Abstract
Understanding the phase behavior of biological membranes is helped by the study of more simple systems. Model membranes that have as few as 3 components exhibit complex phase behavior that can be well described, providing insight for biological membranes. A number of different studies are in agreement on general findings for some compositional phase diagrams, in particular, those that model the outer leaflet of animal cell plasma membranes. These model mixtures include cholesterol, together with one high-melting lipid and one low-melting lipid. An interesting finding is of two categories of such 3-component mixtures, leading to what we term Type I and Type II compositional phase diagrams. The latter have phase regions of macroscopic coexisting domains of {Lα+Lβ+Lo} and of {Lα+Lo}, with domains resolved under the light microscope. Type I mixtures have the same phase coexistence regions, but the domains seem to be nanoscopic. Type I mixtures are likely to be better models for biological membranes.
Keywords: Bilayer phase behavior, Lipid mixing, Nanodomain, Nonideal mixing, Membrane phase, Cholesterol
1. Introduction
In a biological membrane, which molecules are able to make physical contact with their substrates, which are kept apart? Are there patches of the membrane that behave as a unit, for example during fusion or fission or in interaction with viruses? Understanding such events in and on cell membranes at the molecular level benefits by describing which membrane molecules are clustered together and which are kept separated. For an animal cell plasma membrane a starting point for this description on the most basic level is the fundamental unit – the lipid bilayer – with the leaflets of some of the cell membranes having different lipid and protein compositions [1]. The leaflets are coupled together in ways that are currently under study but not yet well understood [2–4]. Even without knowing the factors that control the nature and the strength of the coupling between the two leaflets, lipid mixtures that mimic each leaflet can profitably be studied separately. One approach, described here, is to find out some of the main characteristics of the more simple systems, the individual leaflets, as a prerequisite to understanding the more complex coupled asymmetric system.
For the membrane proteins, we find additional considerations beyond what we think about for water-soluble proteins: When we consider the activity of a water-soluble protein, and its interactions with other proteins and with small molecules, we rarely need to pay attention to the phase behavior of its water environment. But for membrane-bound proteins, the activity of the protein as well as the access of the protein to its targets can depend upon the phase behavior of the membrane, and for coexisting phases, which are continuous and which are isolated, discrete entities. For example, a protein kinase that can be regulated by reversible phosphorylation can be kept close to or apart from its protein target, and likewise can be kept active or inactive depending upon its access to its regulating kinases and phosphatases [5].
As to its chemical constituents, the lipids that form biological membranes comprise one of the major classes of biomolecules, along with amino acids, nucleic acids, and carbohydrates. But unlike these three types of polymers that have their information contained in their covalent structure, the lipids manifest their biological behavior not as polymers but instead as physical mixtures with other lipids and with the membrane proteins. Therefore the physical chemistry of mixing has something to tell us about the behavior of biological membranes [6]. Although we pay some price by examining such chemically simplified mixtures of few components as can be treated rigorously by equilibrium thermodynamics, at a minimum we gain the clarity of agreed-upon terminology, and with progress we gain the predictive power of thermodynamic theory.
In particular, we want to know if different areas of a biomembrane have distinct physical and chemical properties that last long enough to be felt by a membrane protein. We want to know if any such compositionally distinct “domain” is stable, or is merely the transient clustering of molecules in a single-phase nonrandom mixture. And we want to know the rules that describe how membrane proteins partition among any stable lipid phase domains, or how proteins control domain properties, including which phase domains are connected together to form a continuous area that can be rapidly explored by its constituent lipids and proteins, and which phases are dispersed into domains that are separated from each other.
2. Membrane phase behavior
A key property of any system is its phase. The characteristics of the phase depend in general on the variables of temperature, pressure, and composition. Other variables can also be significant, including pH, ionic strength, and the presence of particular multivalent ions. In particular situations, any of these variables can be especially important (see [7]). In this review we consider mainly what can happen to the membrane phases when the lipid composition changes. Pioneering studies of phase dependence on lipid composition in mixtures containing cholesterol by Vist and Davis [8] and early theoretical work by Zuckermann, Mouritsen, Ipsen and others (see for example [9]) have raised our appreciation and understanding of how the important property of phase depends upon lipid details. Indeed, local lipid composition actually does change as a normal aspect of membrane recycling and as a result of the activity of the enzymes of membrane lipid synthesis and breakdown. We emphasize a thermodynamic picture but with the caveat that the system of interest is an ill-defined area of a cell membrane that can be considered to be at quasi equilibrium over the timescale of interest. An entire cell membrane is certainly not at equilibrium because the normal life of the cell includes both removing and adding patches of membrane that contain thousands of molecules, as well as individual molecules, on a timescale of tens of minutes [10]. These changes occur, for example, during fusion or fission of the membrane, or in regions involved in signaling or metabolic activity. Moreover, regions of the membrane can be compositionally isolated from each other and therefore have different phase behavior, even while remaining physically connected, as in the case of the apical and basolateral regions of the plasma membrane of epithelial cells [11].
A compositional phase diagram summarizes and organizes the identities of each phase, including the presence of coexisting phases, at all compositions [12]. The information contained in such a diagram is necessarily limited, since it does not include descriptions of how the properties of each individual phase depend upon composition, although of course this information can be obtained. Rather, the compositional phase diagram can be thought of as a minimal description of a bilayer that is necessary for interpreting the behavior. The entire range of compositions that is accessible to the components, the so-called composition space, increases in a multiplicative way for each compositional variable. As a consequence, although biological membranes contain so many different species in the bilayer, we are limited by practical experimental reasons to examining only a small subset of this vast array as a model for a biological membrane. And even though we seek an understanding of real biological membranes in all of their complexity, this review is lipid-centered in that we omit discussion of mixtures in which a protein or peptide is one of the mixture components. In fact, no such 3- or 4-component phase diagrams that cover significant composition space have ever been reported for bilayer mixtures containing a membrane protein. And so we focus here on the poorly understood phase behavior of lipid mixtures. With this view, once the lipid-only phase behavior is sufficiently well described, then proteins can be more profitably examined as an additional mixture component. Otherwise, we run the unnecessary risk of ascribing lipid-dependent behaviors to the proteins. Indeed, this is exactly the situation with membrane nanodomains, which are one of the behaviors of lipid-only mixtures, but whose small size some researchers had thought must depend upon membrane proteins [13].
For several decades now, the phase behaviors of binary mixtures of lipids have been described as functions of composition and temperature [14]. In briefest summary, the result is largely a story of miscibility in the solid and liquid when chains are sufficiently similar, and conversely immiscibility in the solid, and solid+liquid, when chains are sufficiently dissimilar. But the phases of mixtures that contain cholesterol are more varied and more interesting. Because animal cell plasma membranes are so rich in cholesterol, which is 35–45 mol% of the total lipid, mixtures that model plasma membranes have been extensively studied, necessitating a minimum of 3 components.
3. Phase diagrams to model cell membranes
Mixtures of 3 lipid components, with one being cholesterol, one a high melting temperature lipid, and one a lower melting lipid, have the minimal number of components that yield rich phase behavior [15,16]. In fact, we choose to limit our discussion here to just this one variety of 3-component bilayer mixtures. The most complete phase diagrams of this sort have been determined for DPPC/DOPC/ cholesterol at temperatures from 15 to 45° [17], DPPC/diphytanoyl-PC/cholesterol [18] from 10 to 60°, and for DSPC/DOPC/cholesterol at ambient temperature [19].
Precisely determined phase boundaries have special value: (i) A determination of possible stoichiometries, e.g. ratios of integers, requires a phase boundary location precision of two significant figures; (ii) Straight lines, which always have special significance in phase diagrams, e.g., to identify the triangle of 3-phase coexistence, can be identified; (iii) Narrow but distinct compositional regions can be identified; (iv) Most important, systematic changes in boundaries of different phase diagrams can be found as components are systematically changed.
There are currently enough data from different laboratories, using different measurements and different methods of sample preparation, that one particular type of consensus phase diagram has been firmly established. We have termed this diagram “Type II” [20]. There are also enough data from enough different research groups (see below) that we can describe a different but closely related type of phase diagram, albeit with somewhat less certainty. Solving this second type, which we have termed “Type I”, is more difficult because the phase regions {Lα+Lβ+Lo} and {Lα+Lo} readily show artifactual, and therefore misleading, macroscopic domains by fluorescence imaging methods [21,22]. Unfortunately for experimentalists, when separated phases coexist as nanodomains, we have lost the use of fluorescence microscopy as one of our tools for detecting such a phase region.
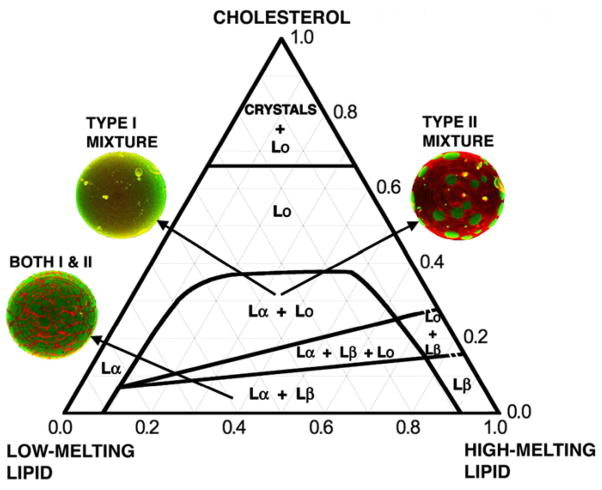
A generic phase diagram of these two types of mixtures is shown in Fig. 1 [19,23]. This figure shows that the overall phase behavior of these two types of mixtures is remarkably similar, even though of course the exact positions of boundaries depend upon the precise chemical nature of the components. The locations of the phase boundaries differ for each particular mixture within Type I or Type II, with more miscible components showing smaller regions where separate phases coexist. Both types of phase diagrams contain a region of {La+Lb} phase coexistence, which is easily identified using optical microscopy by the sharp, angular features of the gel phase domains. Type II mixtures also show visible domains of {Lα+Lβ+Lo} and {Lα+Lo}, minimally about a half micron in the smallest dimension and typically several microns, but in Type I mixtures these phase coexistence regions appear uniform. However, experimental techniques that are sensitive to nanometer length scales confirm {Lα+L-β+Lo} and {Lα+Lo} coexistence in Type I mixtures. The primary difference between these two types of mixtures thus appears to be the size scale of fluid domains, with Type I mixtures having much smaller domain size in regions where Lo is one of the coexisting phases [22]. Later below we describe more fully the mixing information that is summarized in these phase diagrams.
Fig. 1.
Compositional phase diagrams of 3-component bilayer mixtures that contain cholesterol together with a high-melting point lipid and a low-melting point lipid are useful models for animal cell plasma membrane outer leaflets. To date, the various mixtures of these types all seem to display the same pattern of phases. The most important distinction among such 3-component mixtures is the behavior designated here as Type I or Type II. The Type I mixtures have nanoscopic domain dimensions for the coexistence regions {Lα+Lo} and {Lα+Lβ+Lo}. Fluorescence microscope images giant unilamellar vesicles, GUVs, of the Type II mixtures reveal visible domains, whereas Type I mixtures appear uniform in these phase regions. The exact positions of the phase boundaries depend upon the particular lipids. Dashed lines for boundaries involving the solid Lβ phase indicate uncertainty as to whether the phase transition is first-order or higher order (continuous). Some lipids form the untilted solid Lβ phase as shown, others are in the solid tilted Lβ′ phase, and make a transition (not shown) to the Lβ phase. Although large regions of the composition space are found to have a single phase of Lα or Lo, these single phases might have local structure, as yet uncharacterized.
Various physical methods can be used to define the location of phase regions by detecting a more-or-less abrupt change in the measurement at a phase boundary [23–25]. A more limited set of methods are useful for determining the size of domains. Yet, the possibility of very small domain size is currently thought to be of great significance in linking these chemically simple model mixtures with real biological membranes. Nicolini et al. used small angle neutron scattering to detect a broad distribution of domain sizes at the particular composition of SM/POPC/cholesterol= 1/1/1 [26]. This technique is not practical for finding the phase boundaries because of the relatively small number of compositions that can be examined, but the shape of the scattering profile can be modeled to provide some information about the nature of the scattering entities. In this case, data could be fit to scattering from disks of phase domains in the size range of 20–200 nm. Filippov et al. used NMR measurements of lateral diffusion to conclude that SM/POPC/cholesterol mixtures have small domains, but the size was indeterminate [27].
FRET experiments are inherently sensitive to distance in the size range up to perhaps an order of magnitude greater than the Forster energy transfer length, that is, up to ~50 nm [28–30]. Frazier et al. studied SM/POPC/cholesterol, using Monte Carlo simulations of FRET data to produce snapshots of lipid distributions, from which lipid cluster/domain size is found [31]. They report that domain sizes from tens to hundreds of nm fit the FRET data. In another study of domain size, using time-resolved FRET data, also for SM/POPC/cholesterol, de Almeida et al. found a range of domains sizes from ~20 up to ~100 nm, depending upon the composition within the 2-phase region {Lα+Lo} [30].
4. Significance of small domain size for {Lα+Lo}
A straightforward explanation for FRET experiments showing coexisting phases in the raft region even though GUVs look uniform when examined by fluorescence microscopy, is that separate phases exist, but that the phase domains are small. This “phase explanation” is challenged by an alternative interpretation that small domains are in fact not equilibrium phase domains but instead are critical fluctuations that occur even at temperatures and compositions that are distant from a critical point [32–34].
Here, we take a more simple, equilibrium thermodynamic approach to the question of what is a compositionally distinct domain. First, let us consider how small these domains would be. We can reason that if the domains are only slightly smaller than the wavelength of fluorescence excitation/emission, but with a Gaussian distribution about that size, then we would still detect some fraction of the domains in optical microscopy images. From this reasoning, we expect domain size to be much smaller than ~300 nm, not merely slightly smaller: If the average size is actually close to ~300 nm, then the domains would need to have an unusually narrow range of sizes since no large domains are observed.
If small domains correspond to genuine phase separation, then they are distinctly different from the small clusters that occur in ordinary nonideal mixing. Clusters from nonideal mixing change continuously in size and composition in time and in composition space, as can be seen in snapshots from Monte Carlo simulations of mixing [35]. In contrast, tiny phase domains would be more robust than clusters, having well-defined physical and chemical properties. These properties show up in FRET experiments, which reveal that lipid probe behavior fits a model of coexisting domains along a tieline, instead of a model of continuous compositional change, as occurs in ordinary nonideal mixing [36]. The small size might be required for the domains to have their roles in the life of a cell. But if nanodomains are actually a critical phenomenon, then we expect increased domain size as the critical point is approached in either temperature or composition starting far away from the critical point in a 1-phase region [3]. This size increase is observed for macroscopic domains [34] but has not yet been observed for nanodomains, whose size is difficult to measure.
If nanoscale domains describe the coexistence of {Lα+Lo} phases, then we have a conceptual problem: Why would such phase domains be stable? After all, the driving potential to reduce excess energy along the domain boundaries, expressed by the line tension, might be expected to result in essentially unlimited domain growth [37]. We require a mechanism that would drive formation of small domains. Such small domains could occur when the attractive short-range interactions that give rise to clustering and phase separation are combined with a long-range repulsive interaction that outweighs the free energy cost of forming excess domain interface [38], as is indeed found for small domains in monolayers that do not coalesce because of the long-range dipolar repulsion [39]. Combination of short-range attraction together with long-range repulsion is well studied nowadays because of intense research in nanotechnology. For example, such attraction plus long-range repulsion gives rise to spontaneous pattern formation that has proven useful for nanofabrication. The repulsive interaction can be electrostatic, as occurs with lipid monolayers [39], or can arise from contractile tension from adsorption on a substrate [40]. In addition, highly curved bilayer regions at domain interfaces can form a barrier to coalescence of small domains [41]. However, no such repulsive interactions that overwhelm interfacial line tension occur for uncharged and flat lipid bilayers. One plausible candidate for the underlying cause of stable nanodomains is low line tension, low enough to be dominated by the favorable entropy of having many small domains [37]: The free energy penalty incurred by forming more interface could be offset by the entropy gain from having more ways to have the phase. Yet at one extreme, a line tension of zero means no phase separation [42]! Perhaps there is a critical line tension where macroscopic phases break up into the small domains [37]. Or, more generally, the modeling of phospholipid phase transitions in terms of a single order parameter that describes only the acyl chains, might be insufficient; perhaps an order parameter for the headgroup region behaves differently from that for the acyl chains, and frustration might yield phase patterns or nanodomains (see discussion in [43]).
In live cells, unless extensive crosslinking of proteins or lipids occurs, any phase-separated domains that might exist have a dimension below optical resolution [44]. Despite the lack of direct visual evidence, and based in part on evidence from lipid and protein motional measurements, some researchers have ascribed nanoscopic raft size as caused by proteins that in effect create a cage around the lipid domain [21,45,46]. Picturing a membrane as having nanoscopic domains also fits other types of data, including FRET [47] and EM observations [42]. Others suspect that certain membrane proteins adsorb at the raft interface, lowering the interfacial tension and shrinking the raft size [48]. These mechanisms to promote the small size of domains might indeed be operating in cell membranes, with their rich content of different lipids and proteins. But one value of the model studies on lipid-only mixtures is to discover that small size of phase domains can be driven entirely by lipid mixing behaviors.
If the nanometer scale describes the phase-separated domains in animal cell plasma membranes, then perhaps these small structures, rather than micron-size domains, are the ones best suited for model studies. A domain that is a 5 nm diameter circle would contain only ~30 lipids in the Lα phase or ~40 lipids in the Lo phase, and ~1/2 to 2/ 3 would be at the interface. For a 10 nm diameter circle, with ~120 lipids in the Lα phase or 150 in the Lo phase, closer to 1/3 of the total domain lipids are at the interface. The high fraction of lipids that are at the domain perimeter and therefore not entirely surrounded by molecules of the same phase provides a potential mechanism for the nanodomains to have different properties from macro-domains. For example, the partitioning behavior of fluorescent or spin-labeled probes might be different. But far more important and interesting, the partitioning behavior of membrane proteins might be different from that observed by visual examination of GUV images. If this is the case, then efforts should be directed toward characterizing the partitioning properties of proteins in nanodomains. Whereas one or a small number of proteins would not greatly affect the properties of many 1000 s or millions of lipids of a macro-domain, the protein might well influence the properties of the lipids in a couple of layers around its boundary, which is comparable to the total number of lipids in a small nanodomain. Because of the likelihood that size and lifetime of any lipid-driven nanodomains would be under the control of membrane proteins, these questions might have useful answers only when studies of domain size and lifetime are compared in the presence and absence of membrane proteins.
Another issue for biological membranes is the connectivity question for coexisting phases —which type of phase is continuous, enabling membrane proteins within the same phase to find each other. For example, when phases coexist, a protein target of a kinase and a phosphatase could be accessible to both, but only if all three proteins are found appreciably in one continuous phase [5]. In contrast, if that phase is small and discontinuous, the enzyme cannot find its target. Therefore, in addition to the thermodynamic question of whether or not a region of a cell membrane is phase-separated, a different question is which phase is the connected one. And this question might have different answers for macro-domains compared with nanodomains.
In a general sense, these phase diagrams inform us that lipids in bilayer mixtures arrange themselves into characteristic structures because the lipids have energetic preferences for their neighbors. In principle, these energy preferences, or “microscopic interaction energies”, can be recovered from the phase boundaries and tielines of these diagrams [49,50]. However, at this time we know only a handful of what are without doubt the many rules that describe lipid mixing. We know that cholesterol molecules in the bilayer repel each other [45,49,50]; furthermore, the neighboring phospholipids of each cholesterol adopt a particular conformation that is not compatible with the sharing of that phospholipid as a neighbor by more than one cholesterol; saturated acyl chains mix better with other saturated chains than they do with unsaturated chains; cholesterol packs into the gel phase up to about 16 mol%, and at higher concentrations either separates out as the Lo phase, or else transforms continuously into the Lo phase [19,20].
If macroscopic phase domains provide the information we seek in order to understand the rules that describe protein partition between phases, then the GUVs provide a fine model system. Images of fluorescent dyes can be qualitatively or semi-quantitatively analyzed to determine protein partition behavior. But so far, a puzzle has emerged from such partitioning studies of chemically simple model membranes: With the exception of GPI-anchored proteins, other proteins all partition out of Lo, into Lα [51–55]. In the SM-containing mixtures, even long-chain dyes having no double bonds or moieties protruding from the chains favor Lα over Lo, even when that dye favors Lβ over Lα [56]. These behaviors inform us about the properties of the domains in our model mixture, but are they reliable guides for the behaviors of any coexisting domains in real animal cell plasma membranes? We need the partition information involving nanodo-mains for proteins, even just for fluorescent dyes, to compare with existing results for macro-domains.
5. Detailed descriptions of Type I and Type II phase diagrams
5.1. Pure components at the vertices
At ambient temperatures, the phase of the high-melting lipid is typically Lβ for the sphingomyelins, and Lβ′ (the chain-tilted solid phase) for DPPC or DSPC.
The phase of DOPC, POPC, or SOPC is Lα.
Cholesterol is the monohydrate crystal.
5.2. Binary mixtures along the triangle sides
5.2.1. Compositions between the low- and the high-melting lipid
The width of the 2-phase region depends upon the temperature, the identities of these two lipids, and χCHOL. This width shrinks as the two lipids mix better, whether because the temperature or χCHOL increases, or else the two are more compatible, e.g. SM+POPC is narrower than DSPC+DOPC.
If the pure high-melting lipid is in the Lββ phase, then the Lα phase can separate from it directly, as with DOPC added to DSPC at 23 °C. Or, a solid Lβ phase containing some of the low-melting lipid can form, yielding a 2-phase region of coexisting Lβ′+Lβ. This Lβ phase can accommodate a small additional amount of the low-melting lipid, then an Lα phase separates. This occurs, for example, with DSPC+POPC or SOPC at ambient temperature.
Apparently, the Lβ′ phase does not accommodate either the low-melting lipid or cholesterol.
5.2.2. High-melting lipid+cholesterol
This phase transforms from the solid gel (either Lβ′ or Lβ) to the Lo phase as cholesterol is added. The nature of the phase transition(s) has not been firmly established: The phase transition is either first-order or continuous (higher order). In Fig. 1, dotted lines indicate the uncertainty in the transition order. At 25 °C the order parameter remains high along this binary axis from solid to Lo, decreasing somewhat at higher χCHOL [57]. Finally, crystals of cholesterol monohydrate precipitate from the Lo phase at χCHOL =0.67 [58].
5.2.3. Low-melting lipid+cholesterol
GUVs show uniform fluorescence up to at least χCHOL =0.5, the highest cholesterol composition examined for GUVs. Dilute dye and FRET measurements at 0.02 mole fraction compositional resolution up to χCHOL =0.6 show no changes that might indicate phase separation [19]. Compositional phase diagrams that are constructed from samples that are far apart in composition (e.g. compositional increments much greater then ~0.05 mole fraction units) can appear to show abrupt transitions between samples [59], but when examined at higher compositional resolution (e.g. mole fraction 0.02 or better, but at least 0.05) continuous changes become apparent. We must be cautious because a lack of evidence of phase separation could merely indicate insensitivity to a particular type of phase change. But if there are indeed no abrupt phase changes at higher cholesterol concentrations mixed with low-melting phospholipids, then we conclude that the Lα phase can accommodate a great deal of cholesterol without a first-order phase change, until precipitation of crystals of cholesterol monohydrate at χCHOL =0.67 [58]. In other words, we observe that the Lα phase changes to the Lo phase continuously, without a first-order phase transition.
5.3. Ternary mixtures
The Lα phase accommodates an unlimited amount of cholesterol without a first-order phase transition, until χCHOL =0.67, whereupon crystals of cholesterol monohydrate precipitate [58]. The 2-phase coexistence of Lα+Lβ appears to terminate in a straight line at increased χCHOL.
The saturating cholesterol concentration for Lβ phase that is in equilibrium with Lα phase is χCHOL = ~0.16. At this value, an Lo phase separates from an Lβ phase that retains approximately the same ratio of the two PCs, but with the higher cholesterol concentration of χCHOL ~ 0.27. The region of 3-phase coexistence of Lα+ Lβ+Lo terminates in a straight line at increased χCHOL. Type II mixtures such as DPPC/DOPC/chol or DSPC/DOPC/chol show macroscopic domains when imaged using fluorescent dyes that partition among the phases, although rarely are all three different phases distinguishable. In distinct contrast, Type I mixtures such as DSPC/POPC/chol, DSPC/SOPC/chol, DPPC/POPC/chol, DPPC/SOPC/chol, DPPC/DLPC/chol, SM/POPC/chol show uniform fluorescence in this 3-phase region. Phase domains are small.
Similarly, Type II mixtures show a region of coexisting macroscopic domains of Lα+Lβ when examined by fluorescence microscopy using a wide range of different fluorescent dyes [56]. In addition to these GUV images, the boundaries of this compositional region also show up clearly in FRET or dilute single-dye experiments or NMR spectroscopy. But Type I mixtures appear to be uniform in fluorescence microscopy imaging, even while the various spectroscopies reveal clear phase boundaries.
The Lo phase occupies a wide region of composition space until χCHOL =0.67, whereupon crystals of cholesterol monohydrate precipitate, in equilibrium with a cholesterol-saturated Lo phase.
6. Usefulness of phase view for biological membranes
In favorable cases, we know the lipid composition of the combined leaflets of the membrane of a given organelle [60]. In no case do we know the lipid composition of the local vicinity of a protein in a biological membrane. Yet, we want to understand the possible membrane behaviors when cholesterol is added or removed, when lipids are hydrolyzed to fatty acids and lysolipids, or to ceramides or diacylglycerols that all remain as membrane components. The phase diagrams are useful here. We can imagine starting at any particular composition, and we can follow the phase changes as the composition is varied.
Consider compositional variations involving cholesterol. What can happen to a region of the membrane when cholesterol concentration locally decreases? Starting from a typical cholesterol mole fraction of ~0.45 [60], and with Fig. 1 as a guide, this region of the membrane is likely to be in a single phase, Lo. (i) As cholesterol concentration is decreased, by whatever means, this region of the membrane would separate into two phases, Lα+Lo. Some proteins that had been mixed and accessible to each other in the single Lo phase would now separate from each other, some preferring the Lα phase, others the Lo phase. Other proteins, the ones that prefer the same phase, become more concentrated than they were in the single Lo phase; (ii) As cholesterol concentration is decreased further, solid Lβ phase would separate in this region of membrane. Proteins in the membrane must respond to this new phase environment, but in this case almost nothing is known about protein preferences when the three phases coexist, Lα+Lo+Lβ, or the possible interfacial locations of the protein. We note that even though some cell biologists do not consider the Lβ phase to occur in biological membranes to any significant extent, if we simply base our reasoning on the measured compositions of animal cell plasma membranes, then this solid phase cannot be ruled out a priori; (iii) In fact, when cholesterol concentration is further decreased to below mole fraction ~0.16, the two phases that are likely to coexist in the outer leaflet would be Lα+Lβ. In other words, if cholesterol depletion (or enhancement) occurs in some region of a plasma membrane, the membrane phase behavior can be complex and involve drastic and perhaps unexpected changes in bilayer phases.
The compositional phase diagram would also be useful for understanding local membrane changes when lipids are hydrolyzed. However, so far no appropriate phase diagrams have been determined that can guide us. At a minimum, we would need a 4-component diagram – a tetrahedron at constant temperature – with such components as SM/POPC/ceramide/cholesterol.
Acknowledgments
The author is grateful to F. Heberle for reading the manuscript and to F. Heberle and E. Farkas for many useful discussions. Support was received from the National Science Foundation (MCB-0315330) and the National Institutes of Health (R01 GM077198).
Footnotes
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–3165. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan C, Kiessling V, Tamm LK. Coupling of cholesterol-rich lipid phases in asymmetric bilayers. Biochemistry. 2008;47:2190–2198. doi: 10.1021/bi7021552. [DOI] [PubMed] [Google Scholar]
- 3.Putzel GG, Schick M. Phase behavior of a model bilayer membrane with coupled leaves. Biophys J. 2008;94:869–877. doi: 10.1529/biophysj.107.116251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins M, Keller SL. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc Nat Acad Sci USA. 2008;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young RM, Zheng X, Holowka D, Baird B. Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. J Biol Chem. 2005;280:1230–1235. doi: 10.1074/jbc.M408339200. [DOI] [PubMed] [Google Scholar]
- 6.Guggenheim EA. Mixtures: The Theory of the Equilibrium Properties of Some Simple Classes of Mixtures, Solutions and Alloys. Clarendon Press; Oxford: 1952. [Google Scholar]
- 7.Cevc G, Marsh D. Phospholipid Bilayers. John Wiley and Sons; New York: 1987. [Google Scholar]
- 8.Vist MR, Davis JH. Phase equilibria of cholesterol/dipalmitoylphosphatidylcho-line mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- 9.Finegold L, editor. Cholesterol in membrane models. CRC Press; Boca Raton: 1993. [Google Scholar]
- 10.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons K, van Meer G. Lipid sorting in epithelia cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 12.Tamas F, Pal I. Phase equilibria spatial diagrams. In: Ward LS, editor. Phase diagrams, their interpretation and anaglyph representation. Iliffe Books, the Butterworth Group; London: 1970. [Google Scholar]
- 13.Anderson RGW, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 14.Tenchov BG. Nonrandom lipid distributions in membranes. Prog Surf Sci. 1985;20:273–340. [Google Scholar]
- 15.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samsonov VV, Mihalyov I, Cohen FS. Characterization of cholesterol-sphingo-myelin domains and their dynamics in bilayer membranes. Biophys J. 2001;81:1486–1500. doi: 10.1016/S0006-3495(01)75803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple mixtures. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Veatch SL, Gawrisch K, Keller SL. Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys J. 2006;90:4428–4436. doi: 10.1529/biophysj.105.080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Wu J, Heberle FA, Mills TT, Klawitter P, Huang G, Costanza G, Feigenson GW. Phase studies of model biomembranes: complex behavior of DSPC/DOPC/Cholesterol. Biochim Biophys Acta. 2007;1768:2764–2776. doi: 10.1016/j.bbamem.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feigenson GW. Phase boundaries and biological membranes. Annu Rev Biophys Biomol Struct. 2007;36:63–77. doi: 10.1146/annurev.biophys.36.040306.132721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayuyan AG, Cohen FS. Lipid peroxides promote large rafts: effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys J. 2006;91:2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Wu J, Shao H, Kong F, Jain N, Hunt G, Feigenson GW. Phase studies of model biomembranes: macroscopic coexistence of Lα+Lβ, with light-induced coexistence of Lα+Lo phases. Biochim Biophys Acta. 2007;1768:2777–2786. doi: 10.1016/j.bbamem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heberle FA, Buboltz JT, Stringer D, Feigenson GW. Fluorescence methods to detect phase boundaries in lipid bilayer mixtures. Biochim Biophys Acta. 2005;1746:186–192. doi: 10.1016/j.bbamcr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Detecting micro-domains in intact cell membranes. Ann Rev Phys Chem. 2005;56:309–336. doi: 10.1146/annurev.physchem.56.092503.141211. [DOI] [PubMed] [Google Scholar]
- 26.Nicolini C, Thiyagarajan P, Winter R. Small-scale composition fluctuations and microdomain formation in lipid raft models as revealed by small-angle neutron scattering. Phys Chem Chem Phys. 2004;6:5531–5534. [Google Scholar]
- 27.Filippov A, Oradd G, Lindblom G. Domain formation in model membranes studied by pulsed-field-gradient NMR: the role of lipid polyunsaturation. Biophys J. 2007;93:3182–3190. doi: 10.1529/biophysj.107.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvus JR. Fluorescence energy transfer reveals microdomain formation at physiological temperatures in lipid mixtures modeling the outer leaflet of the plasma membrane. Biophys J. 2003;85:1034–1045. doi: 10.1016/S0006-3495(03)74542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towles KB, Brown AC, Wrenn SP, Dan N. Effect of membrane microheterogeneity and domain size on fluorescence resonance energy transfer. Biophys J. 2007;93:655–667. doi: 10.1529/biophysj.106.090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Almeida RRM, Loura LMS, Fedorov A, Prieto M. Lipid rafts have different sizes depending on membrane composition: a time-resolved fluorescence resonance energy transfer study. J Mol Biol. 2005;346:1109–1120. doi: 10.1016/j.jmb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Frazier ML, Wright JR, Pokorny A, Almeida PFF. Investigation of domain formation in sphingomyelin/cholesterol/POPC mixtures by fluorescence resonance energy transfer and Monte Carlo simulations. Biophys J. 2007;92:2422–2433. doi: 10.1529/biophysj.106.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esposito C, Tian A, Melamed S, Johnson C, Tee SY, Baumgart T. Flicker spectroscopy of thermal bilayer domain boundary fluctuations. Biophys J. 2007;93:3169–3181. doi: 10.1529/biophysj.107.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. Critical fluctuations in plasma membrane vesicles. ACS Chem Biol. 2008;3:287–293. doi: 10.1021/cb800012x. [DOI] [PubMed] [Google Scholar]
- 34.Honerkamp-Smith AR, Veatch SL, Keller SL. An introduction to critical points for biophysicists; observations in lipid membranes. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2008.09.010. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Feigenson GW. Monte Carlo simulation of lipid mixtures: finding phase separation. Biophys J. 1993;65:1788–1794. doi: 10.1016/S0006-3495(93)81234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florine KI, Feigenson GW. On the use of partition coefficients to characterize the distribution of fluorescent membrane probes between coexisting gel and fluid lipid phases. Biochim Biophys Acta. 1988;941:102–106. doi: 10.1016/0005-2736(88)90218-0. [DOI] [PubMed] [Google Scholar]
- 37.Frolov VAJ, Chizmadzhev YA, Cohen FS, Zimmerberg J. “Entropictraps” in the kinetics of phase separation in multicomponent membranes stabilize nanodomains. Biophys J. 2006;91:189–205. doi: 10.1529/biophysj.105.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisy V, Brutovsky B. Interpretation of static and dynamic neutron and light scattering from microemulsion droplets: effects of shape fluctuations. Phys Rev E. 2000;61:4045–4053. doi: 10.1103/physreve.61.4045. [DOI] [PubMed] [Google Scholar]
- 39.McConnell HM. Structure and transitions in lipid monolayers at the air–water interface. Ann Rev Phys Chem. 1991;42:171–195. [Google Scholar]
- 40.Plass R, Bartelt NC, Kellogg GL. Dynamic observations of nanoscale self-assembly on solid surfaces. J Condens Matter. 2002;14:4227–4240. [Google Scholar]
- 41.Groves J. Bending mechanics and molecular organization in biological membranes. Ann Rev Phys Chem. 2007;58:697–717. doi: 10.1146/annurev.physchem.56.092503.141216. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin I. Molecular architecture and dynamics at liquid–liquid interfaces. Ann Rev Phys Chem. 1997;48:407–451. doi: 10.1146/annurev.physchem.48.1.407. [DOI] [PubMed] [Google Scholar]
- 43.Seul M, Andelman D. Domain shapes and patterns: the phenomenology of modulated phases. Science. 1995;267:476–483. doi: 10.1126/science.267.5197.476. [DOI] [PubMed] [Google Scholar]
- 44.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dietrich C, Yang B, Fujiwara T, Kusumi A, Jacobson K. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J. 2002;82:274–284. doi: 10.1016/S0006-3495(02)75393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 47.Rao M, Mayor S. Use of Forster′s resonance energy transfer microscopy to study lipid rafts. Biochim Biophys Acta. 2005;1746:221–233. doi: 10.1016/j.bbamcr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Nicolini C, Baranski J, Schlummer S, Palomo J, Lumbierres-Burgues M, Kahms M, Kuhlmann J, Sanchez S, Gratton E, Waldmann H, Winter R. Visualizing association of N-Ras in lipid microdomains: influence of domain structure and interfacial adsorption. J Am Chem Soc. 2006;128:192–201. doi: 10.1021/ja055779x. [DOI] [PubMed] [Google Scholar]
- 49.Huang J, Feigenson GW. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J. Exploration of molecular interactions in cholesterol superlattices: effect of multibody interactions. Biophys J. 2002;83:1014–1025. doi: 10.1016/S0006-3495(02)75227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 52.Shogomori H, Hammond AT, Ostermeyer-Fay AG, Barr DJ, Feigenson GW, London E, Brown DA. Palmitoylation and intracellular-domain interactions both contribute to raft targeting of linker for activation of T cells (LAT) J Biol Chem. 2005;280:18931–18942. doi: 10.1074/jbc.M500247200. [DOI] [PubMed] [Google Scholar]
- 53.Silvius JR. Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies. Biochim Biophys Acta. 2005;1746:193–202. doi: 10.1016/j.bbamcr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Horton MR, Radler J, Gast AP. Phase behavior and the partitioning of caveolin-1 scaffolding domain peptides in model lipid bilayers. J Coll Int Sci. 2006;304:67 –76. doi: 10.1016/j.jcis.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 55.Sengupta P, Hammond A, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochim Biophys Acta. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baumgart T, Hunt G, Farkas ER, Webb WW, Feigenson GW. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim Biophys Acta. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang YW, Costa-Filho AJ, Freed JH. Dynamic molecular structure and phase diagram of DPPC-cholesterol binary mixtures: a 2D-ELDOR study. J Phys Chem B. 2007;111:11260–11270. doi: 10.1021/jp0732110. [DOI] [PubMed] [Google Scholar]
- 58.Huang J, Buboltz JT, Feigenson GW. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1999;1417:89–100. doi: 10.1016/s0005-2736(98)00260-0. [DOI] [PubMed] [Google Scholar]
- 59.de Almeida RFM, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]