Abstract
The construct of craving has been central to addiction research for more than 50 years. Only recently have investigators begun to apply functional neuroimaging techniques to the study of drug cue reactivity, and a small but growing number of studies implicate a distributed system of brain regions in the pathogenesis of craving. The internal consistency of this burgeoning literature has thus far been disappointing, however, leaving open the question of which brain regions contribute to craving. Here we review neuroimaging studies of cue-elicited craving in the context of a framework drawn from behavioral research indicating that perceived drug use opportunity significantly affects responses to the presentation of drug cues. Using this framework provides a way to reconcile discrepant findings among brain-imaging studies of cue-elicited craving.
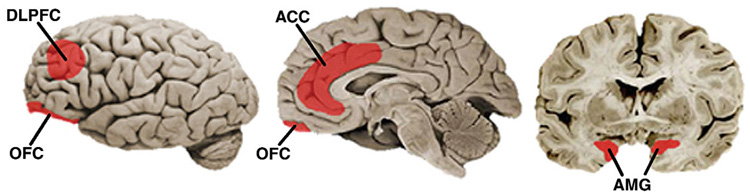
The cue-reactivity paradigm has been among the most prominent methods for investigating drug craving for the past several decades1,2. Cue reactivity involves exposing drug-addicted individuals to stimuli designed to elicit craving while assessing concomitant changes in one or more response systems (such as self-reported urge, cognitive task performance or physiological reactivity). Within the past 5 years, a rapidly growing body of functional neuroimaging studies has adopted the traditional cue-reactivity procedure as a means for elucidating the neural bases of craving. Thus far, a distributed network of brain regions has been linked to cue-elicited craving; in 19 neuroimaging studies of cue reactivity, the amygdala, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC) are the most commonly reported loci of activation (Fig. 1)3,4.
Figure 1.
Lateral (left), mid-sagittal (middle) and coronal (right) sections of the brain illustrating neural regions that have been implicated in cue-elicited craving. DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; AMG, amygdala. (Images modified from ref. 50.)
Although activation of these regions is frequently reported, the data has unfortunately been inconsistent across studies. For example, significant cue-elicited activation of the OFC—a region that has a prominent role in contemporary neurobiological models of craving5–7—has been reported by only 6 of 18 studies (OFC activity not assessed by ref. 8)9–14. Results are similarly discrepant for the amygdala (7 of 18 studies; not assessed by ref. 15)9,11,12,16–19, DLPFC (8 of 19)8,9,11,14,17,20–22 and ACC (10 of 19)8,10,12,14,16,18,20,22–24. To date, conflicting results have been largely ignored or vaguely attributed to inter-study methodological variance9,19,21,25. No framework has yet been offered that sufficiently accounts for the pattern of results observed across studies.
Contextual modulation of cue reactivity
Many of these contradictory findings may be reconciled by attending to psychological factors that affect drug craving. Recent behavioral studies have shown that cue reactivity may be modulated by the context associated with cue presentation. One contextual factor that significantly influences the response to drug cues is whether or not participants anticipate actually using the drugs to which they are being exposed (perceived drug use opportunity)26. When instructed that drugs are available for consumption during an experiment, drug users report substantially higher craving on being presented with drug cues than when instructed that drugs are not available for an extended period of time or until after the experiment has concluded27–29. Affective27,30 and cognitive processes28,31 are also differentially influenced by drug cue presentation as a function of whether or not drug use is expected. Similarly, physiological responses thought to reflect arousal, such as skin conductance2, heart rate32 and asymmetrical frontal electrocortical activity33, are heightened in contexts predictive of impending drug use. Many of these effects transfer to arbitrary stimuli (for example, colored cards) that come to be associated with the opportunity, or lack thereof, to consume32,34,35.
Effects of treatment status mirror the effects observed with manipulations of perceived availability in laboratory settings. Samples of drug users not seeking treatment (and thus perceiving drug use opportunity after experimental participation) consistently show greater cue reactivity (that is, greater cue-elicited craving) than samples of drug users who are actively seeking or undergoing treatment (which presumably precludes the anticipation of post-study drug use)26. It also is possible that treatment status may affect neurobiological responses to drug cues.
Prefrontal cortex contributes to context-dependent processing
If treatment-seeking status does affect neural activity elicited by drug cues, such effects would be most readily apparent in neural regions capable of integrating motivational or affective (e.g., current desires) and cognitive information (e.g., knowledge of the probability and means of acquiring desired outcomes). The prefrontal cortex (PFC), an area thought to be largely responsible for supporting such flexibility36,37, has not been well investigated in the context of human drug craving. However, an emerging literature indicates that the neural basis for adaptive processing of incentive stimuli may be mediated by specific regions of the PFC37–39. The two PFC regions that have received the most attention vis-à-vis craving are the OFC6,40 and DLPFC9,11,41. The OFC is thought to contribute to goal-directed behavior through the assessment of the motivational significance of stimuli and the selection of behavior to obtain desired outcomes42. The OFC has extensive connections with the striatum as well as limbic regions (such as the amygdala) and, as a result, is well situated to integrate the activity of several limbic and subcortical areas associated with motivational behavior and reward processing36.
The DLPFC also contributes to regulatory processing under conditions requiring the integration of cognitive and motivationally relevant information43, possibly by integrating information provided by the OFC and other regions with which it is connected36. The DLPFC is centrally involved in reward processing and decision making, particularly when information must be maintained over a delay or when multiple sources of information must be used to guide behavior37.
Taken together, converging evidence indicates that treatment-seeking status may influence neurobiological responses to drug cues, particularly in specific subregions of PFC. Notably, of 19 neuroimaging studies of cue-elicited craving, 10 recruited individuals actively using drugs8–13,17,20–22 and the other 9 recruited individuals seeking or receiving treatment for drug addiction14,16,18,19,23,24,44,45. Thus, variability across these studies may reflect, in part, an unappreciated effect of drug use expectations on cue-elicited neural activity46.
Table 1 presents DLPFC and OFC activation in studies categorized according to whether or not participants were seeking drug treatment at the time of study participation. Studies were included if they exposed participants to drug-related cues. Cue exposure could be accomplished through a variety of methods (such as holding a cigarette or viewing a video of cocaine use). As shown, activation of the DLPFC and OFC has been found in the majority of studies in which participants have, with one exception, not found significant activation of the DLPFC and OFC.
Table 1.
Activation of DLPFC and OFC during drug-cue exposure
| Study | Imaging modality | Addictive substance | Drug cue | DLPFC | OFC |
|---|---|---|---|---|---|
| Drug users currently not seeking treatment | |||||
| Bonson et al. (2002) | PET | Cocaine | Video, script, paraph. | Y | Y |
| Brody et al. (2002) | PET | Cigarette | Video, tactile | Y | |
| Due et al. (2002) | fMRI | Cigarette | Pictures | Y | |
| Garavan et al. (2000) | fMRI | Cocaine | Video | Y | |
| George et al. (2001) | fMRI | Alcohol | Pictures, gust. | Y | |
| Grant et al. (1996) | PET | Cocaine | Video, paraph. | Y | Y |
| Maas et al. (1998) | fMRI | Cocaine | Video | Y | NA |
| Tapert et al. (2003) | fMRI | Alcohol | Pictures | Y | |
| Tapert et al. (2004) | fMRI | Alcohol | Words | Y | |
| Wang et al. (1999) | PET | Cocaine | Script, tactile | Y | |
| Drug users currently seeking treatment | |||||
| Braus et al. (2001) | PET | Alcohol | Video | ||
| Childress et al. (1999) | PET | Cocaine | Video | ||
| Daglish et al. (2001) | PET | Opiate | Script | ||
| Kilts et al. (2001) | PET | Cocaine | Script | ||
| Schneider et al. (2001) | fMRI | Alcohol | Olfact. | ||
| Modell et al. (1995) | SPECT | Alcohol | Gust., olfact. | ||
| Sell et al. (1999) | PET | Opiate | Video, drug | ||
| Wexler et al. (2001) | fMRI | Cocaine | Video | ||
| Wrase et al. (2002) | fMRI | Alcohol | Pictures | Y | Y |
Y, significant within- or between-group activation; NA, not assessed. Abbreviations: OFC, orbitofrontal cortex; DLPFC, dorsolateral prefrontal cortex; fMRI, functional magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography; drug, drug administration; gust., gustatory stimulation with drug-related taste; olfact., olfactory stimulation with drug-related scents; paraph., visual presentation of drug paraphernalia; pictures, visual presentation of drug-related pictures; tactile, tactile stimulation with drug cues; script, drug-related craving induction script/interview; video, audiovisual presentation of drug-related scenes; words, visual presentation of drug-related words.
As mentioned, brain regions besides the DLPFC and OFC have been implicated in cue-elicited craving. Interestingly, the incidence of significant activation of other regions commonly associated with craving (such as amygdala) is approximately equally distributed across studies involving actively using and treatment-seeking drug users, indicating that the effects of treatment status may be most robust in these subdivisions of PFC. One possibility is that the amygdala is less sensitive than are the DLPFC and OFC to aspects of craving that differ between drug-addicted individuals who are seeking treatment and those who are not (for instance, differences in the intensity of the craving state produced by cue exposure). It does not seem, however, that the amygdala is simply activated at a lower threshold than are these subdivisions of PFC. Significant activation of the amygdala, a region strongly linked to conditioned drug-seeking responses in animal models of craving4, has been reported by fewer studies than significant activation of the DLPFC and OFC. A second possibility is that the consideration of methodological factors not addressed here may help explain the conditions under which the amygdala and other regions are activated in response to drug cues, although an inspection of available data shows that obvious candidate factors (such as differences in imaging modality, craving-induction technique and addictive substance) do not readily provide such an explanation.
Functional significance
Increased cue-elicited activation of the DLPFC and OFC when drug use is anticipated may reflect explicit representation of this expectancy (by OFC) and the generation and maintenance of behavioral goals aimed at obtaining drug reward (by DLPFC)5,9,41. OFC neurons are more active during delay periods when rewards are expected than when no such reward is expected47. Similarly, DLPFC neurons encode reward expectancy during a delay38,43. Moreover, delay activity of DLPFC neurons been shown to predict subsequent behavioral responses in rewarded tasks38. Lesions both of the OFC42 and of rat prelimbic cortex39 (the functional homolog of the nonhuman primate and human DLPFC) impair the acquisition and modification of behavior guided by contingencies between responses and outcomes, indicating that these regions may be crucial for the control of goal-directed behavior.
Summary
The burgeoning literature examining neurobiological responses to drug cues thus far has yielded a complex and contradictory pattern of findings. This review makes clear that variables relating to participant characteristics, such as treatment status, are crucial factors that may reconcile otherwise discrepant findings. Specifically, we observed across multiple studies that the treatment-seeking status of the participants influenced PFC activation by drug cues. Our review suggests that the two most investigated prefrontal regions (OFC and DLPFC) may process drug cues in a context-dependent manner. We find that activation in these two areas is reliably produced in cue-exposure studies of drug-addicted individuals who are still actively using. In contrast, these regions are rarely activated among patients preparing to quit.
Our interpretation of this pattern of results has emphasized perceived drug use opportunity as a factor differentiating drug-using participants seeking treatment from those who are not. There are several possible ways in which treatment-seeking and non-treatment-seeking participants may differ, however, that could contribute to the observed findings (including their history of drug use, number of attempts at abstinence and exposure to abstinence promotion materials). Therefore, the degree to which perceived opportunity and other factors related to treatment-seeking status account for these data awaits direct investigation.
There are several potential mechanisms by which drug use expectancy may influence neurobiological responses to drug-cue presentation in addition to that discussed above (that is, goal-directed processing under conditions in which drug use is expected). For example, those seeking treatment may be motivated to maintain abstinence and therefore may attempt to inhibit cue-elicited craving, perhaps through the use of techniques acquired during treatment26. It is quite possible that such efforts to inhibit would produce a different pattern of neural activation compared to the eager anticipation of future drug use. Indeed, in the latter case one might even be motivated to process information in a manner that promotes drug use (i.e., ‘indulging their urge’; see ref. 26).
Moreover, it is likely that perceived drug use opportunity produces different effects in different contexts. For instance, the pattern of neural activity elicited by drug cues in drug-addicted individuals entering treatment may significantly differ from that produced in actively using addicts who are explicitly told that they cannot consume for a long period of time. In the former case, individuals do not intend to consume drugs because they are trying to quit (they are abstinence seeking), whereas in the latter circumstance individuals desire to seek and consume drugs, but are prevented from doing so by situational constraints (they are abstinence avoidant)48. Future research is desirable to examine these different ways in which perceived drug use opportunity can be manipulated and, more importantly, whether they produce different patterns of neural activation.
Nonetheless, it is readily apparent that factors such as treatment-seeking status must be considered in the design and interpretation of neuroimaging studies of craving. Conceptually, these findings indicate that craving is a complex phenomenon involving both cognitive and affective processes. They also indicate that it may be unwise to consider craving to be a unitary construct. Rather there may be more than one type of craving elicited by drug cues (for example, as a function of perceived drug use opportunity), each with its own behavioral presentation and neurobiological underpinnings30,49.
ACKNOWLEDGMENTS
This research was supported in part by grants from the National Institute on Drug Abuse to M.A.S. (R01DA10605) and J.A.F. (R01DA14103), and a Ford Foundation Predoctoral Fellowship to S.J.W.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Contributor Information
Stephen J Wilson, Email: sjwilson@pitt.edu, Department of Psychology, University of Pittsburgh, and at the Center for the Neural Basis of Cognition, Pittsburgh, Pennsylvania, USA..
Michael A Sayette, Departments of Psychology and Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania, USA..
Julie A Fiez, Departments of Psychology and Neuroscience, University of Pittsburgh, and at the Center for the Neural Basis of Cognition, Pittsburgh, Pennsylvania, USA..
References
- 1.Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95:S129–S144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- 2.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 3.Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 4.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol. Biochem. Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychol. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb. Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 7.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 8.Maas LC, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am. J. Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 9.Bonson KR, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 10.Brody AL, et al. Brain metabolic changes during cigarette craving. Arch. Gen. Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 11.Grant S, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapert SF, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch. Gen. Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- 13.Wang GJ, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 14.Wrase J, et al. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur. Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- 15.Modell JG, Mountz JM. Focal cerebral blood flow change during craving for alcohol measured by SPECT. J. Neuropsychiatry Clin. Neurosci. 1995;7:15–22. doi: 10.1176/jnp.7.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Childress AR, et al. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am. J. Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 18.Kilts CD, et al. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 19.Schneider F, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am. J. Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 20.Garavan H, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 21.George MS, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch. Gen. Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 22.Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict. Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Daglish MR, et al. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am. J. Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- 24.Wexler BE, et al. Functional magnetic resonance imaging of cocaine craving. Am. J. Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 25.Hommer DW. Functional imaging of craving. Alcohol Res. Health. 1999;23:187–196. [PMC free article] [PubMed] [Google Scholar]
- 26.Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Exp. Clin. Psychopharmacol. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp. Clin. Psychopharmacol. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- 28.Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Exp. Clin. Psychopharmacol. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict. Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- 30.Sayette MA, et al. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Exp. Clin. Psychopharmacol. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol Addict Behav. 2001;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- 32.Lazev AB, Herzog TA, Brandon TH. Classical conditions of environmental cues to cigarette smoking. Exp. Clin. Psychopharmacol. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating smoking motivation: impact on an electrophysiological index of approach motivation. J. Abnorm. Psychol. 1999;108:240–254. doi: 10.1037//0021-843x.108.2.240. [DOI] [PubMed] [Google Scholar]
- 34.Dols M, Willems B, van den Hout M, Bittoun R. Smokers can learn to influence their urge to smoke. Addict. Behav. 2000;25:103–108. doi: 10.1016/s0306-4603(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 35.Field M, Duka T. Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology. 2002;159:325–334. doi: 10.1007/s00213-001-0923-z. [DOI] [PubMed] [Google Scholar]
- 36.Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog. Brain. Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- 37.Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci. Biobehav. Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 38.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur. J. Neurosci. 2003;18:1–13. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 39.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 41.Anton RF. What is craving? Models and implications for treatment. Alcohol Res Health. 1999;23:165–173. [PMC free article] [PubMed] [Google Scholar]
- 42.Rolls ET. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe M, Hikosaka K, Sakagami M, Shirakawa S. Coding and monitoring of motivational context in the primate prefrontal cortex. J. Neurosci. 2002;22:2391–2400. doi: 10.1523/JNEUROSCI.22-06-02391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braus DF, et al. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J. Neural. Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- 45.Sell LA, et al. Activation of reward circuitry in human opiate addicts. Eur. J. Neurosci. 1999;11:1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 46.Meyer RE. Craving: what can be done to bring the insights of neuroscience, behavioral science and clinical science into synchrony. Addiction. 2000;95:S219–S227. doi: 10.1080/09652140050111780. [DOI] [PubMed] [Google Scholar]
- 47.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 48.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol. Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 49.Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr. Symp. Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- 50.Williams SM. Sylvius: Fundamentals of Human Neural Structure. Sunderland, Massachusetts, USA: Sinauer Associates; 2000. [Google Scholar]