Abstract
Primate dental and postcranial remains from the Eocene Pondaung Formation (Myanmar) have been the subject of considerable confusion since their initial discoveries, and their anthropoid status has been widely debated. We report here a well preserved primate talus discovered in the Segyauk locality near Mogaung that displays derived anatomical features typical of haplorhines, notably anthropoids, and lacks strepsirhine synapomorphies. Linear discriminant and parsimony analyses indicate that the talus from Myanmar is more similar structurally to those of living and extinct anthropoids than to those of adapiforms, and its overall osteological characteristics further point to arboreal quadrupedalism. Regressions of talar dimensions versus body mass in living primates indicate that this foot bone might have belonged to Amphipithecus. This evidence supports hypotheses favoring anthropoid affinities for the large-bodied primates from Pondaung and runs contrary to the hypothesis that Pondaungia and Amphipithecus are strepsirhine adapiforms.
Keywords: talus, anthropoid primates
The famous late middle Eocene Pondaung Formation of Central Myanmar (formerly Burma) has long been known for its abundant large fossil mammals (1, 2) and particularly its primates (3-14). The Burmese Eocene primates, notably the large-bodied forms [Pondaungia (3) and Amphipithecus (4)], have been the subject of a long-standing debate surrounding the critical issue of their possible anthropoid status (7, 8, 10, 14-24) and as such potentially challenge the role of Africa as the ancestral homeland for the anthropoid clade. With the exception of Pondaungia savagei (7), however, the large-bodied primates (Pondaungia cotteri, Pondaungia minuta, and Amphipithecus mogaungensis) and the more recently discovered smaller-bodied forms [Bahinia (9) and Myanmarpithecus (12)] are represented entirely by fragmentary dental and cranial remains, and this incomplete anatomical information has not allowed for a consensus view regarding their anthropoid affinities. This uncertainty stems from the fact that cranial and dental characteristics of these primates (notably Pondaungia) resemble those of anthropoids in some respects, but in other details they resemble those of adapiforms and some omomyids (14, 23, 24). A supplemental line of doubt concerning these primates' anthropoid affinities derives from recently described postcranial elements of a large-bodied form [a complete left humerus, the distal half of a left calcaneus, and fragments of a right humerus and both left and right ulnae assigned to P. savagei based on size and association but conceivably Amphipithecus as well (7)] that display a mosaic of characteristics mostly consistent with those of adapiforms (notharctines and adapines), omomyids, some extant strepsirhines (lorises), haplorhines (cebid platyrrhines), and also, but to a lesser extent, some fossil anthropoids. Ciochon et al. (7) cautioned that, given such a mixture of characteristics, the phylogenetic implications of Pondaungia postcrania remain difficult to assess. Despite the fact that their interpretations relied exclusively on phenetic criteria, these authors supported the idea of adapiform rather than anthropoid affinities for Pondaungia (and by extension for Amphipithecus) (23) but were not explicit about the significance of these anatomical resemblances in terms of being either functional adaptations (similarities related to similar patterns of locomotion) or phylogenetically significant apomorphies. In this way, the fact that the numerous derived dental features aligning the Burmese primates with Eocene-Oligocene anthropoids of both North Africa and Oman are seen as being ”anthropoid-like” but nevertheless are considered to be strictly ”functional convergences” by some authors (7, 23-25) seems premature in the absence of a more comprehensive fossil record.
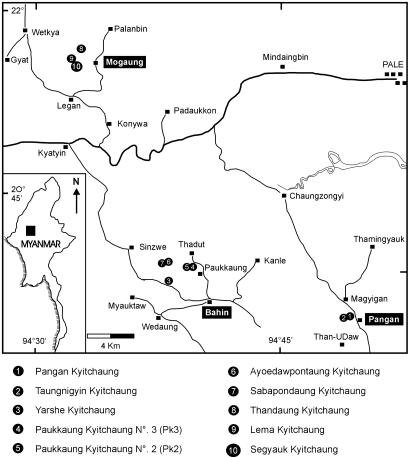
Under the auspices of a paleontological research program led by the joint Myanmar-French Team (Geology Department of the Yangon University and Paleontology Department of Université Montpellier II), an intensive survey in November 2002 of the fossiliferous variegated clays in the new Segyauk Kyitchaung locality (1 km west of Mogaung village; Fig. 1) led to the recovery of an important primate tarsal bone. The specimen [National Museum of Myanmar, Primates (NMMP)-39] is a medium-sized left talus, well preserved enough to show some essential diagnostic characteristics that substantiate the anthropoid status of the larger-bodied Eocene Burmese primates. Several studies, notably those of Gebo (26-28), Beard et al. (29), Gebo et al. (30), Dagosto and Gebo (31), and more recently Gebo et al. (32, 33) and Seiffert and Simons (34), have depicted a set of anatomical traits on tali that are otherwise restricted to living and fossil anthropoids. Following these authors, we will discuss the morphological features of this talus from the Pondaung Formation that provide decisive evidence on the long-standing debate of their higher-level affinities.
Fig. 1.
Location map of the new fossiliferous locality of Segyauk Kyitchaung, 1 km west of Mogaung village in central Myanmar.
Description and Functional Interpretation
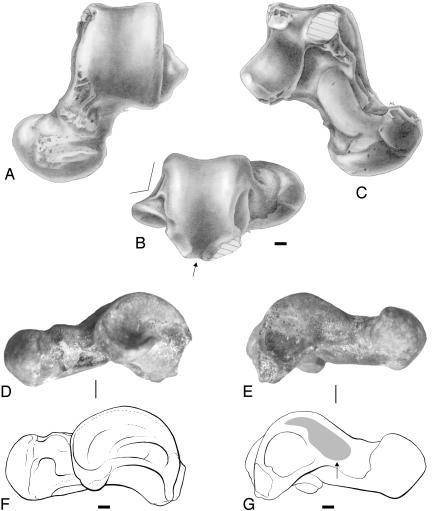
Description and Comparisons. In overall view, NMMP-39 (Figs. 2 and 3) exhibits a long and rather straight talar neck supporting a broad (index, HHT/HW in Table 1) and oval-shaped talar head that is unusually expended medially, a feature that is also seen in Apidium phiomense (DPC 1001), an Oligocene anthropoid from Egypt. The length of the neck and head is ≈57% of the total TL (index, NL/TL in Table 1) and slightly longer than the trochlea (index, NL/TRL in Table 1) as generally observed in haplorhines, notably the anthropoid platyrrhines (33). The long axis of the talar head is oriented mediolaterally relative to the dorsal plane of the trochlear surface. The plantar articular facets on the talar head (anterior navicular and calcaneal facets) are continuous with the sustentacular facet of the talar neck (Fig. 3C). Shallow but well marked depressions occur at the base of the talar head and are located medially and laterally between the facets for the navicular and the sustentaculum tali, and the facets for the calcaneum and the sustentaculum tali, respectively, where the neck of the talus overlies the plantar calcaneo-navicular ligament. The medial deviation (α) of the talar neck relative to the anteroposterior axis of the trochlea is ≈30°. The trochlea is moderately grooved, and its rims are parallel. Both lateral and medial facets of the trochlea are steep (Fig. 3A), a feature that has been described as characteristic of anthropoids, Tarsius, and most omomyids (29). The medial trochlear rim exhibits a long radius of curvature posteriorly. The anterior region of that rim is damaged, but judging from the pattern of breakage it may be expected that it probably extended anteromedially onto the edge of the talar neck. In medial view (Fig. 3E), the talar body is fairly tall dorsoplantarly. The talo-tibial facet for the medial malleolus of the tibia is not deep, broad, and dorsoplantarly extended as in strepsirhines, omomyids, and Tarsius but is reduced, elevated from the plantar surface, and limited in its dorsal border as in Eosimias (26, 32) and more generally as in anthropoids (Fig. 3G). There is no prominent cotylar fossa extending onto the medial side of the talar neck. In lateral view (Fig. 3D), the talo-fibular facet is vertically oriented as in haplorhines (anthropoids and omomyids) rather than sloping gently and laterally as in most strepsirhines, notably Eocene adapiforms (26, 27, 29). On NMMP-39, this facet (the anterior border) is slightly flared in anterior view, but when viewed posteriorly there is a well marked but shallow depression that defines a crease separating the dorsal surface of the facet from the laterally projecting talar process (Fig. 3B). As in basal anthropoids, MNNP-39 lacks the prominent projecting posterior trochlear shelf that is characteristic primarily of extinct (adapids, most prominent in notharctines) and extant (lemurs and lorises) strepsirhines and to a lesser degree of haplorhine omomyids (31, 35, 36). The well curved posterior plantar facet (ectal facet) is not narrow and waisted in the mediolateral plane that slightly extends the lateral process plantarly in the posterior part of the talus (Fig. 3C). The specimen is badly broken medially and posteriorly along its plantar aspect, and its medial tubercle, which buttresses (along with a minute lateral tubercle) the groove for the tendon of flexor fibularis, is missing. The limited surface of breakage suggests that this medial tubercle was probably moderately developed but nevertheless relatively larger than the lateral tubercle (Fig. 3 B and C). As in anthropoids and omomyids, the groove for the tendon of the flexor hallucis longus is in a midline position relative to the posterior trochlear facet (Fig. 3B) rather than lateral to it as in all strepsirhines (26, 27, 29).
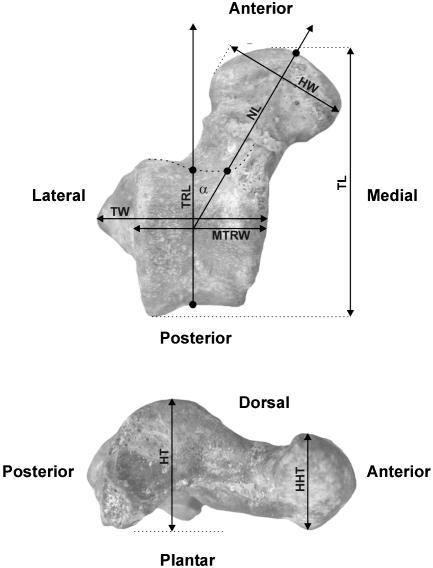
Fig. 2.
Tali measurements.
Fig. 3.
NMMP-39, left talus from Segyauk kyitchaung, in dorsal (A), posterior (B), plantar (C), lateral (D), and medial (E) views; the schematic drawings at the bottom (F and G) are derived from the lateral (D) and medial (E) views, respectively. The arrow shown in the posterior view (B) indicates the midtrochlear position of the flexor fibularis groove. The arrow shown in the schematic drawing of the medial view (G) indicates the elevated position of dorsally limited talo-tibial facet (gray claw-shaped area). Drawings (A-C and F-G) and plate conception are from L. Meslin (Institut des Sciences de l'Évolution de Montpellier). (Scale bars, 1 mm.)
Table 1. Myanmar talus NMMP-39 measurements (in mm) and ratios (×100).
| Talar measurements and ratios | NMMP-39 |
|---|---|
| Talar length (TL) | 16.21 |
| Talar neck length (NL) | 9.25 |
| Trochlear length (TRL) | 8.73 |
| Midtrochlear width (MTRW) | 7.9 |
| Talar width (TW) | 10.52 |
| Lateral body height (HT) | 8.81 |
| Talar head width (HW) | 8.46 |
| Talar head height (HHT) | 6.03 |
| Angle of talar neck to talar body (Tneckangle, α°) | 32 |
| NL/TL | 57 |
| NL/TRL | 106 |
| NL/MTRW | 117 |
| HT/MTRW | 111 |
| HT/TRL | 101 |
| MTRW/TRL | 90 |
| HW/HHT | 140 |
| HW/MTRW | 107 |
| TW/TL | 65 |
Functional Interpretation. In terms of movements associated with these osteological features, the fact that NMMP-39 exhibits a straight and moderately long talar neck, a fairly high talar body, and parallel-sided medial and lateral trochlear facets (not wedged-shaped) with circular rims might be indicative of leaping (27). However, the talar trochlea of NMMP-39 is quite flattened rather than deeply grooved as in most leapers, where only one primary plane of movement is needed at the talocrural joint. Such a trochlear characteristic, associated with the long radius of curvature of the medial trochlear rim, and the absence of posterior trochlear shelf allow much more mobility. Although some frequent leapers such as Avahi and Galago (both extant strepsirhines) possess a flat talar trochlea rather than a central trochlear groove (26, p. 430), on the basis of the osteological features depicted on NMMP-39 it seems too early to claim with certainty that this primate exhibited a specialized leaping mode of locomotion. NMMP-39 does not exhibit any clear osteological adaptations for climbing (27). Taking into consideration the characteristics of the trochlea favoring more joint mobility, NMMP-39 might have belonged to a primate that moved frequently by arboreal quadrupedalism, a form of movement that is intermediate between more extreme leaping and climbing in terms of mechanical requirements (27).
Materials and Methods
A linear discriminant analysis (LDA) and a parsimony analysis (PA) based on the recently published databases (here updated and supplemented with new data) of Gebo et al. (33) [analysis is based on isolated tali from the early middle Eocene of China, Shanghuang (SH)] have been processed to determine which group of primates (extinct or extant) NMMP-39 best fits with in terms of structural talar morphology and shared-derived pedal characteristics, respectively. The PA matrix (Table 2) consists of 12 tarsal characteristics (eight on talus and four on calcaneus; Table 3) observed on 17 terminal taxa [ingroup: 15 taxa (notharctines, adapines, protoadapines, tarsiids, haplorhines SH, omomyines, anaptomorphines, eosimiids, protoanthropoids SH, and anthropoids); outgroup: 3 taxa (Scandentia, Dermoptera, and Plesiadapiformes), which do not differ significantly in the expression of the tarsal traits). The LDA matrix (see Table 4, which is published as supporting information on the PNAS web site) consists of 11 talar variables (10 linear talar dimension ratios and 1 angle) measured on 55 taxa (strepsirhines and haplorhines): 6 adapiforms, 6 lemuriforms, 8 omomyids, 1 eosimiid, 3 tarsiids (1 tarsiid SH indeterminate), 2 haplorhines (indeterminate from SH), and 29 anthropoids (1 oligopithecids, 1 proteopithecid, 1 propliopithecid, 2 parapithecids, 2 cercopithecids, 1 hylobatid, and 21 platyrrhines). All of these primate families or higher taxa were regarded a priori as natural groups and considered as grouping factors in the discriminant analysis. The LDA and PA matrices were analyzed with R-1.6.2 (MASS package) (37) and paup* 4.0 beta 10 for Windows (38), respectively.
Table 2.
Data matrix used in the PA supplemented from ref. 33
| Taxa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outgroups (Scandentia, Dermoptera, and Plesiadapiformes) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eosimias | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 1 |
| Tarsius | 1 | 0 | 0 | 0 | 0 | 0 | 1 | ? | 0 | 1 | 2 | 0 |
| Apidium | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| Platyrrhines (Saimiri, Callicebus, Cebus, Aotus, etc.) | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 0 and 1 | 0 | 1 | 1 | 1 |
| Anaptomorphinae | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| Omomyinae | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| Notharctinae | 2 | 1 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Adapinae | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Protoadapinae | 2 | 1 | ? | ? | 2 | ? | 1 | ? | ? | 0 | 0 | 0 |
| Haplorrhines SH | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| Tarsiids SH | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | ? | ? | ? |
| Protoanthropoids SH | 1 | 0 | 2 | 1 | 1 | 0 | ? | 1 | 0 | 0 | 1 | ? |
| Proteopithecus | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | ? | ? | ? | ? |
| Catopithecus | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 2 | ? | ? | ? | ? |
| Aegyptopithecus | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 2 | ? | ? | ? | ? |
| NMMP-39 | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | ? | ? | ? | ? |
Table 3. Characteristics used in the phylogenetic analysis.
| 1. | Shape of talo tibial facet (0) steep-sided; (1) steep-sided with a plantar lip; (2) sloped |
| 2. | Flexor fibularis groove (0) central to trochlea; (1) lateral to trochlea |
| 3. | Talar neck angle (α) (0) <20°; (1) 20—30°; (2) >30° |
| 4. | Talar body height (HT/MTRW × 100) (0) <100; (1) 100—120; (2) 120—150 |
| 5. | Posterior trochlear shelf (0) none; (1) small; (2) large |
| 6. | TW/TL × 100 (0) <60; (1) >60 |
| 7. | Medial talo tibial facet (0) short (does not reach to plantar edge of bone); (1) long |
| 8. | Medial cotylar fossa (0) absent; (1) moderate; (2) deep |
| 9. | Relative width of posterior calcaneal facet (0) >50; (1) <50 |
| 10. | Relative length of distal calcaneus (0) <45; (1) >45 |
| 11. | Relative length of calcaneus heel (0) long, >30; (1) moderate 25—30; (2) short, <20 |
| 12. | Morphology of calcaneocuboid joint (0) fan-shaped, nonarticular surface; (1) more circular, nonarticular surface present |
Characteristics with more than two states (1, 3—5, and 11) were ordered following ref. 33.
Results
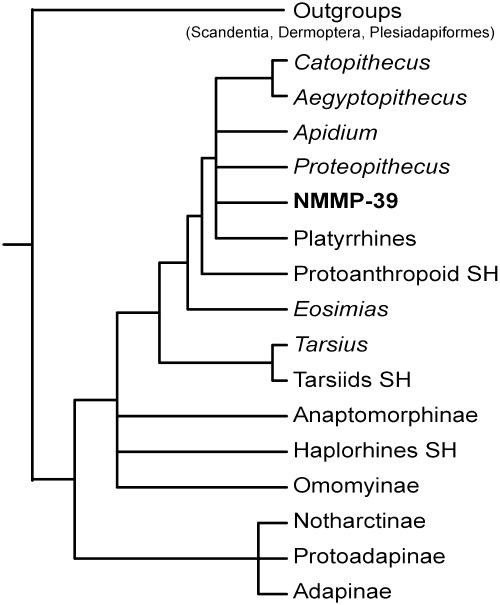
The PA, using a branch-and-bound search algorithm, yielded 225 most-parsimonious trees of 31 steps with a consistency index of 0.613 and a retention index of 0.82. A strict consensus tree is presented in Fig. 4. The lack of resolution in the consensus tree is not due to alternative equally parsimonious and incongruent topologies. Given the high taxon/character ratio (18/11) of the matrix, some cases of polytomy are the consequence of a lack of characteristics for defining nodes. The topology of the tree we present is consistent with that proposed by Gebo et al. (32, 33). Among haplorhines, NMMP-39 falls inside the anthropoid clade sensu Gebo et al. (33), within which Eosimias and the unnamed prothoanthropoid from SH represent the earliest offshoots based on their tarsal morphology. In this tree, tarsiids (Tarsius + tarsiids SH) represent the sister group of the anthropoid clade. Despite the lack of resolution, NMMP-39 belongs to the large clade that includes anthropoids from the Paleogene of both North Africa and Oman (oligopithecids, proteopithecids, propliopithecids, and parapithecids) and modern South American platyrrhines.
Fig. 4.
Strict consensus tree of 225 most-parsimonious trees (each 225 feet; consistency index, 0.613; retention index, 0.82) obtained by a branch-and-bound search performed under paup* 4.0.
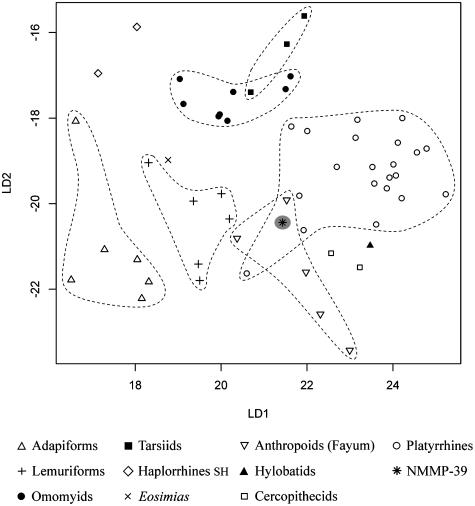
The plot of discriminant factors derived from the LDA is presented in Fig. 5. The position of NMMP-39 was predicted by projecting its corresponding transformed variables (canonical variates) onto the linear discriminants. This prediction clearly shows that the morphology of NMMP-39 is more similar structurally to that of anthropoids (93%, 98% with Eosimias), notably platyrrhines (46%), than to that of strepsirhines (adapiforms + lemuriforms: 1.4%).
Fig. 5.
Plot of discriminant scores derived from the discriminant analysis of the 11 talar variables (10 linear talar dimension ratios and α) depicted in Fig. 2.
Discussion
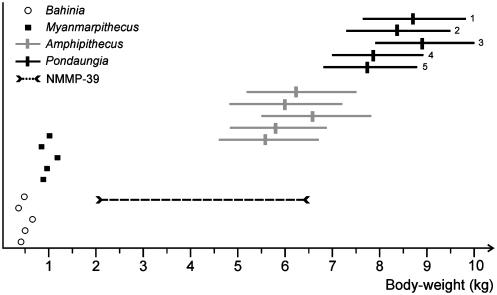
Given the absence of articulated skeletal remains in association with NMMP-39 at Segyauk kyitchaung, it is essential to determine which of the formally designated primate species from the Eocene of Myanmar to which this talus could belong. Regressions of talar dimensions versus body mass in living primates (39) show that NMMP-39 belonged to a primate having a body mass ranging from 2 to 6.5 kg [estimated from all strepsirhine and lemur-only bivariate regression equations based on several linear talar dimensions provided by Dagosto and Terranova (39); Fig. 6]. This body-mass range prediction perhaps is an underestimate, because we have no indication what the age of the individual might be, and furthermore we have only used the available regressions of talar dimensions based on strepsirhines alone (39). On the basis of its size, this talus could be allocated to A. mogaungensis, the body mass of which is estimated to be ≈6 kg based on first lower molar (M1) area [from AMNH 32520, NMMP-2, NMMP-6, and NMMP-7: 4.8-7.2 kg from the all-primate least-squares bivariate regression equation presented by Conroy (40); 5.2-7.5 kg from the equation of Gingerich et al. (41); and 4.6-6.9 kg from the primate least-squares bivariate regression equations presented by Legendre (42); Fig. 6]. Given that no large-bodied primate other than the abundantly sampled Amphipithecus and Pondaungia is known from the Eocene Pondaung Formation to date, such an allocation seems quite probable. In that respect, some of the aforementioned talar characteristics (steep talo-fibular facet, mid-trochlear position of the flexor fibularis groove, dorsoplantarly narrow and dorsally limited talo-tibial facet, and minute posterior trochlear shelf) favoring the haplorhine anthropoid affinities for NMMP-39, and therefore by extension for Amphipithecus, are inconsistent with hypotheses advocating an adapiform status for the large-bodied primates from Myanmar. All known Eocene adapiforms have a derived talar morphology (sloped talo-fibular facet, a flexor fibularis groove that is laterally positioned on the trochlea, dorsoplantarly large and deep talo-tibial facet, and, in some cases, a strong and prominently projecting posterior trochlear shelf) otherwise found only in extant strepsirhines (lemuriforms and lorisiforms). NMMP-39 lacks these adapiform talar synapomorphies. In the broader context of primate evolution, some of these haplorhine talar characteristics exhibited by anthropoids (steep-sided talo-fibular facet, dorsally limited talo-tibial facet, and midtrochlear position of the groove for the tendon of flexor fibularis) would seemingly represent a primitive condition (27, 29), because they occur in the most likely outgroups to Primates (Scandentia, Dermoptera, and Plesiadapiformes). However, Gebo et al. (33) interpreted new tarsal evidence from the early middle Eocene of China (SH) as clearly establishing that these tarsal characteristics ”are actually anthropoid apomorphies (i.e., reversals from the primitive primate condition), and not retentions from a mammalian ancestor.” Following these authors and because of the results of the PA, there is little chance that the characteristics visible on NMMP-39 (notably the limited talotibial facet, the lack of a (or presence of a minute) posterior trochlear shelf (characteristics exclusive to anthropoids, and more specifically to telanthropoids) are primitive and therefore considered as anthropoid-like. It is also highly unlikely that these same characteristics might have been secondarily acquired on NMMP-39 by reversals from the derived adapiform talar condition. This latter point of view is substantiated clearly by the results of the LDA, showing that NMMP-39 exhibits consistent structural differences with the talar morphology of both extinct and extant strepsirhines.
Fig. 6.
Body-weight range prediction of NMMP-39. This weight range was estimated from all strepsirhine and lemur-only regression equations based on several linear talar dimensions (A1, A2, A3, A4, A7, and A81) indicated by Dagosto and Terranova (39). Predicted body-weight ranges of Amphipithecus (NMMP-3250, NMMP-2, NMMP-6, NMMP-7, and NMMP-30) and Pondaungia (NMMP-17, NMMP-25, and NMMP-38) were estimated from the first lower molar (M1) areas by using the equation of Gingerich et al. (41) (line 1), all primate (line 2) and anthropoid (line 3) least-squares regression equations of Conroy (40), and the primate (lines 4 and 5) least-square regression equations of Legendre (42). The body weight of Myanmarpithecus was predicted from NMMP-37, a new fragment of mandible bearing M1-M2 (second lower molar), found in November 2002 in Paukkaung Kyitchaung No. 3 (see Fig. 1) by the Myanmar-French Team.
This tarsal evidence now leaves hypotheses favoring adapiform affinities for the large-bodied primates from Myanmar highly unparsimonious. Furthermore, the dental structure of Amphipithecus and Pondaungia is derived in a direction similar to some or all later anthropoids (including fossil anthropoids from the Paleogene of North Africa and Oman). In light of this tarsal evidence, such a dental resemblance cannot be interpreted exclusively as the result of ”functional convergence” related to dietary specializations (23, 24) but rather should be interpreted as the result of shared ancestry with other living and extinct anthropoids. Contrary to Ciochon and Gunnell (23) or Gunnell et al. (24), we remain proponents of the hypothesis that the large-bodied primates from Myanmar are anthropoids and are not derived strepsirhine adapiforms.
Supplementary Material
Acknowledgments
We are very grateful to the field-work managers of the French Pondaung Fossil Expedition Team in Myanmar (Brigadier General Than-Tun, Major Bo Bo, and other staff of the International Affair and Research Department, Ministry of Defense, Myanmar). We are particularly indebted to the inhabitants of the Mogaung village for their kind hospitality during the field expeditions. K. C. Beard (Carnegie Museum of Natural History, Pittsburgh) and E. R. Seiffert (Duke University, Durham, NC) offered valuable comments and advice on the manuscript. We are thankful to M. Godinot, C. Denys, and J. Cuisin (Museum National d'Histoire Naturelle, Paris) for access to comparative material; L. Meslin [Institut des Sciences de l'Évolution de Montpellier (ISEM)] for art drawings; and Y. Pomes (ISEM), J. Claude (ISEM), and E. Paradis (ISEM) for efficient help in the use of ”R-1.6.2.” We thank M. Richard, E. Fara, and E. R. Seiffert for improving the English version of the manuscript. This work was supported by the program Centre National de la Recherche Scientifique-éclipse and the Wenner-Gren Foundation. L.M. gratefully acknowledges the Fyssen and Singer-Polignac foundations for funding his work. This is publication no. 2003-039 of ISEM, Centre National de la Recherche Scientifique-Unité Mixte de Recherche 5554.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NMMP, National Museum of Myanmar, Primates; TL, talar length; NL, talar neck length; TRL, trochlear length; MTRW, midtrochlear width; TW, talar width; HT, lateral body height; HW, talar head width; HHT, talar head height; LDA, linear discriminant analysis; PA, parsimony analysis; SH, Shanghuang.
References
- 1.Pilgrim, G. E. (1928) Paleontol. Indica 13, 1-39. [Google Scholar]
- 2.Colbert, E. H. (1938) Bull. Am. Mus. Nat. Hist. 74, 255-436. [Google Scholar]
- 3.Pilgrim, G. E. (1927) Mem. Geol. Surv. India 1-26.
- 4.Colbert, E. H. (1937) Am. Mus. Novit. 651, 1-18. [Google Scholar]
- 5.Ba, M., Ciochon, R. L. & Savage, D. E. (1979) Nature 282, 65-67. [DOI] [PubMed] [Google Scholar]
- 6.Ciochon, R. L., Savage, D. E., Tin, T. & Maw, B. (1985) Science 229, 756-759. [DOI] [PubMed] [Google Scholar]
- 7.Ciochon, R. L., Gingerich, P. D., Gunnell, G. F. & Simons, E. L. (2001) Proc. Natl. Acad. Sci. USA 98, 7672-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeger, J.-J., Naing Soe, U. A., Ko Aung, U. A., Benammi, M., Chaimanee, Y., Ducrocq, R.-M., Tun, T., Thein, U. T. & Ducrocq, S. (1998) C. R. Acad. Sci. 321, 953-959. [Google Scholar]
- 9.Jaeger, J.-J., Thein, T., Benammi, M., Chaimanee, Y., Soe, A. N., Lwin, T., Wai, S. & Ducrocq, S. (1999) Science 286, 528-530. [DOI] [PubMed] [Google Scholar]
- 10.Chaimanee, Y., Thein, T., Ducrocq, S., Soe, A. N., Benammi, M., Tun, T., Lwin, T., Wai, S. & Jaeger, J.-J. (2000) Proc. Natl. Acad. Sci. USA 97, 4102-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takai, M., Shigehara, N., Tsubamoto, T., Egi, N., Aung, A. K., Thein, T., Soe, A. N. & Tun, S. T. (2000) Asian Paleoprimatol. 1, 7-28. [Google Scholar]
- 12.Takai, M., Shigehara, N., Aung, A. K., Tun, S. T., Soe, A. N., Tsubamoto, T. & Thein, T. (2001) J. Hum. Evol. 40, 393-409. [DOI] [PubMed] [Google Scholar]
- 13.Gebo, D. L., Gunnell, G. F., Ciochon, R. L., Takai, M., Tsubamoto, T. & Egi, N. (2002) J. Hum. Evol. 43, 549-553. [Google Scholar]
- 14.Shigehara, N., Takai, M., Kay, R. F., Aung, A. K., Soe, A. N., Tun, S. T., Tsubamoto, T. & Thein, T. (2002) J. Hum. Evol. 43, 143-166. [DOI] [PubMed] [Google Scholar]
- 15.Szalay, F. S. (1970) Nature 227, 355-357. [DOI] [PubMed] [Google Scholar]
- 16.Szalay, F. S. (1972) Nature 236, 179-180. [Google Scholar]
- 17.Szalay, F. S. & Delson, E. (1979) Evolutionary History of the Primates (Academic, New York).
- 18.Simons, E. L. (1971) Nature 232, 489-491. [DOI] [PubMed] [Google Scholar]
- 19.Conroy, G. C. (1978) in Recent Advances in Primatology, eds. Chivers, D. J. & Joysey, K. A. (Academic, London), pp. 27-42.
- 20.Gingerich, P. D. (1980) in Evolutionary Biology of the New World: Monkeys and Continental Drift, eds. Ciochon, R. L. & Chiarelli, A. B. (Plenum, New York), pp. 123-138.
- 21.Kay, R. F. (1980) in Evolutionary Biology of the New World: Monkeys and Continental Drift, eds. Ciochon, R. L. & Chiarelli, A. B. (Plenum, New York), pp. 159-188.
- 22.Ciochon, R. D. & Holroyd, P. A. (1994) in Anthropoid Origins, eds. Fleagle, J. G. & Kay, R. F. (Plenum, New York), pp. 143-162.
- 23.Ciochon, R. L. & Gunnell, G. F. (2002) Evol. Anthropol. 11, 156-168. [Google Scholar]
- 24.Gunnell, G. F., Ciochon, R. L., Gingerich, P. D. & Holroyd, P. A. (2002) Contr. Mus. Paleontol. Univ. Michigan 30, 337-372. [Google Scholar]
- 25.Rasmussen, D. T. & Simons, E. L. (1992) Int. J. Primatol. 13, 477-508. [Google Scholar]
- 26.Gebo, D. L. (1986) J. Hum. Evol. 15, 421-430. [Google Scholar]
- 27.Gebo, D. L. (1988) Folia Primatol. 50, 3-41. [DOI] [PubMed] [Google Scholar]
- 28.Gebo, D. L. (1989) J. Hum. Evol. 18, 201-203. [Google Scholar]
- 29.Beard, K. C., Dagosto, M., Gebo, D. L. & Godinot, M. (1988) Nature 331, 712-714. [DOI] [PubMed] [Google Scholar]
- 30.Gebo, D. L., Simons, E. L., Rasmussen, D. T. & Dagosto, M. (1994) in Anthropoid Origins, eds. Fleagle, J. G. & Kay, R. F. (Plenum, New York), pp. 203-233.
- 31.Dagosto, M. & Gebo, D. L. (1994) in Anthropoid Origins, eds. Fleagle, J. G. & Kay, R. F. (Plenum, New York), pp. 567-593.
- 32.Gebo, D. L., Dagosto, M., Beard, K. C., Qi, T. & Wang, J. (2000) Nature 404, 276-278. [DOI] [PubMed] [Google Scholar]
- 33.Gebo, D. L., Dagosto, M., Beard, K. C. & Qi, T. (2001) Am. J. Phys. Anthropol. 116, 83-107. [DOI] [PubMed] [Google Scholar]
- 34.Seiffert, E. R. & Simons, E. L. (2001) J. Hum. Evol. 41, 577-606. [DOI] [PubMed] [Google Scholar]
- 35.Szalay, F. S. (1974) in Phylogeny of the Primates: A Multidisciplinary Approach, eds. Luckett, W. P. & Szalay, F. S. (Plenum, New York), pp. 357-404.
- 36.Godinot, M. & Dagosto, M. (1983) J. Paleontol. 57, 1321-1324. [Google Scholar]
- 37.Ihaka, R. & Gentleman, R. (1996) J. Comput. Graph. Stat. 5, 299-314. [Google Scholar]
- 38.Swofford, D. L. (2002) paup*: Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer, Sunderland, MA), Version 4.
- 39.Dagosto, M. & Terranova, C. J. (1992) Int. J. Primatol. 13, 307-344. [Google Scholar]
- 40.Conroy, G. C. (1987) Int. J. Primatol. 8, 115-137. [Google Scholar]
- 41.Gingerich, P. D., Smith, B. H. & Rosenberg, K. (1980) Am. J. Phys. Anthropol. 52, 231-232. [Google Scholar]
- 42.Legendre, S. (1989) Münchner Geo. Abh. 16, 1-110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.