid may appear to remain supercoiled in the presence of an intercalator not because the intercalator inhibits the catalytic activity of the topoisomerase, but because the DNA relaxed by the topoisomerase is re-supercoiled as a result of the action of the topoisomerase in the presence of the intercalator (Fortune and Osheroff, 1998; Fortune et al., 1999). Thus, we decided to reevaluate the effects of the acridine-based compounds on the catalytic activity of human topoisomerase II using a strand passage assay. The assessment of the inhibitory effect of etoposide on the catalytic activity of human topoisomerase II showed that the etoposide sensitivity of the topoisomerase in the strand passage assay was identical to that in the standard relaxation assay using negatively supercoiled DNA as the substrate (data not shown), indicating that these two assays are equivalent in measuring the drug sensitivity of human topoisomerase II.
The strand passage assay measures the ability of topoisomerase II to convert relaxed plasmid DNA into negatively supercoiled DNA in the presence of intercalative agents (Fortune et al., 1999). Comparison of the levels of supercoils introduced by the human topoisomerase I-catalyzed DNA unwinding reaction (Figs. 4A and 5A) with those of the human topoisomerase II-catalyzed strand passage reaction (Figs. 4B and 5B) allowed us to assess the effects of the acridine-based compounds on the catalytic activity of human topoisomerase II. If a drug does not affect the catalytic activity of human topoisomerase II, as was the case with human topoisomerase I, human topoisomerase II could remove all unconstrained positive supercoils. As a result, the topoisomerase II-catalyzed strand passage reaction would produce the same level of negative supercoiling as that introduced by the topoisomerase I-catalyzed DNA unwinding reaction. On the other hand, if a drug inhibits the catalytic activity of human topoisomerase II, it could not efficiently remove the unconstrained positive supercoils. As a result of the inhibition, the DNA substrate would either remain relaxed (due to the complete inhibition of topoisomerase II activity by the compound) or become supercoiled at a significantly lower level than that introduced by the topoisomerase I-catalyzed DNA unwinding reaction.
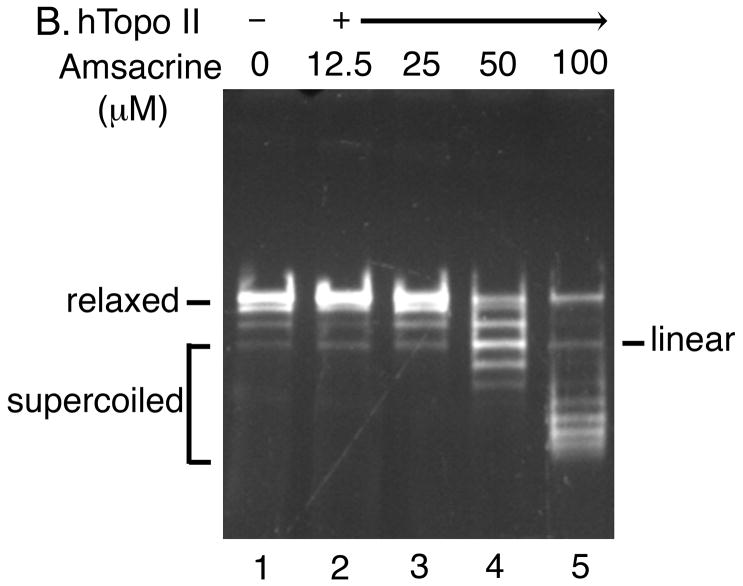
Fig. 4. Determination of the effect of amsacrine on human topoisomerase II catalytic activity using the strand passage assay.
Both the DNA unwinding assay for human topoisomerase I (Panel A) and the strand passage assay for human topoisomerase II (Panel B) were performed in the presence of indicated concentrations of amsacrine.
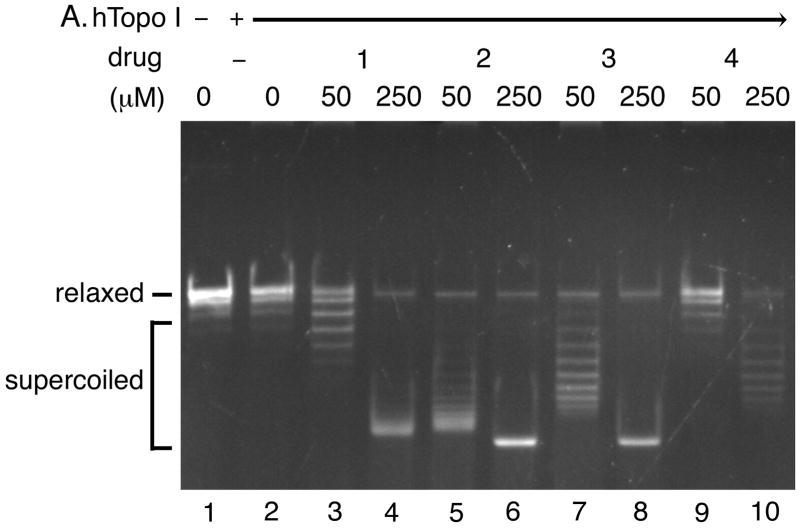
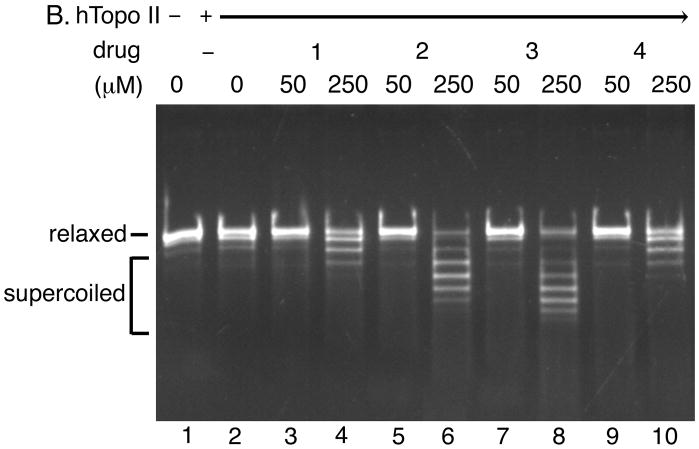
Fig. 5. The acridine-based compounds inhibit the catalytic activity of human topoisomerase II.
The DNA unwinding assay for human topoisomerase I (Panel A) and the strand passage assay for human topoisomerase II (Panel B) were performed in the presence of indicated concentrations of the acridine-based compounds. Three independent experiments for each assay exhibited essentially identical results; representative results are shown. Abbreviations are as indicated in the legend to Fig. 3.
First, we performed control experiments using amsacrine (Fig. 4). As expected, in the presence of amsacrine, the level of supercoiling produced by human topoisomerase II (Fig. 4B) was significantly lower than that produced by human topoisomerase I (Fig. 4A). In addition, linear DNA was generated as a result of the poisoning of human topoisomerase II by amsacrine (Fig. 4B). These results demonstrated that amsacrine could inhibit the catalytic activity of human topoisomerase II.
Next, we assessed the effects of the acridine-based compounds on the catalytic activity of human topoisomerase II (Fig. 5). All four compounds exhibited inhibitory effects on the human topoisomerase II activity. Based on the differences between the levels of supercoils introduced by the human topoisomerase I-catalyzed DNA unwinding reaction (Fig. 5A) and those of the human topoisomerase II-catalyzed strand passage reaction (Fig. 5B), compound 2 (lanes 5 and 6) and compound 3 (lanes 7 and 8) appeared to inhibit the catalytic activity of human topoisomerase II more effectively than compound 1 (lanes 3 and 4) and compound 4 (lanes 9 and 10). Two preparations of human topoisomerase II exhibited essentially identical sensitivities to the acridine-based compounds (data not shown). These results showed that our acridine-compounds were, in fact, catalytic inhibitors of human topoisomerase II. These results also indicated that the ability of a compound to intercalate into DNA and induce constrained negative supercoils (Figs. 3 and 5A) might correlate well with the effectiveness of the compound in inhibiting the catalytic activity of human topoisomerase II (Fig. 5B).
3.4. The acridine-based compounds do not poison human topoisomerase II
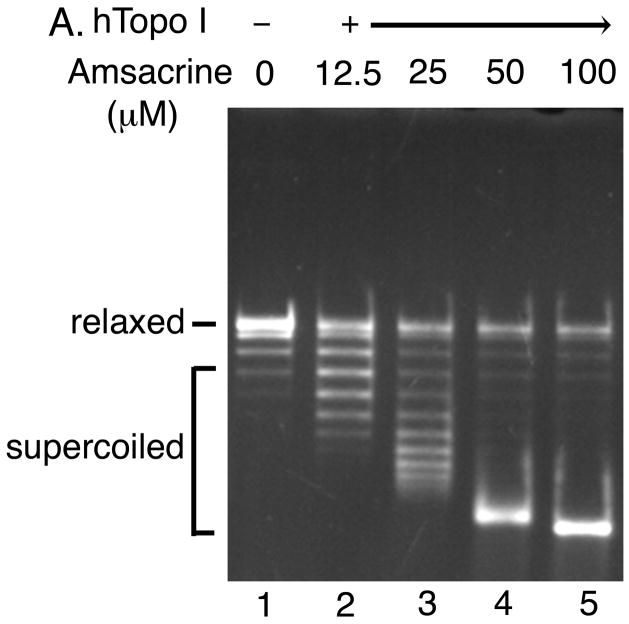
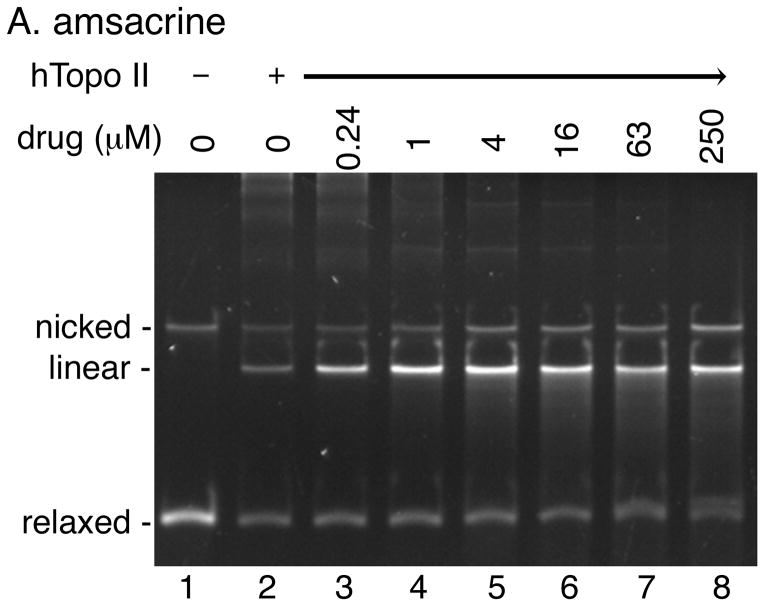
Topoisomerase poisons convert these essential enzymes into cellular poisons by forming a topoisomerase-drug-DNA ternary complex that contains a double-strand break [3–5]. Ternary complex formation causes inhibition of DNA replication, generation of double-strand breaks, and subsequent cell death. Etoposide, amsacrine, and doxorubicin are examples of topoisomerase II poisons, whereas camptothecin poisons topoisomerase I. In a DNA cleavage assay using a purified topoisomerase, addition of a topoisomerase poison would stimulate the formation of the covalent topoisomerase-DNA complexes (Li and Liu, 2001; Froelich-Ammon and Osheroff, 1995; Walker, and Nitiss, 2002; Corbett wt al., 1991). The intercalating topoisomerase II poisons, such as amsacrine, show a bimodal activity in the cleavage assay: a dose dependent increase in cleavage up to a certain concentration and then a progressive loss of the cleavage at higher drug concentrations.
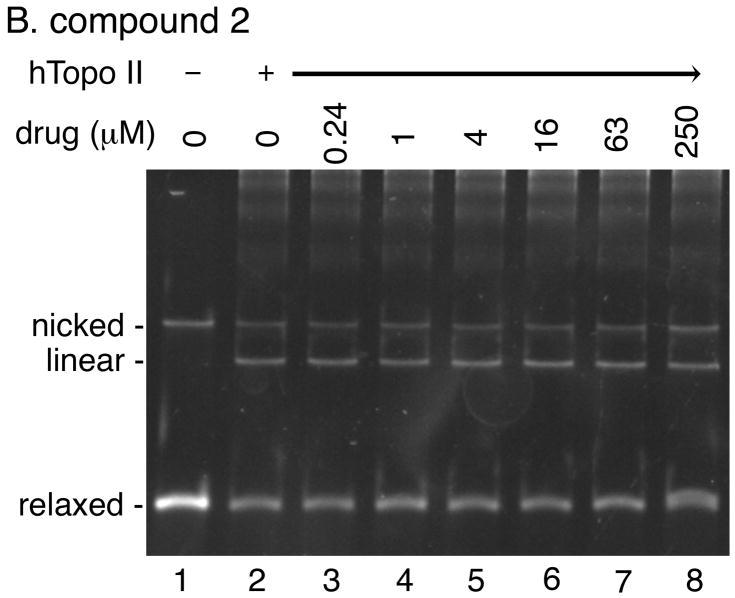
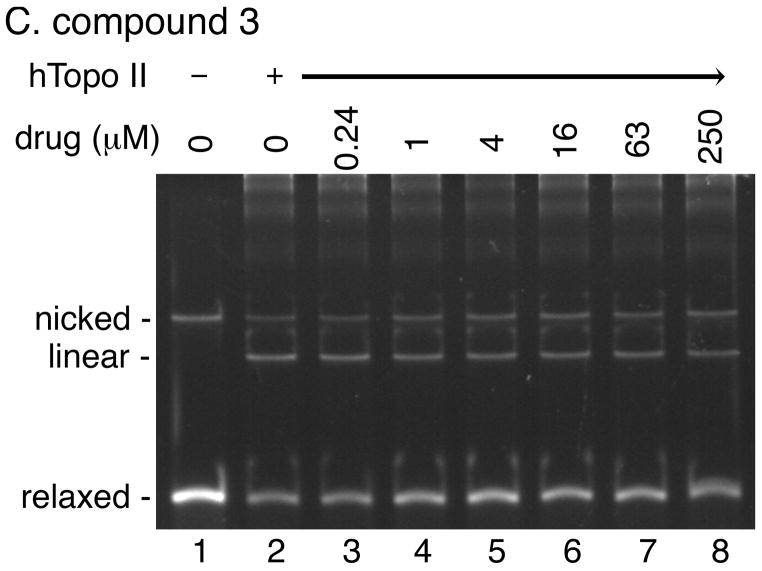
We performed a DNA cleavage assay using relaxed plasmid DNA as the substrate to quantify the overall DNA cleavage activity of human topoisomerase II in the presence of various concentrations of our acridine-based compounds (Fig. 6). If any of the compounds can poison human topoisomerase II, the presence of the compound would stimulate the generation of linear DNA molecules. As expected, amsacrine stimulated human topoisomerase II-catalyzed cleavage of DNA in a bimodal manner (Fig. 6A). In contrast, no stimulation of the DNA cleavage activity of human topoisomerase II was observed at a wide range of concentrations of any of the four compounds (Fig. 6; data not shown). These results demonstrated that our acridine-based compounds did not act as topoisomerase II poisons.
Fig. 6. The acridine-based compounds do not poison human topoisomerase II.
DNA cleavage activity of human topoisomerase II (6 units per reaction) was measured in the presence of the indicated concentrations of the various drugs using relaxed plasmid DNA (0.3 μg) as the substrate. Two independent experiments for each assay exhibited essentially identical results; representative results are shown. Lane 1 in all panels shows the DNA substrate.
4. Discussion
Acridines and their derivatives have been successfully used as chemotherapeutic agents against bacteria, protozoa and fungi, and cancer (Larsen et al., 2003; Denny, 2004; Ferguson and Denny, 2007). In general, this class of compounds intercalates into DNA and exhibits various mutagenic activities in both bacterial and mammalian cells. The cytotoxicity of amsacrine, an acridine with anticancer activity, is primarily caused by its ability to poison human topoisomerase II (Li and Liu, 2001; Froelich-Ammon and Osheroff, 1995; Walker, and Nitiss, 2002). In addition to stimulating covalent topoisomerase II-DNA complex formation, amsacrine also inhibits the catalytic activity of topoisomerase II.
We showed here that the novel acridine-based compounds (Fig. 1) exhibited the antiproliferative activity against pancreatic cancer both in vitro (Table 1) and in vivo (Fig. 2). The DNA unwinding assay demonstrated the ability of these compounds to intercalate into DNA and induce constrained negative supercoils (Fig. 3). The extent of the drug-induced negative supercoils showed that compound 2 and compound 3 could intercalate into DNA more effectively and/or more stably than compound 1 and compound 4 could. These results correlate well with the DNA binding affinities of these compounds (compound 2> compound 3> compound 1> compound 4) measured by the ethidium bromide displacement assay (Goodell et al., 2006).
Intercalative agents could make standard topoisomerase assays, especially the relaxation assay, problematic because intercalation may alter the superhelicity of the plasmid DNA substrate. For instance, TAS-103, an intercalator with anticancer activity, was originally shown to not only poison human topoisomerase II but also act as a catalytic inhibitor of human topoisomerase I (Utsugi et al., 1997). Subsequent studies have revealed, however, that the apparent inhibition of topoisomerase I-catalyzed relaxation resulted from a drug-induced alteration in the topology of the plasmid DNA substrate and demonstrated that TAS-103 functions only as a topoisomerase II poison (Fortune et al., 1999). Thus, although our initial investigation indicated that the acridine-based compounds appeared to inhibit the relaxation activity of human topoisomerase II (Goodell et al., 2006), we needed to reexamine the effects of these compounds on the catalytic activity of human topoisomerase II. We found that all four compounds were able to inhibit the catalytic activity of human topoisomerase II (Fig. 5).
What is the mechanism of inhibition of topoisomerase II activity by the acridine-based compounds? The majority of intercalative topoisomerase II inhibitors function as topoisomerase II poisons by trapping topoisomerase II on DNA as a cleavage complex (Li and Liu, 2001; Froelich-Ammon and Osheroff, 1995; Walker, and Nitiss, 2002). Exceptions include aclarubicin, which acts as a catalytic inhibitor by preventing the binding of topoisomerase II to DNA (Sorensen et al., 1992). Our acridine-based compounds did not poison human topoisomerase II (Fig. 6) and thus the observed inhibition of topoisomerase II was not caused by the poisoning of human topoisomerase II by these compounds. Because both acridines and aclarubicin are intercalators, it is possible that our acridine-based compounds inhibit the binding of human topoisomerase II with DNA. At this point, however, we cannot rule out the possibilities that these acridine-based compounds might inhibit either ATP binding, similar to novobiocin (Reece and Maxwell, 1991; Levine et al., 1998; Drlica and Malik, 2003), or DNA cleavage, similar to merbarone (Larsen et al., 2003). Further studies are necessary to determine the exact mechanism of the inhibition of human topoisomerase II activity by these novel acridine-based compounds. It is worth noting that because our acridine-based compounds are not human topoisomerase poisons, they do not generate double-strand breaks, the etiology of chromosomal translocations and therapy-related cancers, and are likely to exhibit lower toxicity.
Some topoisomerase inhibitors, including pyrazoloacridine and nitro(imidazole/triazole)-linked acridines, have been shown to be dual inhibitors of human topoisomerase I and topoisomerase II (Adjei et al., 1998; Rosenzweig et al., 2005). Dual-targeting drugs may have clinical significance because dual-targeting could reduce the emergence of drug resistance (Denny et al., 2003), as is the case with WCK-1734, a new fluoroquinolone antibacterial drug that targets both DNA gyrase and topoisomerase IV (Strahilevitz and Hooper, 2005). We did not detect, however, any inhibitory effect of our acridine-based compounds on the catalytic activity of human topoisomerase I (data not shown). A recent study on tetra-acridine derivatives has shown that these compounds function as dual inhibitors of human topoisomerase and the human proteasome (Vispé et al., 2007). TAS-103, a human topoisomerase II poison, also targets a component of the signal recognition particle (Yoshida et al., 2008). Furthermore, some DNA intercalators inhibit DNA helicases (Brosh et al., 2000; Pham et al. 2002). Thus, it is possible that in addition to the catalytic inhibition of human topoisomerase II, interference of the functional activity of an unidentified target might contribute to the anticancer activity of our acridine-based compounds.
Acknowledgments
This work was in part supported by a Faculty Research Development Grant from the University of Minnesota Academic Heath Center (to DMF and HH), a Pilot Project Grant from the Specialized Program in Research Excellence grant P20 CA101955 (to HH), a Specialized Program in Research Excellence grant P50 CA102701 from the NCI (to DDB), a Partnership grant from the State of Minnesota (to DDB and DMF), the Mayo Foundation (to DDB), and a fund from the University of Minnesota Medical School (to HH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjei AA, Charron M, Rowinsky EK, Svingen PA, Miller J, Reid JM, Sebolt-Leopold J, Ames MM, Kaufmann SH. Effect of pyrazoloacridine (NSC 366140) on DNA topoisomerases I and II. Clin Cancer Res. 1998;4:683–691. [PubMed] [Google Scholar]
- Azarova AM, Lyu YL, Lin CP, Tsai YC, Lau JYN, Wang JC, Liu LF. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc Natl Acad Sci USA. 2007;104:11014–11019. doi: 10.1073/pnas.0704002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeker PL, Super HG, Thirman MJ, Pomykala H, Yonebayashi Y, Tanabe S, Tanabe S, Zeleznik-Le N, Rowley JD. Distribution of 11q23 breakpoints within the MLL breakpoint cluster region in de novo acute leukemia and in treatment-related acute myeloid leukemia: correlation with scaffold attachment regions and topoisomerase II consensus binding sites. Blood. 1996;87:1912–1922. [PubMed] [Google Scholar]
- Brosh RM, Jr, Karow JK, White EJ, Shaw ND, Hickson ID, Bohr VA. Potent inhibition of werner and bloom helicases by DNA minor groove binding drugs. Nucleic Acids Res. 2000;28:2420–2430. doi: 10.1093/nar/28.12.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Maitra A, Saavedra JA, Klimstra DS, Adsay NV, Hruban RH. Expression of novel markers of pancreatic ductal adenocarcinoma in pancreatic nonductal neoplasms: additional evidence of different genetic pathways. Mod Pathol. 2005;18:752–761. doi: 10.1038/modpathol.3800363. [DOI] [PubMed] [Google Scholar]
- Capranico G, Tinelli S, Austin CA, Fisher ML, Zunino F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta. 1992;1132:43–48. doi: 10.1016/0167-4781(92)90050-a. [DOI] [PubMed] [Google Scholar]
- Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- Classen S, Olland S, Berger JM. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc Natl Acad Sci USA. 2003;100:10629–10634. doi: 10.1073/pnas.1832879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett AH, Zechiedrich EL, Lloyd RS, Osheroff N. Inhibition of eukaryotic topoisomerase II by ultraviolet-induced cyclobutane pyrimidine dimers. J Biol Chem. 1991;266:19666–19671. [PubMed] [Google Scholar]
- Cornarotti M, Tinelli S, Willmore E, Zunino F, Fisher LM, Austin CA, Capranico G. Drug sensitivity and sequence specificity of human recombinant DNA topoisomerases IIα (p170) and IIβ (p180) Mol Pharmacol. 1996;50:1463–1471. [PubMed] [Google Scholar]
- Denny WA. Chemotherapeutic effects of acridine derivatives. Medicinal Chemistry Reviews-Online. 2004;1:257–266. [Google Scholar]
- Denny WA, Baguley BC. Dual topoisomerase I/II inhibitors in cancer therapy. Curr Top Med Chem. 2003;3:339–353. doi: 10.2174/1568026033452555. [DOI] [PubMed] [Google Scholar]
- Drlica K, Malik M. Fluoroquinolones: Action and resistance. Curr Top Med Chem. 2003;3:249–282. doi: 10.2174/1568026033452537. [DOI] [PubMed] [Google Scholar]
- Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400:233–255. doi: 10.1016/s0167-4781(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Ferguson LR, Denny WA. Genotoxicity of non-covalent interactions: DNA intercalatiors. Mutat Res. 2007;623:14–23. doi: 10.1016/j.mrfmmm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J Biol Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- Fortune JM, Velea L, Graves DE, Utsugi T, Yamada Y, Osheroff N. DNA Topoisomerases as Targets for the Anticancer Drug TAS-103: DNA Interactions and Topoisomerase Catalytic Inhibition. Biochemistry. 1999;38:15580–15586. doi: 10.1021/bi991792g. [DOI] [PubMed] [Google Scholar]
- Froelich-Ammon SJ, Osheroff N. Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J Biol Chem. 1995;270:21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- Goodell JR, Madhok AA, Hiasa H, Ferguson DM. Synthesis and evaluation of acridine- and acridone-based anti-herpes agents with topoisomerase activity. Bioorg Med Chem. 2006;14:5467–5480. doi: 10.1016/j.bmc.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Goodell JR, Ougolkov AV, Hiasa H, Kaur H, Remmel R, Billadeau DD, Ferguson DM. Acridine-based agents with topoisomerase II activity inhibit pancreatic cancer cell proliferation and induce apoptosis. J Med Chem. 2008;51:179–182. doi: 10.1021/jm701228e. [DOI] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Maitra A, Shen-Ong GL, van Heek T, Ashfaq R, Meyer R, Walter K, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am J Pathol. 2002;160:1239–1249. doi: 10.1016/S0002-9440(10)62551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer Statistics 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer Statistics 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Larsen AK, Escargueil AE, Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol Ther. 2003;99:167–181. doi: 10.1016/s0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Larson RA, LeBeau MM, Vardiman JW, Rowley JD. Myeloid leukemia after hematotoxins. Environ Health Perspect. 1996;104(Suppl 6):1303–1307. doi: 10.1289/ehp.961041303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine C, Hiasa H, Marians KJ. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim Biophys Acta. 1998;1400:29–43. doi: 10.1016/s0167-4781(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Li TK, Liu LF. Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen Synthase Kinase-3β Participates in Nuclear Factor B–Mediated Gene Transcription and Cell Survival in Pancreatic Cancer Cells. Cancer Res. 2005;65:2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- Pfeiffer ES, Hiasa H. Determination of the primary target of a quinolone drug and the effect of quinolone resistance-conferring mutations by measuring quinolone sensitivity based on its mode of action. Antimicrob Agents Chemother. 2007;51:3410–3412. doi: 10.1128/AAC.00362-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham XH, Tuteja N. Potent inhibition of DNA unwinding and ATPase activities of pea DNA helicase 45 by DNA-binding agents. Biochem Biophys Res Commun. 2002;294:334–339. doi: 10.1016/S0006-291X(02)00481-3. [DOI] [PubMed] [Google Scholar]
- Reece RJ, Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26:335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Roca J, Ishida R, Berger JM, Andoh T, Wang JC. Antitumor bisdioxopiperazines inhibit yeast DNA topoisomerase II by trapping the enzyme in the form of a closed protein clamp. Proc Natl Acad Sci USA. 1994;91:1781–1785. doi: 10.1073/pnas.91.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig HS, Papadopoulou MV, Bloomer WD. Interaction of strong DNA-intercalating bioreductive compounds with topoisomerases I and II. Oncol Res. 2005;15:219–231. doi: 10.3727/096504005776382288. [DOI] [PubMed] [Google Scholar]
- Shore S, Raraty MG, Ghaneh P, Neoptolemos JP. Chemotherapy for pancreatic cancer. Aliment Pharmacol Ther. 2003;18:1049–1069. doi: 10.1111/j.1365-2036.2003.01781.x. [DOI] [PubMed] [Google Scholar]
- Sorensen BS, Sinding J, Andersen AH, Alsner J, Jensen PB, Westergaard O. Mode of action of topoisomerase II-targeting agents at a specific DNA sequence. Uncoupling the DNA binding, cleavage and religation events. J Mol Biol. 1992;228:778–786. doi: 10.1016/0022-2836(92)90863-f. [DOI] [PubMed] [Google Scholar]
- Strahilevitz J, Hooper DC. Dual targeting of topoisomerase IV and gyrase to reduce mutant selection: direct testing of the paradigm by using WCK-1734, a new fluoroquinolone, and ciprofloxacin. Antimicrob Agents Chemother. 2005;49:1949–1956. doi: 10.1128/AAC.49.5.1949-1956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Tsutsui K, Okada S, Watanabe M, Shohmori T, Seki S, Inoue Y. Molecular cloning of partial cDNAs for rat DNA topoisomerase II isoforms and their differential expression in brain development. J Biol Chem. 1993;268:19076–19083. [PubMed] [Google Scholar]
- Turley H, Comley M, Houlbrook S, Nozaki N, Kikuchi A, Hickson ID, Gatter K, Harris AL. The distribution and expression of the two isoforms of DNA topoisomerase II in normal and neoplastic human tissues. Br J Cancer. 1997;75:1340–1346. doi: 10.1038/bjc.1997.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi T, Aoyagi K, Asao T, Okazaki S, Aoyagi Y, Sano M, Wierzba K, Yamada Y. Antitumor activity of a novel quinoline derivative, TAS-103, with inhibitory effects on topoisomerases I and II. Jpn J Cancer Res. 1997;88:992–1002. doi: 10.1111/j.1349-7006.1997.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vispé S, Vandenberghe I, Robin M, Annereau JP, Créancier L, Pique V, Galy JP, Kruczynski A, Barret JM, Bailly C. Novel tetra-acridine derivatives as dual inhibitors of topoisomerase II and the human proteasome. Biochem Pharmacol. 2007;73:1863–1872. doi: 10.1016/j.bcp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Walker JV, Nitiss JL. DNA topoisomerase II as a target for cancer chemotherapy. Cancer Invest. 2002;20:570–589. doi: 10.1081/cnv-120002156. [DOI] [PubMed] [Google Scholar]
- Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nature Rev. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tsutsui K, Inoue Y. Differential expressions of the topoisomerase IIα and IIβ mRNAs in developing rat brain. Neurosci Res. 1994;19:51–57. doi: 10.1016/0168-0102(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Willmore E, Frank AJ, Padget K, Tilby MJ, Austin CA. Etoposide targets topoisomerase IIα and IIβ in leukemic cells: isoform-specific cleavable complexes visualized and quantified in situ by a novel immunofluorescence technique. Mol Pharmacol. 1998;54:78–85. doi: 10.1124/mol.54.1.78. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kabe Y, Wada T, Asai A, Handa H. A new mechanism of TAS-103 action discovered by target screening with drug-immobilized affinity beads. Mol Pharmacol. 2008;73:987–994. doi: 10.1124/mol.107.043307. [DOI] [PubMed] [Google Scholar]