Abstract
Background
Thoracic aortic dissection (TAD) is characterized by dysregulated extracellular matrix. Little is known about the alterations of collagen and stimulators of collagen synthesis, eg, connective tissue growth factor (CTGF), in patients with TAD. In this study, we examined their roles in TAD.
Methods and Results
Surgical specimens of the aortic wall of TAD patients (n=10) and controls (n=10) were tested for collagen types I and III and CTGF expression. When compared with controls, protein levels of type I and III collagen and CTGF were significantly increased by 3.2-, 3.7-, and 5.3-fold, respectively (P<0.05 for all). Similar patterns were shown in mRNA levels of type Iα and Iα2 collagen and CTGF. Using immunohistochemistry and trichrome staining, we also observed elevated levels of collagen in the aortic media and adventitia. Treatment with recombinant human CTGF increased collagen synthesis in cultured aortic smooth muscle cells in a dose- and time-dependent fashion, in which expression of collagens increased from 506±108 counts per minute to 2764±240 cpm by 50 ng/mL CTGF, and from 30±43 cpm to 429±102 cpm at 48 hours.
Conclusions
TAD patients exhibited significantly increased expression of aortic collagen types I and III as well as CTGF, which is likely to be responsible for the compromised aortic distensibility and systemic compliance. Because CTGF can increase collagen expression, CTGF may be a new target molecule in the pathogenesis and progression of TAD.
Keywords: aorta, collagen, defects, growth substances, muscle, smooth
Thoracic aortic dissection (TAD) is one of the leading causes of aortic catastrophe in the United States. TAD begins with a tear through the intima and into the media, which leads to medial separation along the length of the vessel and creates a false lumen. The freshly torn aorta is severely weakened and may rupture. Ascending aortic involvement may result in death from wall rupture, hemo-pericardium and tamponade, occlusion of the coronary ostia with myocardial infarction, or severe aortic insufficiency. Chronic aortic dissections are also a common cause of thoracic aortic aneurysm.1 The mortality rate of untreated dissection approaches 1% per hour during the first 48 hours. Medical management alone is associated with a mortality rate of 50% within the first month after presentation.2 Despite extensive research, the mechanisms responsible for initiating dissection remain elusive. TAD patients often have a weakened aortic media with some types of extracellular matrix (ECM) disorders.1
Collagen and elastin are the most abundant ECM proteins of the aortic wall, and they are responsible for the characteristic mechanical properties—tensile strength and elasticity.3 Among at least 25 different collagens, 13 may be present in the blood vessel wall. However, type I and III collagens are the predominant fiber-forming species in the arterial wall, which in general fulfill the mechanical function of imparting tensile strength and elastic resilience. Type I collagen is believed to impart arterial stiffness, whereas type III collagen is associated with extensibility of the vessel wall.4
Although most studies have documented the decreased and fragmented elastin in aortic aneurysmal tissues,1 collagen content is variable in such patients, including those with abdominal aortic aneurysm.5,6 Little is known about the alteration of collagen and stimulators of collagen synthesis in patients with aortic dissections. Connective tissue growth factor (CTGF), which can be activated by upstream transforming growth factor-β signaling, plays an important role in stimulating the proliferation of connective tissue cells, eg, fibroblasts, and ECM production. CTGF belongs to the CCN family of proteins, which includes CTGF/Hcs24, CYR61, Nov, and the newly discovered WISP1/elm1, WISP2/rCop1, and WISP3.7 CTGF is overexpressed in a variety of fibrotic disorders, such as scleroderma.7 Studies of diseased tissues from human clinical specimens and animal models have also shown that CTGF is overexpressed in atherosclerotic arteries and infarcted myocardium.8–10 However, the presence, localization, and function of CTGF in aortic dissection remain unknown.
Vascular smooth muscle cells (VSMCs), the most abundant cell type in the aortic media, are the main source for the ECM proteins, including collagen, elastin, laminin, gelatin, and proteoglycans in the arterial tunica media, which confer strength and elasticity to the aortic wall. Although overexpression of CTGF can stimulate collagen synthesis in fibroblasts, the relationship between CTGF and collagen production in VSMCs is unclear.
In the present study, we investigated the relevance of collagen and CTGF to aortic dissection both in vivo in TAD patients and in vitro in cultured aortic VSMCs. We demonstrate that TAD patients have increased types I and III collagen in the aortic wall, as well as elevated CTGF expression.
Methods
For details, please refer to the online-only Data Supplement available at circ.ahajournals.org.
Patient Enrollment and Tissue Collection
Written consent was obtained from all patients, as approved by the institutional review board, Baylor College of Medicine. Full-thickness (both outer wall and inner dissecting membrane) ascending aortic wall specimens were obtained from 10 patients undergoing surgical repair of aortic dissection; repair was elective in 9 patients and urgent in 1 patient with acute symptoms. Patients with Ehlers-Danlos syndrome, Marfan syndrome, and other known connective tissue disorders were excluded. None of the patients had dissection induced by trauma or cardiovascular surgery. As shown in Table 1, the original dissection involved the proximal aorta (DeBakey type I or II) in 9 (90%) patients. One patient initially had a distal aortic dissection (DeBakey type III) that progressed with proximal extension into the ascending aorta. For comparison, control specimens of ascending aorta (n=10) were obtained from organ donors (n=7), heart transplant recipients (n=1), and patients undergoing coronary artery bypass surgery (n=2).
Table 1.
Patient Characteristics
| TAD Patients (n=10) | Control Patients (n=10) | |
|---|---|---|
| Characteristics | ||
| Age, y | 57.6±9.6* | 47.7±13.5 |
| Sex, male:female | 9:1 | 9:1 |
| Race | ||
| White | 6 (60%) | 8 (80%) |
| Hispanic | 3 (30%) | 1 (10%) |
| Other | 1 (10%) | 1 (10%) |
| Taking antilipid medications | 6 (60%) | 3/7 (42%) |
| Hypertension | 10 (100%) | 3/4 (75%) |
| Diabetes mellitus | 0 | — |
| Smoking history (past or current) | 6 (60%) | 5/5 (100%) |
| Chronic obstructive pulmonary disease | 0 | — |
| Initial dissection type | ||
| DeBakey type I | 3 (30%) | — |
| DeBakey type II | 6 (60%) | — |
| DeBakey type III | 1 (10%) | — |
| Arteritis | 1 (10%) | 0 |
| Bicuspid aortic valve | 1 (10%) | — |
| Family history of aneurysms | 1 (10%) | 0 |
| Family history of dissection | 0 | 0 |
| Rupture | 0 | 0 |
| Maximal aortic diameter, cm | 6.55±1.41 | — |
| Interval since dissection, y | 5.9±4.5 | — |
| Acute symptomatic aneurysms | 1 (10%) | — |
| Chronic symptoms | 5 (50%) | — |
| Elective operation | 9 (90%) | — |
P<0.05 vs control group.
Immunohistochemical and Biochemical Measurements
Recombinant human (rh) CTGF was a generous gift from Drs Gary Grotendorst and Matthew Duncan (Lovelace Respiratory Research Institute, Albuquerque, NM). Human aortic VSMCs were purchased from Cambrex BioScience, Inc (Walkersville, Md). Collagens Iα1, Iα2, and IIIα1 and CTGF mRNA levels from aortic tissue were analyzed by quantitative real-time reverse transcription—polymerase chain reaction (RT-PCR) with use of a Bio-Rad iCycler system (Bio-Rad, Hercules, Calif). Western blot analysis and immunohistochemical staining were used to evaluate expression of collagen I and III and CTGF at the protein level. The aortic wall was stained with Masson’s trichrome to investigate the localization of collagen. Apoptosis in the human aortic wall was detected by terminal deoxynucleotidyl transferase (TdT)–mediated dUTP nick end-labeling (TUNEL) with use of an ApoAlert DNA fragmentation assay kit (BD Biosciences, Palo Alto, Calif). With [smcap]l-[2,3,4,5-3H]proline incorporation, collagen synthesis was measured from the supernatant of VSMCs stimulated by rhCTGF.
Statistical Analysis
Continuous variables are presented as the mean±SEM. Intergroup differences were analyzed by 1-way ANOVA for comparisons of 3 or more groups and independent Student’s t test for comparisons between 2 groups. A Bonferroni-adjusted probability value <0.05 was regarded as significant. χ2 analysis was used to examine the relationships among categorical variables. Statistical analysis was performed with SPSS 12.0 for Windows (SPSS Inc, Chicago, Ill).
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Elevated Collagen and Apoptosis in the Aortic Wall of TAD Patients
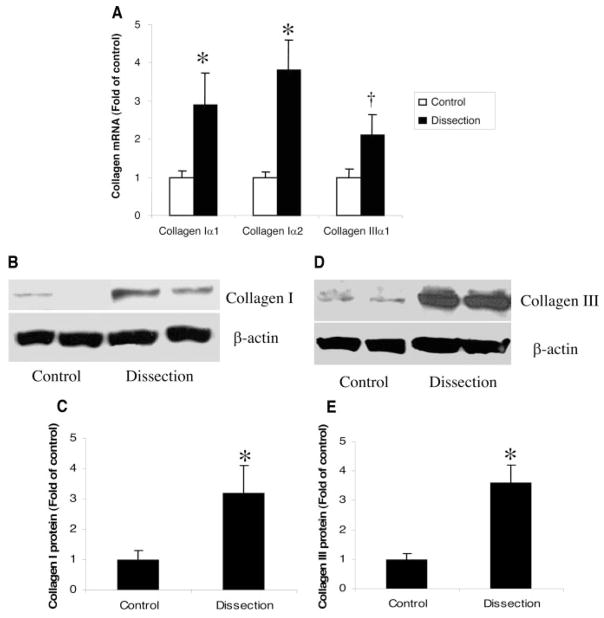
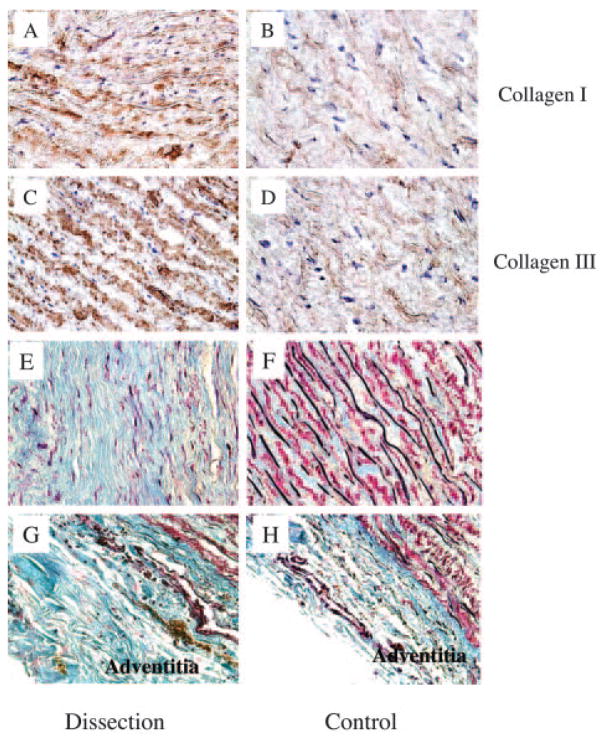
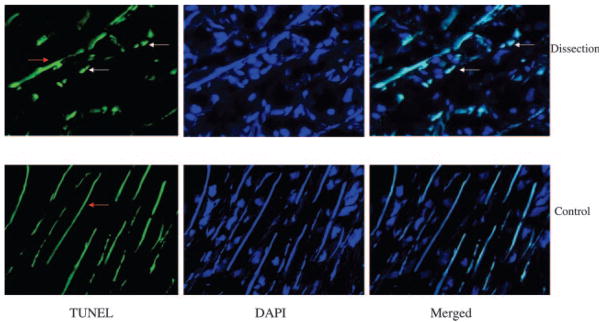
To compare collagen expression in TAD patients with that in controls, we measured both mRNA and protein levels of collagens in the aortic wall. Compared with controls, mRNA levels of both collagen Iα and Iα2 were significantly increased in TAD patients (P=0.04 and P=0.02, respectively). Collagen IIIα1 mRNA levels were also increased but did not reach statistical significance in TAD patients (P=0.09; Figure 1A). Moreover, analysis by Western blot showed significant increases in both type I and III collagen protein levels in TAD patients compared with controls (P=0.02 and P=0.01, respectively; Figure 1B through 1E). Immunohistochemistry also showed increased levels of collagens I and III in the media of the aortic wall from patients with TAD (Figure 2A–2D). To confirm the elevated collagen levels in TAD, the aortic wall was stained with Masson’s trichrome. As shown in Figure 2E and 2F, increased collagen and decreased elastin were localized to the aortic media from patients with TAD. Increased levels of collagen were also seen in the adventitia of the TAD aorta (Figure 2G and 2H). To analyze apoptosis in the aortic wall of TAD, TUNEL staining was used to identify the apoptotic cells in the aortic wall, which revealed increased apoptosis in the media of the aortic wall of TAD patients compared with controls (14.8±2.8 versus 3.5±1.1 cells/field, P=0.01; Figure 3).
Figure 1.
Expression of collagen in patients with TAD and controls. When compared with controls, TAD patients showed increased levels of collagen types Iα, Iα2, and IIIα1 mRNA by quantitative real-time RT-PCR (A). Similarly, on Western blots, patients with TAD showed significantly increased levels of type I (B, C) and type III (D, E) collagens. Densitometric measurement was performed to quantify the relative expression of collagens after Western blotting with the use of imaging densitometry and AlphaEaseFC software (C, E). Results shown represent mean±SEM of 10 samples. *P<0.05, †P=0.09 by Student t test.
Figure 2.
Representative immunohistochemical sections of aorta show increased staining for type I and III collagens in the medial VSMCs of TAD patients (A, C) compared with the normal aortas of controls (B, D). Immune complexes were detected with diami-nobenzidine substrate. Representative sections of aorta stained with Masson’s trichrome demonstrate increased collagen and decreased elastin in the media of the aortic wall of TAD patients (E) compared with controls (F). Similarly, collagen was also increased in the adventitia of TAD patients (G) compared with controls (H). Collagens are stained blue and elastins are stained black. Magnification ×40.
Figure 3.
Representative sections of aorta stained with the TUNEL method demonstrate increased apoptotic VSMCs in the media of TAD aortas. Tissue samples were stained with TUNEL (green, white arrows) and DAPI for nuclei (blue). Merged images show colocalization. Elastin was also non-specifically stained as green lines by TUNEL (red arrow). Magnification ×40.
Increased Expression of CTGF in the Aortic Wall of TAD Patients
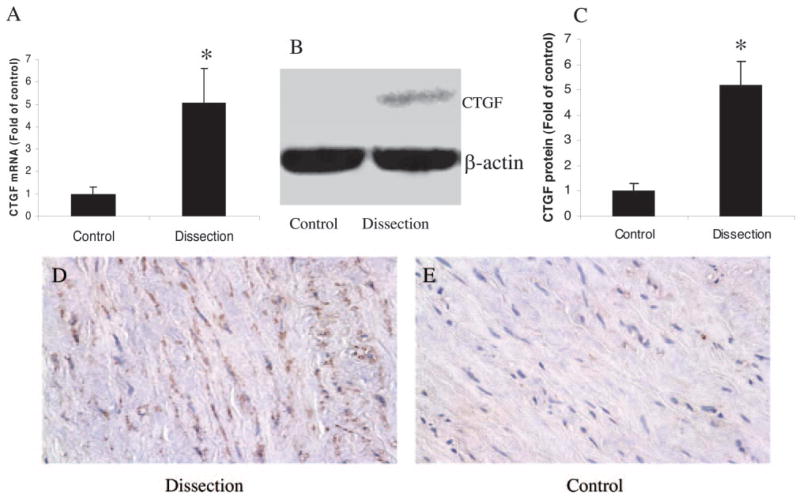
To compare CTGF expression in TAD patients with that in controls, we measured both mRNA and protein levels of CTGF in the aortic wall. Analysis of real-time RT-PCR and Western blot data showed that mRNA and protein levels of aortic CTGF expression were significantly increased compared with those in controls (P=0.02 and P=0.01, respectively; Figure 4A through 4C). Immunohistochemistry revealed increased positive staining for CTGF, which was mainly localized in medial VSMCs (Figure 4D and 4E).
Figure 4.
Expression of CTGF mRNA and protein in TAD patients and controls. Patients with TAD showed significantly increased mRNA (A) and protein (B, C) levels. Densitometric measurement was performed to quantify the relative expression of CTGF after Western blotting by imaging densitometry and AlphaEaseFC software (C). Results shown represent mean±SEM of 10 samples. Representative immunohistochemical sections of aorta demonstrate increased positive staining for CTGF in the medial VSMCs from TAD patients (D) compared with controls (E). Magnification:×40. *P<0.05.
Stimulating Effect of rhCTGF on Collagen Synthesis in VSMCs
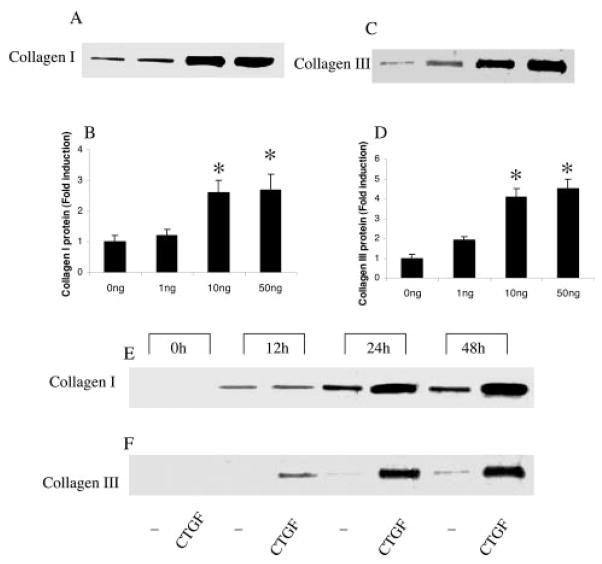
To evaluate the contribution of CTGF to vascular wall remodeling and aortic dissection, we explored collagen synthesis in VSMCs by treating them with rhCTGF. Total collagen synthesis was measured by [3H]proline incorporation. After a 48-hour incubation, rhCTGF significantly increased collagen synthesis in a dose-dependent fashion (with 0 ng/mL, 506±108 counts per minute [cpm]; 1 ng/mL, 501±110 cpm; 10 ng/mL, 1987±230 cpm; and 50 ng/mL, 2764±240 cpm; P=0.03 and 0.02 for 10 ng/mL and 50 ng/mL, respectively, versus 0 ng/mL). At 50 ng/mL, rhCTGF significantly increased collagen synthesis in a time-dependent fashion (rhCTGF versus controls, 30±43 versus 24±54 cpm at 0 hours; 66±55 versus 17±36 cpm at 12 hours; 1844±170 versus111±72 cpm at 24 hours; 2312±299 versus 429±102 cpm at 48 hours; P=0.03 and 0.01 at 24 and 48 hours, respectively, versus 0 hours). To further identify collagen I and III protein expression in VSMCs stimulated by rhCTGF, their levels were measured by Western blotting from the supernatants of these cells. The rhCTGF induced dose- and time-dependent increases in the expression of collagen I synthesized by VSMCs (Figure 5A, 5B, and 5E). Similarly, collagen III expression by VSMCs was also induced by rhCTGF dose- and time-dependently (Figure 5C, 5D, and 5F). When the ratios of collagen I to III were compared, there were no significant differences among different doses (P=0.75).
Figure 5.
rhCTGF induces type I and III collagens in cultured VSMCs in a dose- and time-dependent fashion. VSMCs were maintained in serum-free conditions for 48 hours. In the presence of 50 μg/mL ascorbic acid and 0.2 mmol/L [l]proline, VSMCs were incubated with increasing doses of rhCTGF for 48 hours or at a concentration of 50 ng/mL for up to 48 hours. Unstimulated medium was used as a negative control (0 ng rhCTGF). Conditioned supernatants of cultured cells were collected for use in the Western blot analyses. rhCTGF induced type I collagen synthesis, as shown by the increased levels in the supernatant of treated VSMCs, in a dose- (A, B) and time-(E) dependent fashion. Similarly, type III collagen also increased in a dose- (C, D) and time- (F) dependent fashion. Results shown represent mean±SEM of 3 independent experiments. One-way ANOVA was used to compare the differences among groups and *P<0.05 in comparison with baseline levels (0 ng CTGF or at 0 hour).
Discussion
The present study reports 3 novel findings (1): patients with TAD have increased type I and III collagens in both the media and adventitia of the aortic wall; (2) aortic wall tissues of TAD patients have elevated CTGF expression; and (3) CTGF induces collagen oversynthesis, including types I and III, in cultured VSMCs in a dose- and time-dependent fashion.
Under normal physiological conditions, the ECM undergoes constant metabolism, with a relatively low basic turnover rate. Damage to aortic tissue triggers repair responses; and a balanced ECM regulation is essential for the functional integrity of the vessel wall.3 It is logical to understand that defects in collagen predispose patients to the occurrence of aneurysms and dissection. Indeed, a deletion mutation of intron 1 in the α1 chain of type I collagen in mice results in an age-dependent aortic dissection and rupture.11 Collagen as the principal ECM component of the aortic wall interacts actively with other ECM components, eg, elastin or fibrillin. Proper amounts of each of these individual ECM proteins are essential for the arterial integrity to withstand the outward forces exerted by blood pressure. Fragmentation and decreased content of elastin have been implicated in aneurysmal diseases by most studies as well as a study from our laboratory.1,2,12
Compared with elastin, collagen may play a bigger role in certain aspects of arterial wall integrity. The tensile strength of collagen is ≈1000 times greater than that of elastin.13 However, the collagen content of the aortic wall has been the subject of extensive study and conflicting reports.14 Some reports show decreased levels of collagen,11 whereas others show increased levels of collagen.5,15,16 It appears that either increased or decreased collagen in the aorta may weaken the aortic wall and predisposes to aortic aneurysmal diseases. Our current study also showed an increased collagen content in TAD tissue, which was most likely due to increased production rather than reduced degradation, because collagen mRNA was also upregulated. Different etiological triggers of aortic aneurysmal disease, homogeneity of the selected patients, timing of sample collection, and methods of collagen measurements are all possible explanations for this apparent discrepancy. Although adequate collagen content in the aortic wall is essential for its functional integrity, overaccumulated collagen in the aorta may lead to medial fibrosis, which could hypothetically result in decreased arterial distensibility and increased susceptibility to dissection.17 Our data showed excess collagens localized in both the media and adventitia of the aortic wall. Fibrosis in the adventitia may obstruct vessels feeding the arterial wall as well as small intramural vasa vasorum, resulting in necrosis of VSMCs and leading to stiffness and a vulnerability to pulsatile forces, hence, dissections.17,18
CTGF as an active growth factor can be secreted by human endothelial cells and VSMCs,10,19 which make CTGF highly relevant to arterial diseases. CTGF stimulates collagen synthesis20 and causes fibroblast proliferation, migration, and adhesion, leading to fibroproliferative diseases via transforming growth factor-β pathways.19 Our study is the first to show that CTGF is overexpressed in TAD tissues, and this overexpression is associated with elevated collagen levels. We further demonstrated in cultured aortic VSMCs that CTGF can dose-dependently stimulate collagen expression, consistent with that observed in fibroblasts.20 Although it is tempting to suggest that CTGF overexpression may predispose to TAD, our clinical observation is limited by the nature of the study’s cross-sectional design. Our data do not distinguish whether overexpression of CTGF and collagens occurred before or after the aortic dissection. An animal model will be essential to evaluate the causal relationship in which the effects of CTGF knockdown or overexpression on aortic collagen accumulation and formation of dissection can be properly explored. Other limitations of our study are the overrepresentation of male subjects and the age differences between patients and controls.
Together with the findings of collagen and CTGF overexpression, our study also showed excess VSMC apoptosis in aortic tissues of TAD patients. Recent studies have demonstrated that apoptosis is a prominent feature of aneurysm and dissection.21 Some studies have indicated that collagen may actually induce apoptosis in endothelial cells, epithelial cells, and VSMCs.22 These cells undergo apoptosis when cultured on a collagen gel.23 Therefore, the observed excessive VSMC apoptosis could be the result of overexpressed collagen.
In conclusion, our results demonstrate that CTGF and collagens are overexpressed in the aortic wall of TAD patients. CTGF in turn stimulates collagen synthesis in cultured aortic VSMCs. Increased aortic collagen will likely result in aortic fibrosis, which contributes to the pathogenesis of aortic dissection. Although more studies are clearly needed to resolve their causal relationships, a delicate balance of collagen, elastin, and VSMCs in the aortic wall is clearly essential for a compliant aorta, and CTGF may play a vital role in this balance.
Acknowledgments
Sources of Funding
The study was supported by research grants from the Michael E. DeBakey Research Foundation, American Heart Association EIA Award (AHA 0440001N) to Dr X.L. Wang, and the Thoracic Surgery Foundation for Research and Education/National Heart, Lung, and Blood Institute Co-sponsored Mentored Clinical Scientist Development Award (1K08 HL080085) to Dr LeMaire.
Footnotes
Presented at the American Heart Association Scientific Sessions, Dallas, Tex, November 13–16, 2005.
Disclosures
None.
References
- 1.Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17:615–635. vii. doi: 10.1016/s0733-8651(05)70105-3. [DOI] [PubMed] [Google Scholar]
- 2.Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management, part I: from etiology to diagnostic strategies. Circulation. 2003;108:628–635. doi: 10.1161/01.CIR.0000087009.16755.E4. [DOI] [PubMed] [Google Scholar]
- 3.Jensen LT, Host NB. Collagen: scaffold for repair or execution. Cardiovasc Res. 1997;33:535–539. doi: 10.1016/s0008-6363(96)00247-7. [DOI] [PubMed] [Google Scholar]
- 4.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol. 1999;34:513–525. doi: 10.1016/s0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 5.Baxter BT, Davis VA, Minion DJ, Wang YP, Lynch TG, McManus BM. Abdominal aortic aneurysms are associated with altered matrix proteins of the nonaneurysmal aortic segments. J Vasc Surg. 1994;19:797–802. doi: 10.1016/s0741-5214(94)70004-4. discussion 803. [DOI] [PubMed] [Google Scholar]
- 6.Mesh CL, Baxter BT, Pearce WH, Chisholm RL, McGee GS, Yao JS. Collagen and elastin gene expression in aortic aneurysms. Surgery. 1992;112:256–261. discussion 261–262. [PubMed] [Google Scholar]
- 7.Moussad EE, Brigstock DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 8.Oemar BS, Werner A, Garnier JM, Do DD, Godoy N, Nauck M, Marz W, Rupp J, Pech M, Luscher TF. Human connective tissue growth factor is expressed in advanced atherosclerotic lesions. Circulation. 1997;95:831–839. doi: 10.1161/01.cir.95.4.831. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi H, Oka T, Kusachi S, Nakanishi T, Takeda K, Nakahama M, Doi M, Murakami T, Ninomiya Y, Takigawa M, Tsuji T. Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol. 1998;30:2411–2422. doi: 10.1006/jmcc.1998.0799. [DOI] [PubMed] [Google Scholar]
- 10.Cicha I, Yilmaz A, Klein M, Raithel D, Brigstock DR, Daniel WG, Goppelt-Struebe M, Garlichs CD. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005;25:1008–1013. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- 11.Rahkonen O, Su M, Hakovirta H, Koskivirta I, Hormuzdi SG, Vuorio E, Bornstein P, Penttinen R. Mice with a deletion in the first intron of the Col1a1 gene develop age-dependent aortic dissection and rupture. Circ Res. 2004;94:83–90. doi: 10.1161/01.RES.0000108263.74520.15. [DOI] [PubMed] [Google Scholar]
- 12.Lemaire SA, Wang X, Wilks JA, Carter SA, Wen S, Won T, Leonardelli D, Anand G, Conklin LD, Wang XL, Thompson RW, Coselli JS. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123:40–48. doi: 10.1016/j.jss.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Clark JM, Glagov S. Transmural organization of the arterial media: the lamellar unit revisited. Arteriosclerosis. 1985;5:19–34. doi: 10.1161/01.atv.5.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RW. Reflections on the pathogenesis of abdominal aortic aneurysms. Cardiovasc Surg. 2002;10:389–394. doi: 10.1016/s0967-2109(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 15.Bode MK, Soini Y, Melkko J, Satta J, Risteli L, Risteli J. Increased amount of type III pN-collagen in human abdominal aortic aneurysms: evidence for impaired type III collagen fibrillogenesis. J Vasc Surg. 2000;32:1201–1207. doi: 10.1067/mva.2000.109743. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo RJ, McCarthy WJ, Dixit SN, Lilly MP, Shively VP, Flinn WR, Yao JS. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg. 1989;10:365–373. doi: 10.1067/mva.1989.13151. [DOI] [PubMed] [Google Scholar]
- 17.Stefanadis CI, Karayannacos PE, Boudoulas HK, Stratos CG, Vlachopoulos CV, Dontas IA, Toutouzas PK. Medial necrosis and acute alterations in aortic distensibility following removal of the vasa vasorum of canine ascending aorta. Cardiovasc Res. 1993;27:951–956. doi: 10.1093/cvr/27.6.951. [DOI] [PubMed] [Google Scholar]
- 18.Reed D, Reed C, Stemmermann G, Hayashi T. Are aortic aneurysms caused by atherosclerosis? Circulation. 1992;85:205–211. doi: 10.1161/01.cir.85.1.205. [DOI] [PubMed] [Google Scholar]
- 19.Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids: identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272:20275–20282. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- 20.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 21.Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(suppl II):II-329–II-334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 22.Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the αvβ3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang MJ, Hu JJ, Lin HH, Chiu WT, Jiang ST. Collagen gel overlay induces apoptosis of polarized cells in cultures: disoriented cell death. Am J Physiol. 1998;275:C921–C931. doi: 10.1152/ajpcell.1998.275.4.C921. [DOI] [PubMed] [Google Scholar]