Abstract
Background and Purpose
5′ adenosine monophosphate-dependent protein kinase (AMPK) acts as a metabolic sensor. AMPK is elevated under ischemic conditions, but the role of AMPK in ischemic brain remains controversial. In this study, we examined the effects of AMPK inhibition using both pharmacological and genetic approaches in an in vivo stroke model.
Methods
Focal stroke was induced by reversible middle cerebral artery occlusion in male wild-type mice as well as mice deficient in one of the isoforms of the catalytic subunit of AMPK, AMPK α-1 or α-2.
Results
AMPK inhibition was neuroprotective after focal stroke. Mice deficient in AMPK α-2 demonstrated significantly smaller infarct volumes compared with wild-type littermates, whereas deletion of AMPK α-1 had no effect. Phosphorylation of a major upstream regulator of AMPK, LKB1, was also induced in stroke brain.
Conclusions
AMPK activation is detrimental in a model of focal stroke. The AMPK catalytic isoform α-2 contributes to the deleterious effects of AMPK activation. AMPK inhibition leads to neuroprotection even when these agents are administered poststroke.
Keywords: AMP-activated protein kinase, animal model, neuroprotection, stroke
Stroke is the third leading cause of death behind heart disease and cancer and now ranks as the leading cause of long-term disability in the United States. As our population ages, it is expected that the prevalence and incidence of cerebrovascular disease will continue to increase.1 No neuroprotective agents have demonstrated benefit in clinical trials, suggesting that novel pathways and targets need to be explored. We report that adenosine monophosphate-activated kinase may play an important role in the neuronal response to ischemic stress.
Glucose and oxygen depletion after ischemia cause disruption of cell ion homeostasis, leading to increased intracellular calcium and neuronal cell death.2 In an attempt to repair cell damage, numerous energy-consumptive pathways are activated, which may further contribute to cell death. Most recently, it has been demonstrated that manipulating the neuronal energy metabolism by altering the activity of adenosine monophosphate-activated protein kinase (AMPK) may provide neuroprotection after ischemia.3
AMPK is a known sensor of peripheral energy balance. AMPK is activated when cellular energy supply is low as signaled by increasing intracellular adenosine monophosphate and declining adenosine triphosphate levels. Peripherally, AMPK acutely regulates cellular metabolism and chronically regulates gene expression, reducing energy utilization (fatty acid, lipid and protein biosynthesis) and increasing energy production (fatty acid oxidation).4–7 AMPK is a heterotrimeric protein composed of a catalytic α subunit and 2 regulatory subunits, β and γ The catalytic α contains a serine/threonine protein kinase catalytic domain and exists as 2 isoforms, α-1 and α-2. The α-1 isoform is primarily cytoplasmic, whereas α-2 is predominantly nuclear and plays a role in transcriptional regulation.8–10 The 2 catalytic isoforms are distinct although highly homologous. Studies have indicated that AMPK α-2, not AMPK α-1, is induced under hypoxia conditions in human glioma cells.12 Mice deficient in AMPK α-2 demonstrate glucose intolerance and reduced insulin sensitivity, whereas the AMPK α-1 null mice do not show such alterations.13,14 Therefore, it is becoming clear that the 2 isoforms of AMPK α have different functions.
AMPK is rapidly activated in the brain in energy-deprived states such as fasting and ischemia.4 In the periphery, activation of AMPK and its downstream pathways lead to glycolysis and fatty acid oxidation, providing adenosine triphosphate for the energy-poor cell. However, unlike most peripheral tissue, neurons are exquisitely sensitive to even brief periods of ischemia. Neurons lack the enzymatic machinery necessary for effective glycolysis,15,16 and anaerobic lactate production rapidly leads to acidosis. Pharmacological inhibition of AMPK using either the selective inhibitor Compound C or C75, a fatty acid synthase inhibitor that modulates neuronal adenosine triphosphate levels,17 provided neuroprotection.3 However, pharmacological studies must be interpreted with caution, because unexpected, nonselective effects are possible. The limitations of pharmacological studies of AMPK have been recently highlighted.18
After cerebral ischemia, neuronal cell death can occur through necrosis, apoptosis, or a combination of mechanisms. 19 Because apoptosis is an energy-dependent process, inhibition of AMPK may only delay cell death. Additionally, these agents have previously only been evaluated when given before the ischemic insult. As noted by the Stroke Therapy Academic Industry Roundtable (STAIR), stringent and complete animal studies are required if we are to identify the most promising neuroprotective targets for translation to clinical studies. Therefore, in this study, we examined the effects of postischemic inhibition of AMPK, evaluated behavioral outcomes, and used genetic models of AMPK deficiency to further evaluate the link between AMPK and neuroprotection. We hypothesized that AMPK inhibition, whether through pharmacological inhibition or genetic deletion, would lead to neuroprotection.
Methods
Adenosine Monophosphate-Dependent Protein Kinase Mice
The present study was conducted in accordance with National Institutes of Health guidelines for the care and use of animals in research and under protocols approved by the Center for Laboratory Animal Care of University of Connecticut Health Center. The AMPK α-1 and AMPK α-2 knockout mice were bred in-house from strains previously described (AMPK α-1 background mice were C57Bl6/129 and AMPK α-2 mice were C57Bl6).12,20 All genetically modified mice were compared with their appropriate wild-type littermates. All studies used male animals age- and weight-matched (21 to 25 g, 10 to 12 weeks of age).
Animal Genotyping
Genotypes of the knockout mice were determined by polymerase chain reaction as previously described13,14,20 for primers.
Compound C and C75 Treatments
Compound C ((6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyyrazolo[1,5-a] pyrimidine; 20 mg/kg) was dissolved in distilled water and was administered intraperitoneally to the wild-type or AMPK α-2-deficient mice at the onset or 2 hours after the onset of stroke, depending on the individual experiment.21 C75 (3-carboxy-s4-octyl-2-methylenebutyrolactone) was dissolved in RPMI medium (Roswell Park Memorial Institute medium) and injected intraperitoneally to wild-type mice at the onset or 2 hours after the onset of stroke.22 The dose volume for every compound was 0.2 mL per 20 g body weight as previously described.3
Focal Cerebral Ischemia Model
Focal transient cerebral ischemia was induced in AMPK-null mice or background-matched wild-type littermates for 90 minutes followed by reperfusion as described previously.3,23,24 In separate nonsurvival cohorts of AMPK α-1 and α-2 knockout mice (n=4 per group), femoral arterial blood pressure and physiological measurements, including blood pH, PO2, PCO2, and blood glucose, were obtained. Cortical perfusion using laser Doppler flowmetry was evaluated throughout middle cerebral artery occlusion (MCAO) and early reperfusion as described previously.3,23
Behavioral Scoring
Neurological deficits were scored at 24 or 72 hours or 7 days poststroke. The score system was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by the tail; 2, circling to affected side; 3, unable to bear weight on the affected side; and 4, no spontaneous locomotor activity.
Histological Assessment
At 24 hours, 72 hours, or 7 days after stroke, the mice were euthanized and the brains were removed and cut into 5 2-mm slices. The slices were stained with 1.5% 2,3,5-triphenyltetrazolium solution at 37°C for 30 minutes. The stained slices were fixed with formalin (4%), images were digitalized, and the infarct volumes (corrected for edema) were analyzed using computer software (Sigmascan Pro5) as previously described.23
Western Blots
Western blots were done as described previously.3 Brains were homogenized using lysis buffer and protein was loaded on a 4% to 15% gradient sodium dodecyl sulfate–polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. AMPK, p-AMPK, and p-LKB1 (Thr189) were detected using corresponding antibodies from cell signaling (1:1000). β-actin (1:5000; Sigma) was used as the loading control. All blots were incubated overnight in primary antibody at 4°C in Tris-buffered saline buffer containing 4% bovine serum albumin and 0.1% Triton X-100. The secondary antibodies (goat anti-rabbit IgG1:5,000 for pLKB1, AMPK, and p-AMPK; Chemicon) were diluted and the ECL (pico) detection kit (Amersham Biosciences) was used for signal detection.
Statistics
Data from individual experiments were presented as mean±SE of mean. One-way analysis of variance (with Tukey post hoc correction, when appropriate) was used for the comparison of the means between the experimental groups except the neurological deficit scores, which were done by Mann-Whitney U test. P<0.05 was considered statistically significant. Behavioral and histological assessments were done by an investigator blinded to genotype/drug treatment.
Results
Stroke Outcome in Adenosine Monophosphate-Dependent Protein Kinase α-1 and Adenosine Monophosphate-Dependent Protein Kinase α-2 Knockout Mice
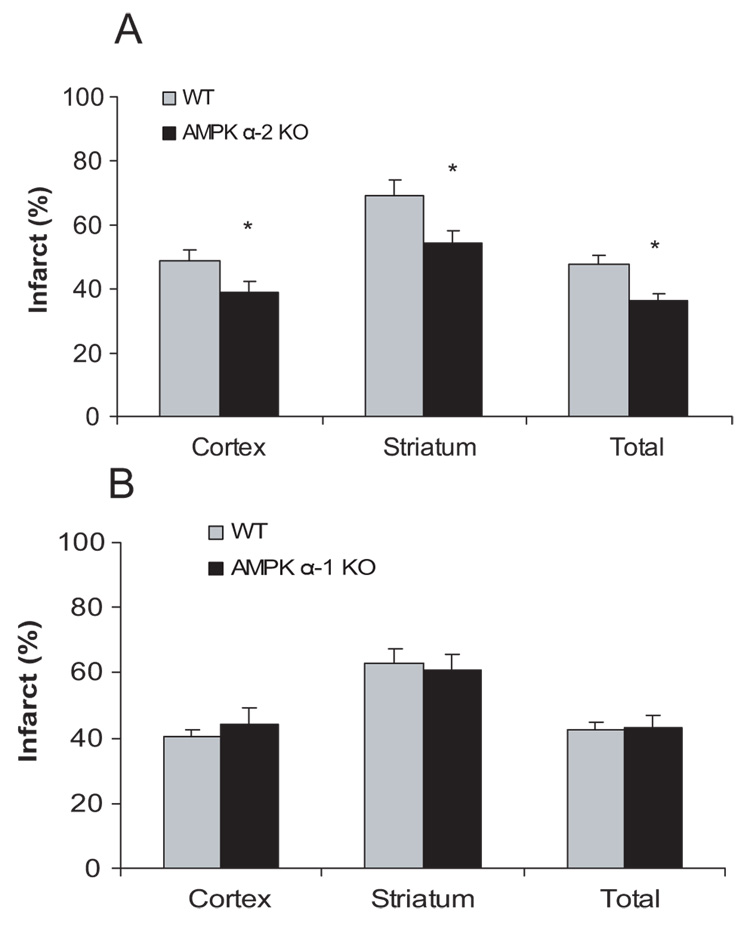
To determine whether the 2 isoforms of AMPK catalytic subunit play a different role in cerebral ischemia, stroke outcomes in AMPK α-1 knockout, α-2 knockout, or matched wild-type littermate controls were determined. AMPK α-2 mice (n=12) demonstrated significantly smaller cortical, striatal, and total (knockout 36.1±2.2% versus wild-type 47.7±2.7%, P<0.05) infarct volumes compared with wild-type mice (n=10; Figure 1A). There was no difference in the stroke outcome between AMPK α-1 knockout mice (n=9) and wild-type littermates (n=11) in total, cortical, or striatal infarct volume (Figure 1B).
Figure 1.
Mice deficient in AMPK α-2 (A), not α-1 (B) had reduced cortical, striatal, and total infarct volumes at 24 hours (shown as percentage of the nonischemic hemisphere). Mean±SEM. *P<0.05, one-way analysis of variance, compared with corresponding wild-type control.
No differences in mean arterial pressure, pH, pO2, pCO2, or blood glucose were seen between the AMPK α-1/α-2 and their corresponding wild-type controls (Table 1). Laser Doppler flow was equivalently reduced during ischemia (10.9±0.54% in AMPK α-2 knockout versus 11.5±0.55% in wild-type, n=4/gp) and was restored equally in early reperfusion (80.1%±4.9% in AMPK α-2 knockout versus 83.6%±7.3% in wild-type, n=4/gp) as was laser Doppler flow in AMPK α-1 knockout and wild-type littermates (Table 2).
Table 1.
Physiological Parameters Measured in the Wild-Type and AMPK α -1 Knockout and AMPK α -2 KO Mice
| pH | PCO2, mm Hg | PO2, mm Hg | Glucose, mg/dL | Mean Arterial Blood Pressure, mm Hg | |
|---|---|---|---|---|---|
| Preischemia | |||||
| WT | 7.34±0.027 | 40.8±2.9 | 121±7.5 | 124±4.6 | 75±2.8 |
| AMPK α-1 KO | 7.33±0.014 | 45.6±0.48 | 116±9.5 | 108±7.2 | 78±3.6 |
| Ischemia | |||||
| WT | 7.32±0.013 | 39.5±2.0 | 117±8.6 | 120±9.0 | 79±4.2 |
| AMPK α -1 KO | 7.30±0.030 | 44.9±3.1 | 120±18 | 103±13 | 84±2.3 |
| Preischemia | |||||
| WT | 7.37±0.034 | 39.4±4.5 | 113±8.7 | 134±12 | 73±2.8 |
| AMPK α -2 KO | 7.33±0.022 | 45.5±1.7 | 109±6.7 | 165±8.8 | 78±1.8 |
| Ischemia | |||||
| WT | 7.35±0.008 | 39.0±2.9 | 112±6.8 | 132±13 | 75±4.2 |
| AMPK α -2 KO | 7.33±0.025 | 43.5±3.0 | 115±5.2 | 159±5.7 | 82±0.65 |
There were no significant differences between the AMPK-null mice and their corresponding wild-type controls. The physiological parameters were measured before and 30 minutes after MCAO.
KO, knockout; WT, wild-type.
Table 2.
Laser Doppler Flow Measured in the Wild-Type and AMPK α -1 Knockout and AMPK α -2 KO mice
| LDF (ischemia period) | LDF (reperfusion period) | |
|---|---|---|
| WT | 13.1%±1.1% | 82.7%±3.7% |
| AMPK α-1 KO | 12.9%±0.72% | 87.7%±3.9% |
| WT | 11.5%±0.55% | 83.6%±7.3% |
| AMPK α-2 KO | 10.9%±0.54% | 80.1%±4.9% |
There were no significant differences between the AMPK-null mice and their corresponding wild-type controls. LDF was shown averaged over 90 minutes ischemia or 30 minutes reperfusion and expressed as the percentage of the base line (n=4 per group).
LDF indicates laser Doppler flow; KO, knockout; WT, wild-type.
Compound Treatment C Led to Sustained Neuroprotection
To evaluate if AMPK inhibition provides sustained neuroprotection, we assessed ischemic damage at 72 hours after cerebral ischemia. Because our previous work demonstrating robust neuroprotection with AMPK inhibition was done with a longer ischemic time (2 hours3), we also evaluated the effect of Compound C given at the onset of ischemia 24 hours after stroke using a 90-minute ischemic insult. Treatment with Compound C led to significant neuroprotection after stroke at both 24 (P<0.05 n=7/gp; Table 3) and 72 hours compared with vehicle-treated mice (P<0.05 n=8; Table 3). These improvements were reflected in improved behavior scores in Compound C-treated MCAO mice at both 24 hours and 72 hours (Table 3).
Table 3.
The Neuroprotective Effect of Compound C in Wild-Type Mice After MCAO (24- or 72-hour survival)
| Cortical (%) | Striatal (%) | Total (%) | Scores | |
|---|---|---|---|---|
| 24-hour survival | ||||
| Control (n=7) | 36.8±3.2 | 48.0±4.9 | 41.3±4.5 | 2.6±0.21 |
| Compound C (n=7) | 18.1±4.1* | 30.0±5.6* | 22.4±3.9* | 1.9±0.24* |
| 72-hour survival | ||||
| Control (n=6) | 54.1±3.6 | 73.6±7.8 | 61.7±3.6 | 3.0±0.4 |
| Compound C (n=8) | 31.8±2.1* | 49.4±3.0 | 38.3±2.3* | 1.7±0.1* |
Mice were treated intraperitoneally with 20 mg/kg per body weight of Compound C or vehicle at the onset of the stroke. Cortical, striatal, and total infarct volumes were measured (shown as percentage of the nonischemic hemisphere). Mean±SEM.
P<0.05, one-way analysis of variance, compared with corresponding vehicle-treated mice control. The neurological deficit scores were analyzed by Mann–Whitney U test.
Delayed Administration of Compound C or C75 Reduced Stroke Damage
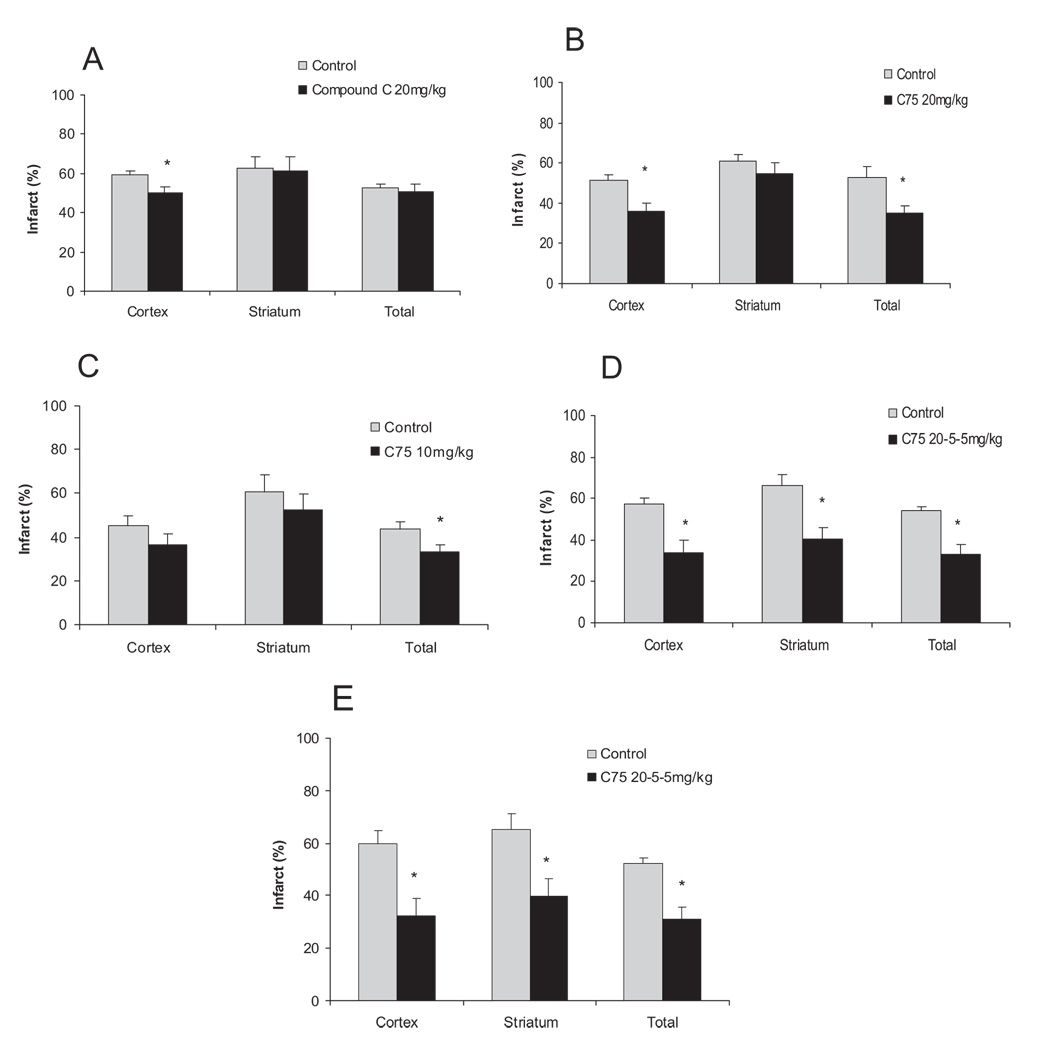
To assess if AMPK inhibition using Compound C or C75 protects when administered after stroke, Compound C or C75 (10 mg/kg or 20 mg/kg) was injected 2 hours after ischemia and outcome was evaluated at 24 hours. To evaluate if delayed administration of C75 is neuroprotective in longer survival models, we also assessed the stroke outcome at 72 hours and 7 days after stroke (first dose 20 mg/kg at 2 hours, second dose 5 mg/kg at 24 hours, and 5 mg/kg at 48 hours after the onset of stroke). Delaying administration of Compound C (n=7) attenuated its neuroprotective effect, because only a reduction in cortical infarct volume was seen (n=9; Figure 2A). Poststroke administration of C75 (20 mg/kg; n=7) significantly reduced the total as well as the cortical infarct volumes compared with the vehicle-treated group (n=6, P<0.05; Figure 2B), demonstrating a more potent effect of C75 in this model. The neuroprotection was also seen at a lower dose of C75 (10 mg/kg; n=10), which significantly reduced the total infarct volumes (n=10/gp, P<0.05; Figure 2C). Delayed administration of C75 (n=8) with daily treatment also significantly decreased the cortical, striatal, and total infarct at 72 hours after stroke compared with vehicle control (n=8, P<0.05; Figure 2D). The neuro-protection of C75 (n=7) administrated poststroke was still seen at day 7 as it reduced cortical, striatal, and total infarct volume compared with vehicle-treated control (n=6, P<0.05; Figure 2E).
Figure 2.
Delayed treatment of Compound C (A) or C75 (B–D) was neuroprotective. A, Mice were treated intraperitoneally with 20 mg/kg per body weight of Compound C or vehicle 2 hours after onset of stroke (24 hours survival). B through C, C75 was injected at 20 mg/kg (B) or 10 mg/kg (C) at 2 hours after onset of stroke and stroke volumes were compared with vehicle controls at 24 hours. D through E, C75 was given 2, 24, and 48 hours after the onset of MCAO at doses of 20 mg/kg, 5 mg/kg, and 5 mg/kg, respectively, and compared with vehicle-treated controls at 72 hours or 7 days. Cortical, striatal, and total infarct volumes were measured (corrected for edema percentage of the nonischemic hemisphere). Mean±SEM. *P<0.05, one-way analysis of variance, compared with corresponding vehicle-treated mice control.
The Neuroprotective Effect of Compound C Is Attenuated in Adenosine Monophosphate-Dependent Protein Kinase α-2 Mice
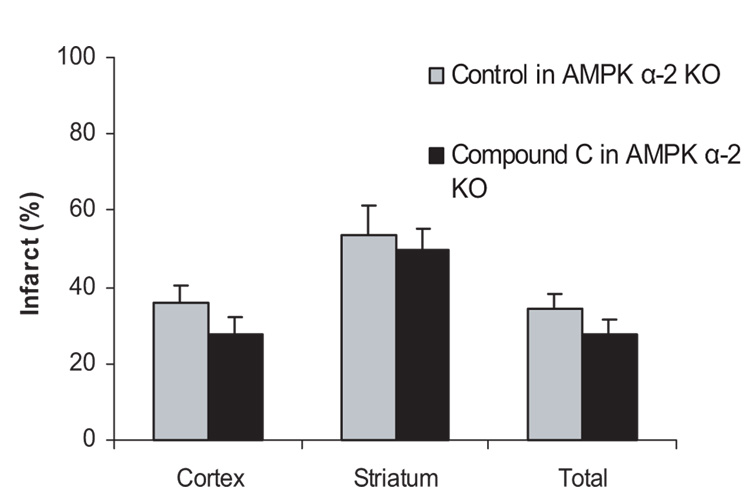
To further investigate the role of AMPK α-2 in stroke, the effect of Compound C was also studied in AMPK α-2 knockout mice. If AMPK α-2 contributes to the AMPK toxicity in ischemia, Compound C would lose, at least in part, its effect in the knockout mice. In AMPK α-2 knockout mice, Compound C (n=9) did not reduce the infarct volumes when compared with the vehicle-treated knockout mice (n=8; Figure 3).
Figure 3.
The neuroprotective effect of Compound C was attenuated in AMPK α-2 knockout mice. AMPK α-2 knockout mice were treated intraperitoneally with 20 mg/kg per body weight of Compound C or vehicle at stroke onset (24 hours survival). Mean±SEM. *P<0.05, one-way analysis of variance, compared with vehicle-treated knockout mice control.
Examination of an Upstream Activator of Adenosine Monophosphate-Dependent Protein Kinase, LKB1
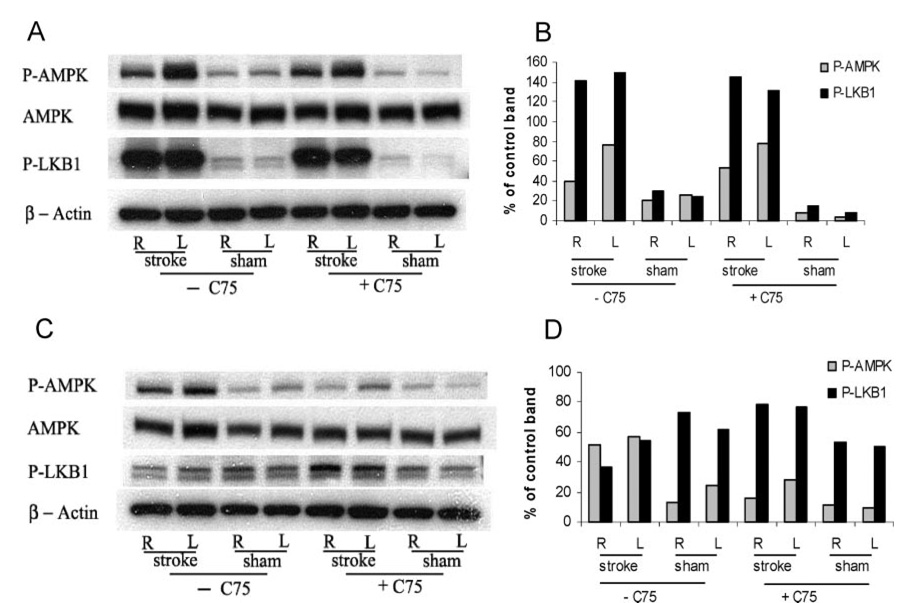
To further explore the AMPK system, we also assessed the expression/activation of one of the major regulators of AMPK, LKB-1, which is activated by rising levels of adenosine monophosphate.6 Elevations in p-AMPK and p-LKB1 were seen in the stroke brain 2 hours after MCAO (lanes 1 and 2 in Figure 4A and Figure 3B) in both the ipsilateral and contralateral hemisphere with minimal changes seen in sham brain. As we have shown previously, p-AMPK levels begin to decline 24 hours after stroke (lanes 1 and 2 in Figures 4C and 4D), an effect that is enhanced with C75 treatment (lanes 5 and 6 in Figures 4C and 4D). p-LKB1 levels decline in vehicle-treated animals at 24 hours, but remain slightly elevated in C75-treated mice (lanes 5 and 6 in Figures 4C and 4D).
Figure 4.
Phosphorylated LKB1 level increased after stroke. Western blot analysis was performed on lysates derived from whole hemisphere homogenates from either stroke animals (R, right/ischemic hemisphere and L, left/nonischemic hemisphere) or nonstroke sham animals 2 hours (A and B) or 24 hours (C and D) after the onset of stroke. The optical density of samples were expressed as the percentage of control band (β-actin). Antibodies detecting either phosphorylated or unphosphorylated AMPK, phosphorylated LKB1, and β-actin, which serves as a loading control (bottom panel), were used. C75 (+C75) was injected to the mice at the onset of the stroke (20 mg/kg intraperitoneally). Controls (−C75) were given vehicle. (n=5 for stroke groups and n=3 for sham groups).
Discussion
This study has several important findings. First, we confirm that inhibition of AMPK in the ischemic brain is beneficial. Second, the neuroprotection seen with AMPK inhibition is sustained for at least 1 week postinfarct. Third, continued neuroprotection is seen even after delayed administration of Compound C and C75. Finally, we demonstrate a significant role for AMPK α-2 isoform in the detrimental response of AMPK activation in ischemic brain, as deletion of this isoform leads to neuroprotection. The combination of pharmacological and genetic methods for inhibiting AMPK activity, combined with the use of relevant in vivo models of ischemia–reperfusion, strongly support our hypothesis that AMPK inactivation in the setting of ischemia is neuroprotective.
AMPK is a known regulator of cellular energy that functions to monitor cell-intrinsic processes that convey energy homeostasis.5–7 The discovery that the hypothalamus expressed high levels of AMPK, especially in nuclei that regulate energy balance and food intake17 positioned AMPK to also serve as a central nervous system sensor of organismal energy balance. Hypothalamic AMPK was also shown to respond to the peripheral adiposity signal, leptin, with alterations in food intake.25 This role in the regulation of energy balance suggested that AMPK may play a more global role in the regulation of energy balance in the central nervous system during severe or pathological energy challenge, like in ischemia.
The consequence of AMPK activation after stress is complex. We have previously shown that AMPK is activated in the mouse brain after MCAO in mice and in hippocampal slice cultures subjected to 2 hours of oxygen glucose deprivation in vitro.3 Pharmacological inhibition of the AMPK provided neuroprotection, whereas its activation exacerbated stroke outcome after MCAO. However, there are numerous at reports that suggest that AMPK activation may be beneficial under conditions of metabolic stress in peripheral tissues such as the heart,26,27 but the action of AMPK appears to be dependent on cell type and the metabolic milieu.28 For example, deletion of the AMPKα-2 isoform had no effect on cardiac function in vivo during normoxic conditions; how-ever, ischemia-induced contracture occurred more rapidly in isolated perfused hearts from AMPK α-2-deficient mice. This was associated with decreased adenosine triphosphate and glycogen content and a reduction in lactate production. Despite this apparently “poor” metabolic adaptation during ischemia, AMPK α-2-deficient hearts recovered as well as wild-type hearts, suggesting that changes in reperfusion and long-term outcomes need to be assessed in these studies.27
The effect of AMPK on neuronal metabolism is even less understood. In contrast to our findings, one study in cultured hippocampal neurons suggested a beneficial effect of AMPK activation after metabolic stress.29 Most recently, in a focal rat MCAO model, phosphodependent functional modulation of the GABA(B) receptor by AMPK30 led to an enhancement of slow synaptic inhibition by activating inwardly rectifying K(+) channels. This was hypothesized to be a protective response to injury, although ischemia-induced phosphorylation of GABA was seen primarily in the CA3 and dentate gyrus. The majority of damage after MCAO is in the cortex and striatum, not the hippocampus. In these structures, a dramatic downregulation of staining was seen,30 suggesting that AMPK-mediated GABA receptor activation occurs at sites distant from the ischemic damage.
To confirm our pharmacological data and determine the importance of each α isoform,8,10 we looked at the infarct volumes of the AMPK α-1- and α-2-null mice. AMPK α-2-null mice were modestly protected after stroke, whereas AMPK α-1-null mice were not. Thus, AMPK α-2 plays a distinctive role in response to stroke and contributes to the neurotoxicity of the overactivation of AMPK. The reduction of the infarct volume, however, is less than that seen with pharmacological inhibitors in wild-type mice. Besides the catalytic α subunit, AMPK also has 2 regulatory subunits, β and γ 6,11 One explanation could be that the 2 regulatory subunits may also contribute to cell damage in stroke through an unknown mechanism such that a pan-AMPK inhibitor such as Compound C could afford more protection than the deletion of one of the AMPK α isoform genes. Another possibility is that the pharmacological inhibitors may have other sites of action that result in neuroprotection in addition to their effect on AMPK; this is especially likely in the case of C75, which has actions on other metabolic pathways.17
When evaluating neuroprotection studies, concerns exist when the damage is evaluated at a short time point (ie, 22 hours). The present study shows that treatment with Compound C leads to continued neuroprotection at 72 hours. The reduction in infarct volume was more modest than at 24 hours, which likely represents the single dosing regimen used (t1/2=12 to 15 hours). Repeated dosing for the first 48 hours may increase the protective effect. This is suggested by our data using a repeated dosing regimen of C75 (first dose 20 mg/kg at 2 hours, second dose 5 mg/kg at 24 hours, and 5 mg/kg at 48 hours after the onset of stroke), which demonstrated neuroprotection at both 72 hours and 7 days.
We also examined the effect of delayed administration of Compound C or C75. Delaying the administration of Compound C led to a reduction in its neuroprotective potential, although a modest yet significant reduction in cortical infarction was still seen. In contrast, delayed administration of C75 at 20 mg/kg or 10 mg/kg dose-dependently decreased the total infarct volumes.
Our Western data suggest that the “signal” for activation of both AMPK and its upstream regulator, LKB1, is transmitted globally throughout the brain as robust contralateral increases in p-AMPK and p-LKB1 are seen in our model. Although focal stroke involves a discrete area of brain, compensatory mechanisms, including changes in cerebral blood flow and metabolic demand occur in areas far removed from the ischemic “core” as we have described previously.3 C75 treatment had no effect on p-AMPK at 2 hours (Figures 4A and 4B), yet C75 treatment reduced p-AMPK levels at 24 hours (Figures 4C and 4D), suggesting a sustained effect of treatment. Most importantly, a dramatic increase in pLKB1 was seen in the ischemic brain, a finding that has not been previously reported. LKB-1 may be the key regulator of AMPK under pathological conditions,19,31 and our findings imply that it is major contributor to stroke-induced increases in p-AMPK. Further studies will investigate the contributions of the 2 major upstream regulators of p-AMPK, LKB1 and CaMKK β, to stroke outcome.32
In summary, our results collectively confirm that activation of AMPK is detrimental in stroke and that it is the α-2 AMPK catalytic isoform, not α-1, that leads to the deleterious effect of AMPK activation in of acute stroke. The use of AMPK knockout mouse models further strengthen our results and clearly shows that AMPK activation is detrimental in the ischemic brain. Pharmacological AMPK inhibition reduced stroke damage even when administered poststroke, suggesting that manipulation of the AMPK pathway may provide a novel approach for the treatment of stroke.
Acknowledgments
Sources of Funding
This work was supported by The American Heart Association (L.D.M.) and the National Institutes of Health (NS050505 to L.D.M.) and the National Institute of Neurological Disorders and Stroke (G.V.R.). Compound C and C75 were provided by FASgen.
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://stroke.ahajournals.org/cgi/content/full/38/11/2992
Disclosures
Under a licensing agreement between FASgen and the Johns Hopkins University, G.V.R. and L.D.M. are entitled to a share of royalty received by the University on sales of products related to compounds described in this article. G.V.R. has an interest in FASgen stock, which is subject to certain restrictions under University policy. The Johns Hopkins University, in accordance with its conflict of interest policies, is managing the terms of this arrangement.
References
- 1. [Accessed March 2007];American Heart Association Statistics 2007. Available at www.americanheart.org.
- 2.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium–apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 3.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of amp-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 4.Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carling D. The amp-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 7.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the a2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie GD. Characterization of the AMP-activated protein kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 10.Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 12.Neurath KM, Keough MP, Mikkelsen T, Claffey KP. AMP-dependent protein kinase alpha 2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. Glia. 2006;53:733–743. doi: 10.1002/glia.20326. [DOI] [PubMed] [Google Scholar]
- 13.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 14.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dienel GA, Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia. 2005;50:362–388. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- 16.Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 17.Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- 18.Hardie DG, Frenguelli BG. A neural protection racket: AMPK and the GABAB receptor. Neuron. 2007;53:159–162. doi: 10.1016/j.neuron.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Chiarugi A. ‘Simple but not simpler’: toward a unified picture of energy requirements in cell death. Faseb J. 2005;19:1783–1788. doi: 10.1096/fj.05-4200rev. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-amp-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Henman MC, Atkinson J, Fixon-Owoo S, Tatlisumak T, Shaw GG, Doyle KM. The pre-ischaemic neuroprotective effects of the polyamine analogues BU43b and BU36b in permanent and transient focal cerebral ischaemia models in mice. Brain Res. 2006;1076:209–215. doi: 10.1016/j.brainres.2005.12.097. [DOI] [PubMed] [Google Scholar]
- 25.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 26.Russell R., 3rd Stress signaling in the heart by AMP-activated protein kinase. Curr Hypertens Rep. 2006;28:446–450. doi: 10.1007/s11906-006-0021-z. [DOI] [PubMed] [Google Scholar]
- 27.Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC, Horman S, Vaulont S, Hoerter J, Viollet B, Hue L, Vanoverschelde JL, Bertrand L. Role of the alpha2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol. 2006;291:H2875–H2883. doi: 10.1152/ajpheart.01032.2005. [DOI] [PubMed] [Google Scholar]
- 28.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574(1):95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 30.Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C-æ by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 32.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]