Abstract
Attention to internal body sensations is practiced inmost meditation traditions. Many traditions state that this practice results in increased awareness of internal body sensations, but scientific studies evaluating this claim are lacking. We predicted that experienced meditators would display performance superior to that of nonmeditators on heartbeat detection, a standard noninvasive measure of resting interoceptive awareness. We compared two groups of meditators (Tibetan Buddhist and Kundalini) to an age- and body mass index-matched group of nonmeditators. Contrary to our prediction, we found no evidence that meditators were superior to nonmeditators in the heartbeat detection task, across several sessions and respiratory modulation conditions. Compared to nonmeditators, however, meditators consistently rated their interoceptive performance as superior and the difficulty of the task as easier. These results provide evidence against the notion that practicing attention to internal body sensations, a core feature of meditation, enhances the ability to sense the heartbeat at rest.
Descriptors: Meditation, Interoception, Heartbeat detection, Awareness, Respiration
Meditation is a form of mental training that has been practiced for thousands of years that can be conceptualized as a family of complex emotional and attentional regulatory training regimens developed for various ends, including the cultivation of well-being and emotional balance (Lutz, Dunne, & Davidson, 2007). Although typically practiced in the context of spiritual traditions, there has been a notable increase in the therapeutic application of meditation as a complement in alternative medicine. (Arias, Steinberg, Banga, & Trestman, 2006; Astin, Shapiro, Eisenberg, & Forys, 2003; Barnes, Powell-Griner, McFann, & Nahin, 2004).
Most meditation traditions incorporate attention to internal body sensations as a component of the practice, particularly in the beginning stages of instruction, possibly because the availability of these sensations from moment to moment makes them a convenient object to focus on. The most commonly attended body sensations include the breath, the position of the joints (proprioception), the degree of muscle tension, and the heartbeat (Kabat-Zinn, 1990; Kornfield, 1996; Nairn, 2000; Selby, 1992). Although attention to internal body sensations is most commonly practiced under conditions of rest, the subjective experience of these interoceptive body sensations is also routinely modulated through manipulations of the breath and musculo-skeletal posture, particularly during the practice of yoga exercises (Arambula, Peper, Kawakami, & Gibney, 2001; Bhajan Y, 2000; Peng et al., 2004). Many traditions state that the practice of attending to interoceptive sensations results in enhanced awareness of these sensations, and also assert that the meditation practice results in enhanced awareness of a variety of other internal events, such as the ongoing experience of thoughts and emotions (Kabat-Zinn, 1990; Kornfield, 1996; Nairn, 2000).
The idea that a meditation practice would enhance interoceptive awareness is certainly plausible, but there is no scientific evidence to support this claim. In the current study, we sought to address this knowledge gap by studying interoceptive awareness in experienced meditators.
Several methods for assessing interoceptive awareness have been described, including gastrointestinal distension (Holzl, Erasmus, & Moltner, 1996), adrenergic stimulation (Cameron & Minoshima, 2002; Khalsa, Rudrauf, Sandesara, Olshansky, & Tranel, in press), and heartbeat perception (Brener & Kluvitse, 1988; Schandry, 1981; Whitehead, Drescher, & Heiman, 1977). The latter, heartbeat perception, is considered the standard and preferred method for the noninvasive assessment of interoceptive awareness, and factors modulating awareness of cardiac sensations have been extensively studied (Brener, Liu, & Ring, 1993; Eichler & Katkin, 1994; Jones, 1994; Knapp, Ring, & Brener, 1997; Ring & Brener, 1992; Rouse, Jones, & Jones, 1988; Schandry, Bestler, & Montoya, 1993). Recently, functional neuro-imaging studies have demonstrated that heartbeat perception tasks activate a network of brain regions including the insula, primary somatosensory cortex, and the anterior cingulate cortex (Craig, 2002; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Pollatos, Schandry, Auer, & Kaufmann, 2007). These brain regions are considered necessary for the representation and maintenance of the internal state of the organism (Craig, 2002; Critchley et al., 2004; Pollatos et al., 2007) and for the conscious experience of emotion and feelings (Damasio et al., 2000), lending further support to the notion that heartbeat perception is a good index of interoception.
Although there are several techniques for assessing heartbeat perception, the most commonly used methods are heartbeat detection and heartbeat tracking. During heartbeat detection, subjects determine whether an exteroceptive stimulus, such as a light or a tone, is contemporaneous with their heartbeat sensation (Brener & Kluvitse, 1988; Schneider, Ring, & Katkin, 1998; Whitehead et al., 1977). Performance is indexed by the number of correct responses reported by the subject (e.g., true positives and true negatives), which also allows measurement of individual and group response accuracy. Subjects are then classified as “good heartbeat detectors” when their performance lies above chance according to the binomial distribution (Katkin, Wiens, & Ohman, 2001; Schneider et al., 1998; Wiens & Palmer, 2001). During heartbeat tracking, subjects silently count their heartbeats during brief, fixed time periods. Performance is indexed by a cardiac perception score, in which the number of counted heartbeats is contrasted with the number of actual heartbeats. Subjects are classified as “good heartbeat perceivers” when their scores fall above a predetermined level (Herbert, Ulbrich, & Schandry, 2007). Heartbeat detection has been the more commonly utilized measure, perhaps because it appears to suffer from less methodological confounds than heartbeat tracking. Such confounds include the lack of a statistical measure to evaluate individual performance, the possible influence of a priori knowledge about average heart rate on the rate of counting (Phillips, Jones, Rieger, & Snell, 1999; Ring & Brener, 1996), and the insensitivity of heartbeat tracking tasks to changes in heart rate (Windmann, Schonecke, Frohlig, & Maldener, 1999). Consequently, we selected heartbeat detection as an index of interoceptive awareness.
We identified experienced meditators from two different meditation traditions that are extensively practiced within the United States: Tibetan Buddhism and Kundalini yoga. These traditions were selected to examine whether the effects of the meditation practice on interoceptive awareness were consistent across traditions, despite the fact that each tradition adopts slightly differing approaches to the cultivation of interoceptive awareness. For example, in Tibetan Buddhism interoceptive awareness is more commonly cultivated while meditating under resting physiological conditions, whereas in Kundalini yoga interoceptive awareness is more commonly cultivated during yoga exercises that elicit conditions of mild physiological arousal.
We hypothesized that the long-term practice of meditation leads to enhanced interoceptive awareness. On this basis, we predicted that experienced meditators from both traditions would display enhanced awareness of heartbeat sensations during performance of a heartbeat detection task at rest. We further hypothesized that experienced meditators would display metacognitive awareness of this enhancement, that is, knowledge of accurate self performance, based on the rationale that meditation cultivates a monitoring of experience at levels beyond mere interoceptive processing. We predicted that metacognitive awareness would be reflected through more accurate subjective ratings of interoceptive task performance in both groups of meditators than in nonmeditators.
Methods
Participants
Seventeen nonmeditators, 17 Kundalini meditators, and 13 Tibetan Buddhist meditators participated in the study (Table 1). Meditators were selected according to three criteria: (1) a minimum of 15 years of formal meditation practice, (2) a self reported strong daily practice, and (3) having attended at least one meditation retreat during the previous year. Nonmeditators were identified as individuals who had never attended a formal yoga or meditation course and did not practice self-taught meditation. All groups were matched with respect to age and body mass index. Any participant reporting a history of neurological or psychiatric disease was excluded from the study. Based on this criterion, 1 Kundalini meditator and 2 nonmeditators were precluded from study participation. This study was approved by the University of Iowa’s Institutional Review Board, and all participants provided informed consent prior to participation.
Table 1.
Demographic Data for All Three Groups (Means ± SD)
| Nonmeditators | Kundalini | Tibetan Buddhist | |
|---|---|---|---|
| Sex | 4 M:13 F | 5 M:12 F | 7 M:6 F |
| Age (years) | 50.6 ± 9.6 | 52.1 ± 8.6 | 48.8 ± 10.1 |
| Body mass index | 24.8 ± 5.1 | 24.0 ± 5.2 | 22.3 ± 3.3 |
| Meditation practice (years) | 0 ± 0 | 29.3 ± 6.4 | 24.7 ± 8.4 |
| Cumulative meditation practice (hours) | 0 ± 0 | 17,660 ± 9128 | 24,903 ± 14,270 |
Tasks
Participants performed two types of tasks: a pulse detection familiarization task and a heartbeat detection task. Each task utilized identical stimuli but required a different attentional focus. During pulse detection, participants took their nondominant wrist pulse and were required to judge whether a train of exteroceptive stimuli (800-Hz, 50-ms tones) were simultaneous or nonsimultaneous with pulse sensations. During heartbeat detection participants were not allowed to take their pulse and were required to judge whether the tones were simultaneous or nonsimultaneous with perceived heartbeat sensations.
Tone Delivery
Tone delivery was triggered by each myocardial contraction, as measured (indirectly) from the R-wave of a lead II electrocardiogram(MP100 acquisition unit, Biopac Systems, Inc.). During simultaneous trials, tones were delivered at the same time as the participant’s own finger pulse, approximately 250–300 ms after the R-wave1 (corresponding to the R-wave to pulse interval, or RPI). The finger pulse was measured with an infrared photo-plethysmograph (TSD123B) attached to the distal phalange of the fifth digit of the dominant hand. The RPI was measured for each participant by calculating the average delay between the peak of the R-wave and the foot of the systolic upstroke measured during a 2-min resting period. Mean resting heart rate and RPI intervals are listed in Table 2.2 During nonsimultaneous trials, tones were delivered 400 ms after the RPI, approximately 650–700 ms after the R-wave. Thus, tone delivery was temporally linked to each participant’s actual heartbeat during each trial. Trial order was randomized within and across each block. Tones were presented through noise canceling headphones (QuietComfort, Bose Inc., Framingham, MA). All participants performed the tasks in the supine position with their eyes closed, and they were given an unlimited time to respond during each trial.
Table 2.
Cardiovascular Parameters for All Three Groups (Means ± SD)
| Nonmeditators | Kundalini | Tibetan Buddhist | |
|---|---|---|---|
| Resting heart rate: Visit 1 (bpm) | 63.3 ± 10.0 | 67.0 ± 8.7 | 65.6 ± 14.9 |
| Resting heart rate: Visit 2 (bpm) | 65.8 ± 8.4 | 68.8 ± 11.5 | 65.3 ± 15.3 |
| R-wave to pulse interval: Visit 1 (s) | 0.269 ± 0.017 | 0.261 ± 0.013 | 0.266 ± 0.016 |
| R-wave to pulse interval: Visit 2 (s) | 0.266 ± 0.015 | 0.260 ± 0.014 | 0.264 ± 0.014 |
Procedure
The study involved two visits, spaced 1–14 days apart. At the beginning of each visit, resting pulse and heart rate were measured for 2 min. After each resting period, the pulse plethysmograph was removed in order to prevent participants from deriving heartbeat information from the finger pulse sensation during the subsequent tasks. During the first visit, participants performed one block of pulse detection followed by two blocks of heartbeat detection, in the same order. During the second visit participants repeated both blocks of heartbeat detection, in the same order as before. All blocks consisted of 23 trials. Any participant not meeting the criterion for good pulse detection (≥ 16 out of 23 trials correct, p<.05 per binomial test) during the first visit was excused from the study (1 nonmeditator was excluded based on this criteria). During the first heartbeat detection block (HB1), participants were instructed to breathe normally. During the second heartbeat detection block (HB2), participants were instructed to practice a yogic breathing pattern: Ujjai breath, a technique that involves symmetric long deep nostril breathing against airway resistance (Brown & Gerbarg, 2005). Respiratory rate was measured during all tasks with a thoracic respiratory belt (RSP100C). Each participant’s respiratory patterns were examined for compliance with the Ujjai breathing condition after completion of the task. All participants demonstrated satisfactory respiratory patterns, indicating accurate performance of the Ujjai breath. Aside from this breathing technique, no formal meditation instruction was given to the participants. We controlled for breathing patterns during heartbeat detection for two related reasons: (1) spontaneous respiratory manipulations have been observed to occur in subjects in the absence of an instruction to breathe normally and have been suggested as a potential strategy for maximizing heartbeat sensations (Jones, 1994; Weisz, Balazs, & Adam, 1988), and (2) several meditators were observed to spontaneously display Ujjai breathing during the piloting phase of the study and this, in and of itself, could be a basis for enhanced interoceptive accuracy. One Kundalini meditator was unable to perform the Ujjai breathing due to a pre-existing respiratory condition and was excluded from the heartbeat detection analysis.
Subjective Ratings
Prior to performing each task, participants were asked to predict task accuracy (e.g., “How good do you think you will be at [task X]?”) and difficulty (“How hard do you think [task X] will be?”). Upon completion of each task participants were also asked to estimate task accuracy (“How good do you think you were at [task X]?”) and difficulty (“How hard do you think [task X] was?”). Accuracy ratings could range from 1 (very bad) to 5 (very good). Difficulty ratings could range from 1 (very hard) to 5 (very easy). To familiarize participants with each task, all participants were instructed to sample tones from each trial type (i.e., one simultaneous and one nonsimultaneous trial) for an unlimited period prior to performing each task. In addition, task-related feedback was withheld from all participants until the conclusion of the study.
Accuracy Measures
Accuracy scores were calculated using A′ = [1/2+((HR − FP)(1+HR − FP))/(4HR(1 − FP))], a nonparametric signal detection analog of d′ ideal for signal detection conditions with low trial numbers (Grier, 1971). In this formula, HR = hit rate and FP = false positive. Following methods commonly utilized in heartbeat detection studies (Brener et al., 1993; Jones, O’Leary, & Pipkin, 1984; Rouse et al., 1988), A′ scores were normalized using the following formula: 2arcsin (sqrt A′), such that performance ranged from 0 to π (chance = π/2). Participants were further classified as “good heartbeat detectors” if they displayed above chance performance during a block of testing, defined as ≥ 16 out of 23 trials correct, p<.05 per binomial test, again following the approach of previous studies (Katkin et al., 2001; Schneider et al., 1998; Wiens & Palmer, 2001). Because no differences were predicted between the two groups of meditators, the overall analyses examined the three groups separately. All univariate repeated measures ANOVA tests were assessed for violations of the sphericity assumption, and when violated, were corrected with the Huynh–Feldt method. In these instances the corrected p values are reported, along with the Huynh–Feldt ε correction.
Results
Participants
Both groups of meditators reported significantly more years of meditation practice, F(2, 44) = 120.5, p<.001, and hours of cumulative meditation practice, F(2, 44) = 31.9, p<.001, than the nonmeditators. The groups did not differ with respect to age F(2, 44) = .45, p = .64, or BMI F(2, 44) = 1.07, p = .35, (Table 1). There were also no differences between the proportion of men and women in the nonmeditators and the Kundalini meditators, χ1 = .15, p = .70, or the Tibetan Buddhist meditators χ1 = 1.76, p = .19.
Cardiovascular Parameters
A 3 × 2 repeated measures ANOVA did not reveal any group differences in resting heart rate, F(2, 44) = 0.42, p = .66. There was no effect of visit on resting heart rate, F(1, 44) = 2.18, p = .15, and there were no Group × Visit interactions, F(2, 44) = 0.86, p = .43. The groups also did not differ with respect to the R-wave to pulse interval, F(2, 44) = 1.34, p = .26. There was no effect of visit on the R-wave to pulse interval, F(1, 44) = 0.10, p = .75, and there were no Group × Visit interactions, F(2, 44) = 0.38, p = .69.
Accuracy Measures
There were no group differences in accuracy on the pulse detection task, F (2, 44) = 1.27, p = .29. A 3 × 2 × 2 ANOVA was run on heartbeat detection accuracy with group (Kundalini, Tibetan Buddhist, nonmeditators) as the between-subjects factor and with block (Block 1 and Block 2) and visit (Visit 1 and Visit 2) as within-subject factors. There were no main effects for group, F(2, 43) = 0.30, p = .74, αp2 = .01, block, F(1, 43) = 0.69, p = .41, or visit, F(1, 43) = 1.38, p = .25, and there were no significant interactions between group and visit, F(2, 43) = 0.77, p = .47, block and visit, F(1, 43) = .00, p = .99, or group and block and visit, F(2, 43) = 1.03, p = .37, (Figure 1). The lack of group differences was not accounted for by group differences in response bias, defined as the tendency to favor one particular response type over another, for either pulse detection, F(2, 44) = 0.1, p = .91, or heartbeat detection, F(2, 43) = 1.3, p = .28.
Figure 1.
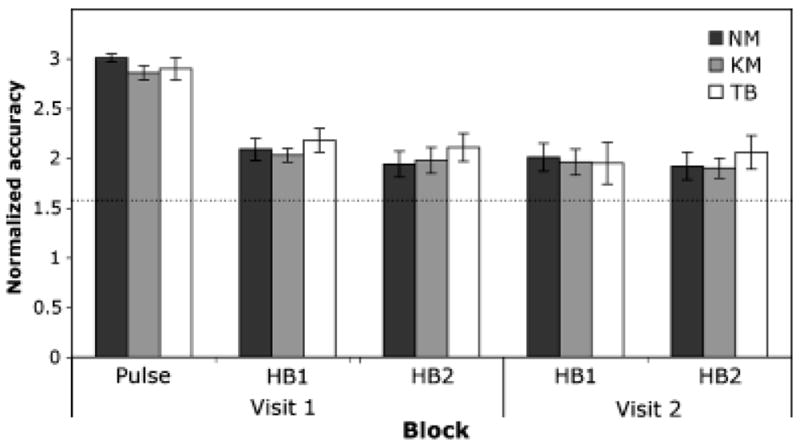
Heartbeat detection and pulse detection accuracy across each block for each group. HB1: heartbeat detection during normal breathing. HB2: heartbeat detection during Ujjai breathing. NM: nonmeditators. KM: Kundalini meditators. TB: Tibetan Buddhist meditators. Performance accuracy could range from 0 to π, with chance performance = π/2. Dotted line = chance, Error bars: SE.
There were also no differences in the proportion of meditators classified as good heartbeat detectors (Table 3). This was equally true when the definition of good heartbeat detection performance was restricted to above chance performance on two out of two visits or loosened to above chance performance on at least one out of two visits, for both tasks (Table 3).
Table 3.
Percentage of Individuals Classified as Good Heartbeat Detectors for Each Block of Heartbeat Detectiona
| No. of visits above chance |
||||||
|---|---|---|---|---|---|---|
| Good heartbeat detectors: HB1 |
Good heartbeat detectors: HB2 |
|||||
| 0/2 | 1/2 | 2/2 | 0/2 | 1/2 | 2/2 | |
| NM (n =17) | 47% (8) | 18% (3) | 35% (6) | 47% (8) | 18% (3) | 35% (6) |
| KM (n =17) | 35% (6) | 47% (8) | 17% (3) | 38% (6) | 50% (8) | 13% (2) |
| visits | , p= .24 | , p= .29 | ||||
| visits | , p= .49 | χ1 = .14, p= .35 | ||||
| TB (n =13) | 46% (6) | 23% (3) | 31% (4) | 31% (4) | 38% (5) | 31% (4) |
| visits | χ1 = .002, p= .48 | χ1 = .28, p= .30 | ||||
| visits | χ1 = .02, p= .44 | χ1 = .11, p= .37 | ||||
The numbers of individuals meeting each criterion are listed in parentheses. A Chi-square with one degree of freedom compares whether the proportion of meditators classified as good heartbeat detectors differs from the nonmeditators. NM: nonmeditators. KM: Kundalini meditators. TB: Tibetan Buddhist meditators. Only 16 Kundalini meditators completed testing in Block 2.
Subjective Ratings
There were no group differences in the ratings of pulse detection accuracy, F(2, 44) = 0.12, p = .89, or pulse detection difficulty, F(2, 44) = .76, p = .48. However, a 3 × 2× 2 ANOVA with two repeated measures factors revealed significant group differences in ratings of heartbeat detection accuracy, F(2, 43) = 5.77, p = .007, αp2 = .21, and heartbeat detection difficulty, F(2, 43) = 4.1, p = .023, αp2 = .16, with both groups of meditators rating their heartbeat detection performance to be more accurate and the task to be less difficult than the nonmeditators (Figures 2 and 3). There were no other significant main effects or interactions for ratings of heartbeat detection accuracy. For ratings of heartbeat detection difficulty, there was a significant effect of visit, F(2, 1) = 4.5, p = .039, αp2 = .10, with all groups rating both tasks as less difficult on the second visit. There was a significant interaction between group and block, F(2, 2) = 4.7, p = .015, αp2 = .18, with both groups of meditators rating each block to be less difficult than the nonmeditators. There was also a significant interaction between block and visit, F(2, 1) = 4.3, p = .045, αp2 = .10, with all groups rating blocks from the second visit to be easier than the first visit.
Figure 2.
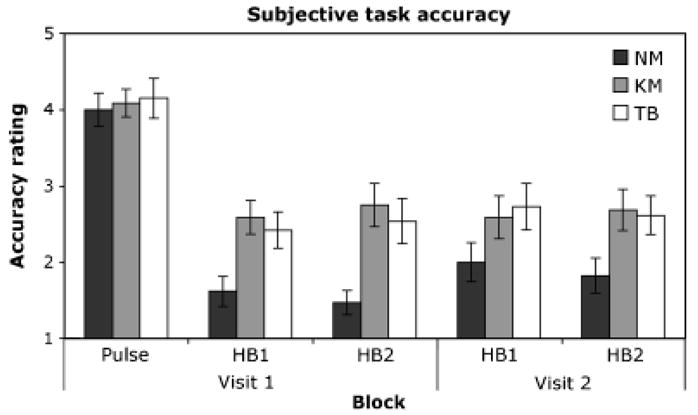
Mean ratings of pulse and heartbeat detection accuracy across each block for each group. Accuracy ratings could range from 1 (very bad) to 5 (very good). NM: nonmeditators. KM: Kundalini meditators. TB: Tibetan Buddhist meditators. Error bars: SE.
Figure 3.
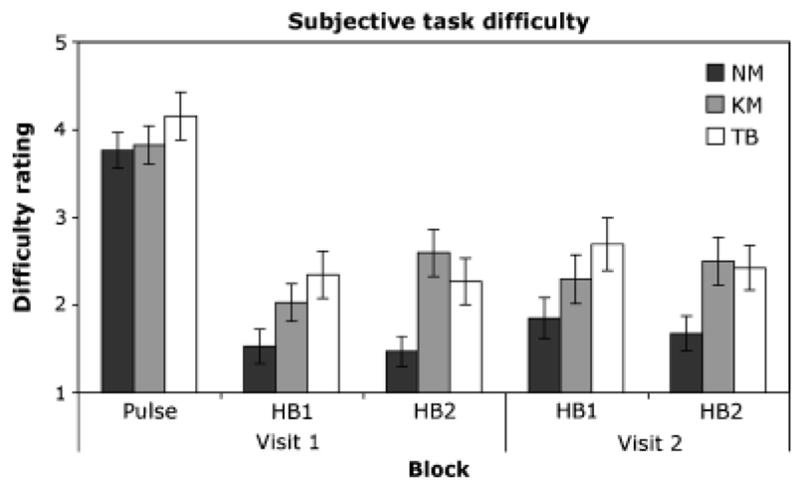
Mean ratings of pulse and heartbeat detection difficulty across each block for each group. Difficulty ratings could range from 1 (very hard) to 5 (very easy). NM: nonmeditators. KM: Kundalini meditators. TB: Tibetan Buddhist meditators. Error bars: SE.
Post Analysis
Both groups of meditators displayed higher subjective ratings of heartbeat detection accuracy and lower subjective ratings of heartbeat detection difficulty than nonmeditators. Because these findings occurred in the absence of an actual difference in heartbeat detection accuracy, we examined the relationship between the objective accuracy scores and the subjective accuracy ratings provided by each participant after the pulse and heartbeat detection tasks. Because maximum objective accuracy was almost always reached for pulse detection, we assumed that the subjective accuracy ratings for pulse detection represented the maximum possible range for subjective accuracy ratings in general. We then normalized each participant’s subjective heartbeat detection rating by the global average subjective pulse detection accuracy ratings measured from all participants using the following formula: (individual heartbeat detection accuracy rating-global mean pulse detection accuracy rating)/global mean pulse detection accuracy rating. We also normalized each group’s heartbeat detection accuracy scores (A′) by the mean pulse detection accuracy scores using this same procedure. Finally, we examined the relationship between these normalized heartbeat detection ratings and normalized accuracy scores for each group for congruency. We found that, on average, meditators’ subjective accuracy ratings appeared more congruent with objective accuracy scores than the nonmeditators (Table 4). There was a nearly significant interaction between group and scale, F(2, 2) = 2.6, p = .08, αp2 = .1, in support of this dissociation.
Table 4.
Normalized Objective Heartbeat Detection Accuracy and Subjective Ratings of Heartbeat Detection Accuracya
| Nonmeditators | Kundalini | Tibetan Buddhist | |
|---|---|---|---|
| Normalized objective heartbeat detection accuracy | 0.45 (0.10) | 0.40 (0.10) | 0.46 (0.14) |
| Normalized subjective heartbeat detection accuracy | 0.27 (0.08) | 0.51 (0.09) | 0.51 (0.10) |
Means (SE), range 0–1.
Discussion
The current findings do not support the hypothesis that experienced meditators would display increased interoceptive awareness, as meditators did not differ from nonmeditators in heartbeat detection accuracy. The lack of an effect of meditation on awareness of heartbeat sensations appears to be a reliable finding. It occurs in two different groups of experienced meditators, measured at two time points, and with two different respiratory manipulation strategies. These results are consistent with recent findings by Nielsen and Kaszniak (2006), who reported a lack of significant differences between a group of Buddhist meditators and a group of nonmeditators on a single session of standard heartbeat detection. The Nielsen and Kaszniak study had a small sample size, did not include comparison subjects matched for age or body mass (Rouse et al., 1988), and was conducted in a small number of sessions, limiting statistical power. The present study, however, did not suffer from any of these limitations and still did not reveal any effect of meditation on interoceptive awareness. We conducted a power analysis based on the observed main effect to determine the sample size required to achieve a statistically meaningful result for heartbeat detection. We found that group sizes would need to be increased by one order of magnitude before reaching the threshold of significance. Thus if the sample sizes were increased 10-fold, the main effect would be as follows: F(2, 457) = 3.21, p = .041, αp2 = .01. Even if this were the case, the presumed effect size suggests that the influence of meditative experience on resting awareness of heartbeat sensations would be quite small.
Sex differences in heartbeat detection ability have sometimes been reported in the literature (Jones & Hollandsworth, 1981; Katkin, Blascovich, & Goldband, 1981; Whitehead et al., 1977; but see Ring & Brener, 1992; Rouse et al., 1988). There were no significant group differences between the proportion of males and females in the current study. However, the sample sizes may not be large enough to test whether sex differences influenced the findings. In any event, it seems unlikely that sex played a role in the current findings: We examined whether there were any sex related differences in interoceptive accuracy and found that none of the results differed with respect to sex.
Although we believe that the current finding is reliable, it is important to consider alternatives that might explain it. First, it is possible that awareness of heartbeat sensations alone is a poor index of the type of interoceptive sensations cultivated by the practice of meditation. Although attention to body sensations such as the heartbeat is practiced at some point of the training in all meditation traditions, attention is more commonly directed toward breathing. Any enhancement of interoceptive sensations that results from the long-term practice of meditation might be specific only to the bodily signals that are attended. Thus the current results do not rule out the possibility that meditation cultivates interoceptive awareness for other body signals such as breathing. Second, attention to nonbodily signals is also frequently practiced in all meditation traditions. For example, in the Tibetan Buddhist tradition, attention is commonly focused on complex mental imagery or external visual objects during meditation (Lutz et al., 2007), and the awareness that develops during such attention training might not translate readily into an enhancement of interoceptive awareness. Third, the current study only examined interoceptive awareness under resting conditions. We chose this starting point because the meditation practice most commonly occurs during those conditions. It is possible that interoceptive awareness for heartbeat sensations is limited at rest by a physiological mechanism not amenable to voluntary modulation, even through a long-standing meditation practice. Of note, none of the groups in the current study displayed heartbeat detection rates above 50%. These rates are consistent with those routinely reported in the heartbeat detection literature (Brener & Kluvitse, 1988; Eichler & Katkin, 1994; Jones, 1994; Knapp et al., 1997; Ring & Brener, 1992; Wiens & Palmer, 2001), suggesting that it is difficult for most individuals to display awareness of heartbeat sensations at rest. However, the current finding does not guarantee that meditation would not be associated with enhanced interoceptive awareness under other physiological conditions. Indeed, visceral sensations do not dominate conscious experience under resting conditions, but quickly develop when conditions such as exercise or stress signal deviations in the homeostatic state (Cameron & Minoshima, 2002; Khalsa et al., in press). Thus it is still possible that meditators would display increased interoceptive awareness under these conditions. Such considerations argue for the development of new measures of interoceptive awareness that take into account nonhomeostatic physiological body states.
Both groups of meditators displayed higher subjective ratings of heartbeat detection accuracy and lower subjective ratings of heartbeat detection difficulty than nonmeditators. In the absence of an actual difference in heartbeat detection accuracy these findings were surprising. The ratings differences did not appear to be due to a general rating bias, as there were no such group differences in the ratings of the pulse detection task. All groups displayed accurate pulse detection performance, rated their accuracy accordingly, and found the task to be easy. With respect to heartbeat detection, the nonmeditators’ ratings were near the bottom of each scale, suggesting that they found the task to be difficult and felt that their performance was very poor. The meditators’ ratings were near the middle of the scale, suggesting that they found the task to be neither easy nor difficult and felt that their performance was neither good nor bad. These rating differences could be explained either by the nonmeditators underestimating their performance or the meditators overestimating their performance. Even though both groups displayed performance within the normally reported range on the heartbeat detection task, “normal” performance is quite often below chance, given the fact that heartbeat detection is most commonly measured at rest. Thus it could be argued that either the meditators’ or the nonmeditators’ ratings were congruent with the literature. An analysis of the relationship between each group’s objective accuracy scores and subjective ratings of accuracy suggests it is more likely that the nonmeditators were underestimating their heartbeat detection performance and provides support, albeit limited, for the notion that experienced meditators’ subjective perceptions of interoceptive states are more in tune with their performance on interoceptive tasks.
Overall, the results of this study provide evidence against the notion that practicing attention to internal body sensations, a core feature of meditation, enhances resting interoceptive awareness.
Acknowledgments
We thank M. Ricard and A. Francis for assistance with participant recruitment. The project was supported by NIH NCCAM F31AT003061 from the National Center For Complementary & Alternative Medicine (NCCAM) (S.K.), by the Mind and Life Institute (S.K.), by NCCAM U01AT002114-01A1 (A.L.), and by NIDA R01 DA022549 (D.T.).
Footnotes
This delay, around 250 to 300 ms, has been shown to lead to the perception by accurate heartbeat detectors that heartbeats and tones are “simultaneous” (Brener et al., 1993; Eichler & Katkin, 1994; Jones, 1994; Knapp et al., 1997; Ring & Brener, 1992; Rouse et al., 1988; Schandry et al., 1993).
These intervals are similar to those observed when measuring the RPI from the finger pulse (Teng & Zhang, 2006). However, shorter intervals have been reported when measuring the RPI from the ear pulse, possibly due to the shorter distance traveled by the pulse wave (de Boer, Ring, Curlett, Ridley, & Carroll, 2007; de Boer, Ring, Wood, et al., 2007).
References
- Arambula P, Peper E, Kawakami M, Gibney KH. The physiological correlates of Kundalini Yoga meditation: A study of a yoga master. Applied Psychophysiology and Biofeedback. 2001;26:147–153. doi: 10.1023/a:1011343307783. [DOI] [PubMed] [Google Scholar]
- Arias AJ, Steinberg K, Banga A, Trestman RL. Systematic review of the efficacy of meditation techniques as treatments for medical illness. Journal of Alternative and Complementary Medicine. 2006;12:817–832. doi: 10.1089/acm.2006.12.817. [DOI] [PubMed] [Google Scholar]
- Astin JA, Shapiro SL, Eisenberg DM, Forys KL. Mind-body medicine: State of the science, implications for practice. Journal of the American Board of Family Practice. 2003;16:131–147. doi: 10.3122/jabfm.16.2.131. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Powell-Griner E, McFann K, Nahin RL. Advance data from vital and health statistics. Vol. 343. Hyattsville, MD: National Center for Health Statistics; 2004. Complementary and alternative medicine use among adults: United States, 2002. [PubMed] [Google Scholar]
- Bhajan Y, Khalsa GS. Breathwalk: Breathing your way to a revitalized body, mind and spirit. New York: Broadway Books; 2000. [Google Scholar]
- Brener J, Kluvitse C. Heartbeat detection: Judgments of the simultaneity of external stimuli and heartbeats. Psychophysiology. 1988;25:554–561. doi: 10.1111/j.1469-8986.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Brener J, Liu X, Ring C. A method of constant stimuli for examining heartbeat detection: Comparison with the Brener-Kluvitse and Whitehead methods. Psychophysiology. 1993;30:657–665. doi: 10.1111/j.1469-8986.1993.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: Part I—Neuro-physiologic model. Journal of Alternative and Complementary Medicine. 2005;11:189–201. doi: 10.1089/acm.2005.11.189. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Minoshima S. Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosomatic Medicine. 2002;64:851–861. doi: 10.1097/01.psy.0000038939.33335.32. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- de Boer D, Ring C, Curlett AC, Ridley M, Carroll D. Mental stress-induced hemoconcentration and its recovery: A controlled study of time course and mechanisms. Psychophysiology. 2007;44:161–169. doi: 10.1111/j.1469-8986.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- de Boer D, Ring C, Wood M, Ford C, Jessney N, McIntyre D, et al. Time course and mechanisms of mental stress-induced changes and their recovery: Hematocrit, colloid osmotic pressure, whole blood viscosity, coagulation times, and hemodynamic activity. Psychophysiology. 2007;44:639–649. doi: 10.1111/j.1469-8986.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Eichler S, Katkin ES. The relationship between cardiovascular reactivity and heartbeat detection. Psychophysiology. 1994;31:229–234. doi: 10.1111/j.1469-8986.1994.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Grier JB. Nonparametric indices for sensitivity and bias: Computing formulas. Psychological Bulletin. 1971;75:424–429. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Ulbrich P, Schandry R. Interoceptive sensitivity and physical effort: Implications for the self-control of physical load in everyday life. Psychophysiology. 2007;44:194–202. doi: 10.1111/j.1469-8986.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Holzl R, Erasmus LP, Moltner A. Detection, discrimination and sensation of visceral stimuli. Biological Psychology. 1996;42:199–214. doi: 10.1016/0301-0511(95)05155-4. [DOI] [PubMed] [Google Scholar]
- Jones GE. Perception of visceral sensations: A review of recent findings, methodologies, and future directions. Vol. 5. London: Jessica Kingsley Publishers; 1994. [Google Scholar]
- Jones GE, Hollandsworth JG. Heart rate discrimination before and after exercise-induced augmented cardiac activity. Psychophysiology. 1981;18:252–257. doi: 10.1111/j.1469-8986.1981.tb03029.x. [DOI] [PubMed] [Google Scholar]
- Jones GE, O’Leary RT, Pipkin BL. Comparison of the Brener-Jones and Whitehead procedures for assessing cardiac awareness. Psychophysiology. 1984;21:143–148. doi: 10.1111/j.1469-8986.1984.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Delta Trade Paperbacks; 1990. [Google Scholar]
- Katkin ES, Blascovich J, Goldband S. Empirical assessment of visceral self-perception: Individual and sex differences in the acquisition of heartbeat discrimination. Journal of Personality and Social Psychology. 1981;40:1095–1101. doi: 10.1037//0022-3514.40.6.1095. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Wiens S, Ohman A. Nonconscious fear conditioning, visceral perception, and the development of gut feelings. Psychological Science. 2001;12:366–370. doi: 10.1111/1467-9280.00368. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2008.08.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp K, Ring C, Brener J. Sensitivity to mechanical stimuli and the role of general sensory and perceptual processes in heartbeat detection. Psychophysiology. 1997;34:467–473. doi: 10.1111/j.1469-8986.1997.tb02391.x. [DOI] [PubMed] [Google Scholar]
- Kornfield J. Living dharma: Teachings of twelve Buddhist masters. Boston: Shambala Publications; 1996. [Google Scholar]
- Lutz A, Dunne JD, Davidson RJ. Meditation and the neuroscience of consciousness: An introduction. New York: Cambridge University Press; 2007. [Google Scholar]
- Nairn R. What is meditation? Buddhism for everyone. Boston: Shambhala Publications; 2000. [Google Scholar]
- Nielsen L, Kaszniak AW. Awareness of subtle emotional feelings: A comparison of long-term meditators and nonmeditators. Emotion. 2006;6:392–405. doi: 10.1037/1528-3542.6.3.392. [DOI] [PubMed] [Google Scholar]
- Peng CK, Henry IC, Mietus JE, Hausdorff JM, Khalsa G, Benson H, et al. Heart rate dynamics during three forms of meditation. International Journal of Cardiology. 2004;95:19–27. doi: 10.1016/j.ijcard.2003.02.006. [DOI] [PubMed] [Google Scholar]
- Phillips GC, Jones GE, Rieger EJ, Snell JB. Effects of the presentation of false heart-rate feedback on the performance of two common heartbeat-detection tasks. Psychophysiology. 1999;36:504–510. doi: 10.1017/s0048577299980071. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Ring C, Brener J. The temporal locations of heartbeat sensations. Psychophysiology. 1992;29:535–545. doi: 10.1111/j.1469-8986.1992.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Ring C, Brener J. Influence of beliefs about heart rate and actual heart rate on heartbeat counting. Psychophysiology. 1996;33:541–546. doi: 10.1111/j.1469-8986.1996.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Rouse CH, Jones GE, Jones KR. The effect of body composition and gender on cardiac awareness. Psychophysiology. 1988;25:400–407. doi: 10.1111/j.1469-8986.1988.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schandry R, Bestler M, Montoya P. On the relation between cardiodynamics and heartbeat perception. Psychophysiology. 1993;30:467–474. doi: 10.1111/j.1469-8986.1993.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Schneider TR, Ring C, Katkin ES. A test of the validity of the method of constant stimuli as an index of heartbeat detection. Psychophysiology. 1998;35:86–89. [PubMed] [Google Scholar]
- Selby J. Kundalini awakening: a gentle guide to Chakra activation and spiritual growth. New York: Bantam Books; 1992. [Google Scholar]
- Teng XF, Zhang YT. The effect of applied sensor contact force on pulse transit time. Physiological Measurement. 2006;27:675–684. doi: 10.1088/0967-3334/27/8/002. [DOI] [PubMed] [Google Scholar]
- Weisz J, Balazs L, Adam G. The influence of self-focused attention on heartbeat perception. Psychophysiology. 1988;25:193–199. doi: 10.1111/j.1469-8986.1988.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM, Heiman P. Relation of heart rate control to heartbeat perception. Biofeedback and Self-Regulation. 1977;2:371–392. [PubMed] [Google Scholar]
- Wiens S, Palmer SN. Quadratic trend analysis and heartbeat detection. Biological Psychology. 2001;58:159–175. doi: 10.1016/s0301-0511(01)00110-7. [DOI] [PubMed] [Google Scholar]
- Windmann S, Schonecke OW, Frohlig G, Maldener G. Dissociating beliefs about heart rates and actual heart rates in patients with cardiac pacemakers. Psychophysiology. 1999;36:339–342. doi: 10.1017/s0048577299980381. [DOI] [PubMed] [Google Scholar]


