Abstract
The clearance of apoptotic cells is a highly regulated mechanism, normally associated with anti-inflammatory response. During early stages of apoptosis the cell is promptly recognized and engulfed by professional phagocytes or tissue cells to avoid the outflow of intracellular content and limit the immunological reaction against released antigens. However, increasing evidences suggest that impairment in the uptake of apoptotic cell debris is linked to the development of autoimmunity. In fact, autoantigens have been demonstrated to be content within apoptotic bodies and apoptotic cells seems to be critical in the presentation of antigens, activation of innate immunity and regulation of macrophage cytokine secretion.
We herein review the known mechanisms for regulating the uptake of the products of apoptosis in the development of autoimmunity.
Keywords: Cell clearance, lupus, autoantibodies
INTRODUCTION
Apoptosis, the major mechanism of programmed cell death, is essential to regulate and maintain tissue growth and maintain homeostasis. Dying cells undergo morphological modifications including chromatin condensation, nuclear fragmentation and generation of apoptotic bodies. Furthermore, they express so called “eat-me” signals on the cell surface that allow macrophage recognition and phagocytosis [1]. Thus, apoptosis is no longer considered a “trash disposal” mechanism as the clearance of apoptotic cells is a highly regulated process, essential to avoid the outflow of intracellular content and limit the immunological response against generated antigens. Within the immune system alone, it has been estimated that more than 109 cells undergo apoptosis daily [2] and these are cleared rapidly by neighboring tissue cells or professional phagocytes, normally without inciting an inflammatory reaction [3–5]. Indeed, the most significant difference between phagocytosis of pathogens and the uptake of apoptotic cells has been traditionally considered the immune response: a pro-inflammatory reaction is often induced after phagocytosis whereas the secretion of anti-inflammatory cytokines follows the engulfment of apoptotic cells [4, 6, 7].
Dysregulation of apoptosis has been associated with the pathogenesis of cancer [8, 9], neurodegeneration [10], cardiovascular disease [11, 12], and other complex diseases. The impact of apoptosis on immunity has been extensively investigated [2, 13] and several reports suggest a correlation between apoptosis and autoimmunity through an impairment of apoptosis [14–16] or an ineffective removal of apoptotic cells [17–20]. Moreover, recent data have demonstrated that autoantigens are found within apoptotic bodies [21] and that apoptotic cells are critical in the presentation of antigens [22], activation of innate immunity and regulation of macrophage cytokine secretion [23]. Apoptotic bodies have been also described as B cell autoantigens [24]. This review article is intended to provide a critical overview of current theories on the consequences of apoptosis and their connection to the breakdown of tolerance.
CLEARANCE OF APOPTOTIC CELLS AND PRODUCTS
The removal of apoptotic cells is mediated by professional phagocytes, i.e. macrophages and immature dendritic cells (DC) [25, 26], and by a wide variety of cells, such as endothelial cells [27], mesenchymal cells [28], or cardiocytes [17]. Professional phagocytes can also ingest opsonized bacteria or pathogens; however, the process and the consequences of these processes are distinct. The most important difference between the up-take of apoptotic cells and the phagocytosis of pathogens is the immune response, and the term engulfment is used to define the uptake of apoptotic cells (Table 1) [1]. Differences in the mechanisms of uptake between professional phagocytes and tissue cells have been reported [29]; these appear to reflect the rates of uptake and the timing of digestion rather than unique uptake mechanisms, receptors and signaling.
Table 1.
Characteristics of the immune response induced after cell phagocytosis. AA: arachidonic acid, LPS: lipopolysaccharide
| Necrotic cells | Apoptotic cells | |
|---|---|---|
| Cytokines |
|
|
| Inflammation |
|
|
| Dendritic Cells |
|
|
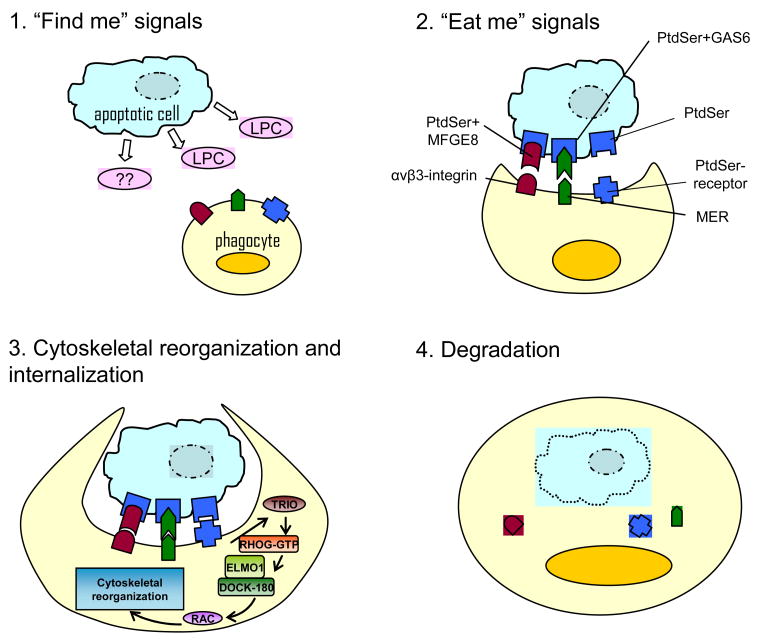
It is generally accepted that the uptake of apoptotic cells involves four essential steps (Figure 1): the release of “find me” signals that advise the phagocytes of the presence of apoptotic cells; the expression of “eat me” signals and recognition of the apoptotic cells by the phagocyte; the cytoskeletal reorganization and internalization of the target; and the degradation of the ingested apoptotic cell.
Figure 1. Phagocytosis of apoptotic cells.
At the early stages of apoptosis the dying cell releases the “find-me” signals that facilitate the recruitment of phagocytes, it is generally accepted that multiple find-me signals work together in this process but only data about lipid lysophodphatidylcholine (LPC) are found. Apoptotic cells then express membrane eat-me signals which are recognized by phagocyte receptors; phosphatidylserine (PtdSer) is the key receptor in this process and binds mainly the Phosphatidylserine-receptor on the phagocyte. The PtdSercan also be expressed by the apoptotic cell conjugated to milk fat globule EGF factor 8 protein (MFGE8) or growth-arrest-specific 6 (GAS6), which in turn can be recognized by the phagocyte receptors αv β3-integrin and MER. The cytoskeletal reorganization involves RHO family GTPases (i.e. RAC), ELMO1 (engulfment and cell motility 1), DOCK180 proteins represent one mode of activation. Other modes of activation may also exist (not shown). After internalization, actin is shed from the phagosome and the phagosome matures by a series of fusion and fission events with components of the endocytic pathway culminating in the mature phagolysosome
At the earliest stages of apoptosis, dying cells release diffusible factors, known as ‘find-me’ signals, which facilitate the recruitment of phagocytes. Lauber et al demonstrated that the lipid lysolysophosphatidylcholine (LPC), a factor released by apoptotic cells, can attract monocytes [30]. The regulation of this signals and the response induced in the phagocytes are still unknown. Apoptotic cells then express “eat me” signals on the cellular surface in the early stages of apoptosis [13] to allow phagocytic recognition. Several receptors have been reported to mediate the binding and uptake of dying cells, the most studied of these factors is phosphatidylserine (PtdSer) [31, 32]. In addition, the phagocytes express receptors that recognize the “eat-me” signals, i.e. the PtdSer-specific receptor [33–35]. The expression of the “eat-me” signals appears to be cell specific and to depend on the coexistence of multiple factors, i.e. by which cell is being engulfed, the receptors that are expressed by the apoptotic cell, the state of activation of the phagocyte [36, 37]. There is also evidence that inhibitors (“don’t eat me” signals) play a role in this process and in its regulation [38]. Finally, to be able to engulf the apoptotic cell the phagocyte requires an actin-dependent cytoskeletal reorganization that involves two signaling pathways: first, the RHO family GTPases, with a fine balance between activating (i.e. RAC) and inhibiting factors (i.e. RHOA) [39]; the second pathway involves the cell surface receptor proteins ABC1/CED-7 and CD-91/CED-1 [40]. After internalization, actin is displaced from the phagosome and the phagosome matures by a series of fusion and fission events with components of the endocytic pathway culminating in the mature phagolysosome. Phagosome trafficking occurs primarily in association with microtubules and therefore requires the coordinated interaction of the actin- and tubulin-based cytoskeleton. The final step of the engulfment of apoptotic cells is the digestion, and importantly, the consequences that the process determines in the cytokine secretion.
The absence of pro-inflammatory response after ingestion of apoptotic cells is considered one of the key points of the engulfment of dying cells [41]. In fact, it has been demonstrated that monocyte secretion of IL-1β, IL-8, granulocyte macrophage colony-stimulating factor, and TNF-α is inhibited after phagocytosis of apoptotic cells whereas the secretion of anti-inflammatory cytokines such as TGF-β1 and IL-10 is increased [7, 42]; interestingly these data have been also confirmed in vivo [6]. However, it is well accepted that impaired clearance of apoptotic cells is related to the development of autoimmunity and promote the secretion of pro-inflammatory cytokines [43]. A recent study seems to clarify in part this paradox [17]. In congenital heart block (CHB) in infants from anti-Ro positive mothers the opsonization of apoptotic cells with maternal antibodies inhibits the clearance of apoptotic cells with consequent accumulation and production of inflammatory cytokines.
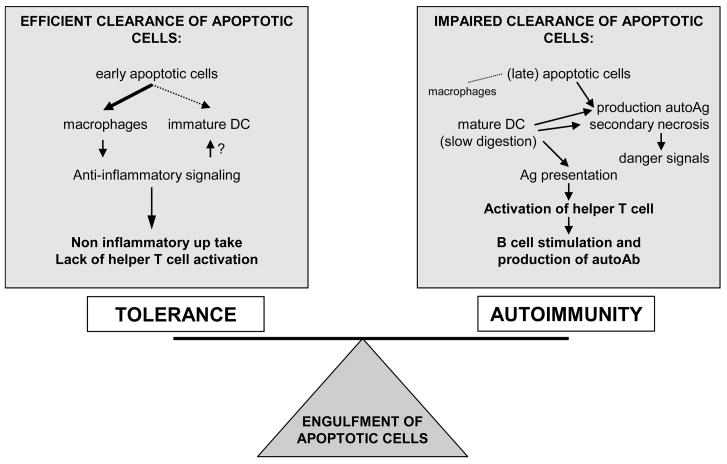
In conclusion, the role of apoptotic cells in the development of autoimmunity seems to be in part related to a defect in their clearance, with consequent impairment of the balance of signals associated to the apoptotic cell ingestion, i.e “eat me” signals [13], the phagocytic cell itself and how long the apoptotic cells persists before and after phagocytosis occurs (Figure 2).
Figure 2.
Consequences of the engulfment of apoptotic cells in the immune system. The uptake of dying cells is usually followed by an anti-inflammatory response and not associated with loss of tolerance. However, an ineffective removal of apoptotic cells is related to generation of “neo-antigensf that, presented to the T cells by mature DC can stimulate the production of autoantibodies. Ag: antigen; Ab: antibody; DC: dendritic cell
THE ADAPTIVE IMMUNE RESPONSE TO APOPTOTIC CELLS
Antigen presentation
A key factor in the induction of immune responses is the ability of DCs to internalize antigens and present antigen-derived peptides on major histocompatibility complex (MHC) molecules for the recognition by T lymphocytes.
Albert et al reported that immature DC are capable of phagocytosis of apoptotic cells, although not as efficiently as macrophages [25, 44] and that they can cross-present viral, tumor, and self-antigens to CD8+ T cells [45]. Moreover, it was recently suggested that apoptotic cells are able to promote maturation of DCs by upregulating costimulatory molecules and inducing proinflammatory cytokine release, while functioning as endogenous adjuvants for the induction of specific T cell responses [46]. Further evidence supporting the presentation of neo-antigens derived from apoptotic cells by DCs has been reported [47–49]. Nevertheless, the issue remains unclear and it has also been demonstrated that the uptake of apoptotic cells by DC suppresses the secretion of IL-12, a paracrine agent that alters the maturation of the DC, resulting in the inability to activate T cells [13]. These discrepancies may be due to the type of apoptotic cell being used, the variable effects of early and late stage apoptotic cells on DC maturation [50], and cytokine secretion [51].
Interestingly, DCs have a limited capacity for lysosomal degradation, due to a poor content of lysosomal protease [52] and apoptotic cells contained in DC phagosomes have been observed in the afferent lymphatics of the intestine directed to the lymphonodes [53]. On this basis, it has been suggested that the slow degradation of ingested apoptotic material by DC may allow an extended period of time to sample the microenvironment for danger signals, which stimulate DC to initiate an immune response [54]. Danger signals include those received through pattern recognition receptors such as Toll-like receptors (TLR) but also necrotic cells [55].
T cells
The first report of antigens expressed by apoptotic cells that were presented to T cells [45, 56] was published almost ten years ago. At the same time, T cell proliferation in PBMC isolated from both healthy controls or patients with systemic lupus erythematosus (SLE) was induced using either apoptotic cells or isolated antigens [57]; cloning of these T cells confirmed that antoantigens typically found in SLE, i.e. histones, were targeted by some of these T cell clones. Since then it has became generally accepted that antigens generated or exposed during apoptosis can lead the production of autoreactive T cells [13, 58]. It is known that the engulfment of necrotic cells strongly stimulate DC to mature and activate T cells; intracellular proteins also activate DC [59] suggesting that cells must lyse before they can promote an immune response. It has been then hypothesized that the autoimmune response is a consequence of the uptake of late apoptotic cells. In fact, phagocytosis of late apoptotic cells by mouse macrophages leads to the production of proinflammatory cytokines [60]. However, this theory is in contrast with the recent report that autoantigens are translocated into small apoptotic bodies during the early stages of apoptosis [21].
B cells
It has been proposed that apoptotic bodies can be targeted by autoantibodies [24] and that B cells with Ig receptors for apoptotic cells are positively selected [61]. Accordingly, B cells that react with apoptotic cells may tip the balance from tolerance to autoimmunity (Figure 2).
THE CONSEQUENCES OF APOPTOSIS IN AUTOIMMUNE DISEASES
It has been demonstrated that dysregulated apoptosis or impaired clearance of dying cells is related to the development of autoimmune diseases. However, most of the data on apoptosis in these conditions derives from observations and the pathogenic role of apoptosis remains to be established. Three different processes involving apoptotic cells have been demonstrated to be related to the development of autoimmunity. First, apoptosis is the mode of cell killing by immune processes e.g. cytotoxic T lymphocyte; it can make autoantigens available for self-perpetuating disease. Second, apoptosis in excess can be a source of autoimunogenic fragments, as discussed here in the context of neonatal lupus syndromes. Finally, genetic faults in apoptosis pathways, prototypically Fas/FasL, can interfere with tolerogenic deletion of lymphocytes and facilitate autoimmunity, a typical example of this is the autoimmune lymphoproliferative syndrome.
Table 2 summarizes the available data about the consequences of apoptosis in autoimmune diseases
Table 2.
Consequences of apoptosis in autoimmunity
| Autoimmune condition | Mechanism | |
|---|---|---|
| Congenital heart block (CHB) | The degeneration of the myocardic conductive system in infants from anti-Ro positive mothers causes the CHB. The maternal autoantibodies pass through the placental filter and recognize the antigen expressed within the blebs of apoptotic cardiocytes. The opsonizzation of the blebs with maternal autoantibodies inhibit their clearance with consequent accumulation and production of inflammatory cytokines. | [70] [17] [69] [74] |
| Systemic lupus erythematosus (SLE) | The pathogenesis of SLE is strongly associated with C1q deficiency, it has been demonstrated that C1q does not bind apoptotic cells isolated from SLE patients. The pathogenic role of the complement in SLE is still unclear, but it seems to be linked to impairment of apoptotic cell removal. | [64] [43] |
| Lupus nephritis (LN) | LN is associated with high titers of circulating high-affinity, IgG anti-double-stranded DNA (anti-dsDNA) antibodies and glomerular immunoglobulin deposits. Large chromatin fragments, derived from apoptotic cells, are located in the membranes of nephritic kidneys from murine and human SLE. Here, they act as targets for nephritic antibodies against components of nucleosomes. | [75] [66] [65] [76] |
| Autoimmune lymphoproliferative syndromes | A defect in lymphocyte apoptosis alters immune homeostasis resulting in an expansion of a normally rare circulating lymphocyte, the αβ double negative T cell. The abnormality in Fas mediated apoptosis underlying ALPS serves as a risk factor for autoimmunity involving blood cells and the development of lymphoma | [71] |
| Autoimmune thyroid diseases |
Hashimoto thyroiditis: self-reactive CD4+ T lymphocytes recruit B cells and CD8+ T cells into the thyroid. Disease progression leads to the death of thyroid cells by apoptosis (CD95) and hypothyroidism. Both autoantibodies and thyroid-specific cytotoxic T lymphocytes have been proposed to be responsible for autoimmune thyrocyte depletion.
Graves’ disease: the production of Th2 cytokines dominates in the thyroid, and thyrocytes are protected from apoptosis via over expression of antiapoptotic molecules such as Bcl-2. |
[77] [78] [79] |
| Type I diabetes | Possible mechanism for β-cell destruction in IDDM. (a) β-cells do not express CD95. During the insulitis process, infiltrating TH1 cells secrete NO, IFN-γ, and IL-1β, inducing CD95 expression on the surface of pancreatic β-cell. Autoreactive CTLs produce CD95L prime β-cell for destruction through CD95/CD95L interaction. (b) In contrast, IL-4 and IL-10 production might protect pancreatic β-cell from CD95-mediated apoptosis by the up-regulation of FLIP and Bcl-xL. | [80] [81] |
| Multiple sclerosis(MS) | MS is a T-cell-mediated inflammatory demyelinating disease of the central nervous system. T-cell apoptosis plays an important role in the spontaneous recovery from disease, but the antigen specificity of the apoptotic T-cells has not been determined. | [82] |
Systemic Lupus Erythematosus (SLE)
The complement protein C1q has been a focus of attention in SLE for many years, since C1q is consumed during acute phases of the disease [62]. In recent years, C1q has taken on greater importance, since it has been implicated in the effective removal of apoptotic products [63]. Several experimental reports demonstrate the relationship between impaired clearance of apoptotic cells and SLE, and especially the involvement of C1q defects in SLE [64]. Kalaaji et al demonstrated that large chromatin fragments, derived from apoptotic cells, localize in the intraglomerular membrane in murine and human SLE [65], and that those intra-glomerular membrane-associated nucleosomes are targeted by anti-dsDNA autoantibodies in human lupus nephritis [66].
Neonatal lupus syndromes (NLS) affect a variety of different organs; the most relevant involvement is represented by the heart, but also disorders of the skin, liver, and blood elements are all linked with anti-SSA/Ro-SSB/La antibodies in the maternal and fetal circulation [67, 68]. The CHB is a rare condition caused by inflammatory degeneration of the myocardic conductive system in infants from anti-Ro positive mothers, with SLE or Sjögren syndrome. This disease is of special interest for the understanding of the role of apoptosis in the development of autoimmune diseases. It has been demonstrated that maternal autoantibodies pass through the placental filter and recognize antigens expressed within the blebs of apoptotic cardiocytes. Interestingly, the opsonization of blebs with maternal autoantibodies inhibits the macrophage clearance with consequent accumulation and production of inflammatory cytokines [17, 69, 70].
Autoimmune lymphoproliferative syndrome (ALPS)
ALPS is a rare defect in lymphocyte apoptosis that alters immune homeostasis resulting in an expansion of a normally rare circulating lymphocyte, the αβ double negative T cell. ALPS is associated with inherited heterozygous mutations in the Fas gene that serves as a risk factor for autoimmunity involving blood cells and the development of lymphoma [71].
Primary biliary cirrhosis (PBC)
A characteristic feature of PBC is the specificity of the immune damage on the small intrahepatic bile ducts, despite the fact that the mitochondrial targets are ubiquitous proteins expressed in all nucleated cells. About 90% of patients with PBC have serum antimitochondrial antibodies (AMA) against the E2 subunit of the mitochondrial pyruvate dehydrogenase complex (PDC-E2). An anomaly of apoptosis in biliary epithelial cells (BEC) has been hypothesized as a source of antigens that could be responsible for activation of autoreactive T cells. It is known that macrophage phagocytosis of opsonized apoptotic cells is delayed in PBC [19] and that oxidative stress [16] induce apoptosis in BEC. However, how the mitochondrial antigens ultimate become exposed to the immune system is still unknown. In apoptotic BECs PDC-E2 is released not modified, whereas other cell types loose the antigenicity of PDC-E2 during apoptosis [72]; this seems to be related to lack of glutathiolation of PDC-E2 in BECs, since glutathiolation prevents autoantibody recognition [72]. Furthemore, BECs have the ability to phagocyte apoptotic BECS and to present novel mitochondrial self-peptides derived from phagocytosed apoptotic BECs [18]. These data support the hypothesis that phagocytosis of apoptotic cell by non-professional phagocytes may contribute to the tissue specificity of autoimmune diseases. Finally, Shimoda el al have recently suggested that BECs are “innocent victims” of autoimmune injury and that the proinflammatory activity of BECs in PBC is secondary to the intervention of liver-infiltrating mononuclear cells [73].
CONCLUSIONS AND FUTURE DIRECTIONS
The clearance of apoptotic cells, initially seen as an uninteresting “waste disposal”, is now accepted as a multipurpose process that is critical to the maintenance of tolerance; indeed, the removal of cellular components potentially antigenic is generally immunologically silent. One of the major issues in autoimmunity has been the intracellular localization of many autoantigens and how these molecules may become exposed to the immune system. An impairment in the clearance of apoptotic cells may resolve this issue. However, there are still contradictions to resolve: how does the anti-inflammatory response that follows the uptake of apoptotic cells become pro-inflammatory? What is the role of DC in the presentation of new antigens? How can we reconcile DC’s suppressive response to ingested apoptotic cells with their role of antigen presenting cells of neoantigens? What are the genetic and environmental factors that regulate apoptosis and antigen cleavage?
Nevertheless, we recognize the exciting prospect that the molecular identification of the factors that promote the initiation and the maintenance of normal apoptosis could provide novel tools for effective and possibly less toxic therapeutic interventions in patients with autoimmune diseases.
ABBREVIATIONS
- DC
dendritic cells
- MHC
major histocompatibility complex
- SLE
systemic lupus erythematosus
- CHB
congenital heart block
- ALPS
autoimmune lymphoproliferative syndrome
- LN
lupus nephritis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303–309. doi: 10.1016/j.jaut.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y. Silent cleanup of very early apoptotic cells by macrophages. J Immunol. 2003;171:4672–4679. doi: 10.4049/jimmunol.171.9.4672. [DOI] [PubMed] [Google Scholar]
- 4.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 5.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 6.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 9.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Leuner K, Pantel J, Frey C, Schindowski K, Schulz K, Wegat T, et al. Enhanced apoptosis, oxidative stress and mitochondrial dysfunction in lymphocytes as potential biomarkers for Alzheimer’s disease. J Neural Transm Suppl. 2007:207–215. doi: 10.1007/978-3-211-73574-9_27. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury RP, Lee JM, Greaves DR. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2:309–315. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]
- 12.Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007;28:69–75. doi: 10.1016/j.jaut.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 14.Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7:113–118. doi: 10.1191/096120398678919804. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Arguelles A, Brito GJ, Reyes-Izquierdo P, Perez-Romano B, Sanchez-Sosa S. Apoptosis of melanocytes in vitiligo results from antibody penetration. J Autoimmun. 2007;29:281–286. doi: 10.1016/j.jaut.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Salunga TL, Cui ZG, Shimoda S, Zheng HC, Nomoto K, Kondo T, et al. Oxidative stress-induced apoptosis of bile duct cells in primary biliary cirrhosis. J Autoimmun. 2007;29:78–86. doi: 10.1016/j.jaut.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, et al. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27:232–241. doi: 10.1016/j.jaut.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allina J, Stanca CM, Garber J, Hu B, Sautes-Fridman C, Bach N, et al. Anti-CD16 autoantibodies and delayed phagocytosis of apoptotic cells in primary biliary cirrhosis. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lleo A, Invernizzi P, Selmi C, Coppel RL, Alpini G, Podda M, et al. Autophagy: highlighting a novel player in the autoimmunity scenario. J Autoimmun. 2007;29:61–68. doi: 10.1016/j.jaut.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008;15:183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 22.Mandron M, Martin H, Bonjean B, Lule J, Tartour E, Davrinche C. Dendritic cell-induced apoptosis of human cytomegalovirus-infected fibroblasts promotes cross-presentation of pp65 to CD8+ T cells. J Gen Virol. 2008;89:78–86. doi: 10.1099/vir.0.83278-0. [DOI] [PubMed] [Google Scholar]
- 23.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 24.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169:159–166. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 25.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang KS, Burow A, Kurrer M, Lang PA, Recher M. The role of the innate immune response in autoimmune disease. J Autoimmun. 2007;29:206–212. doi: 10.1016/j.jaut.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, et al. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108(Pt 3):967–973. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 28.Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, et al. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–5252. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- 29.Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10:857–860. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- 30.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 31.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 32.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 33.Ezekowitz RA, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S. Macrophage-restricted molecules: role in differentiation and activation. Immunol Lett. 1999;65:5–8. doi: 10.1016/s0165-2478(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 36.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 37.Pradhan D, Krahling S, Williamson P, Schlegel RA. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol Biol Cell. 1997;8:767–778. doi: 10.1091/mbc.8.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 39.Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 41.Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leukoc Biol. 1992;52:269–273. [PubMed] [Google Scholar]
- 42.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 43.Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, et al. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Rubartelli A, Poggi A, Zocchi MR. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta3 integrin and requires intracellular and extracellular calcium. Eur J Immunol. 1997;27:1893–1900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- 45.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 46.Johansson U, Walther-Jallow L, Smed-Sorensen A, Spetz AL. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J Immunol. 2007;179:1711–1720. doi: 10.4049/jimmunol.179.3.1711. [DOI] [PubMed] [Google Scholar]
- 47.Rovere P, Manfredi AA, Vallinoto C, Zimmermann VS, Fascio U, Balestrieri G, et al. Dendritic cells preferentially internalize apoptotic cells opsonized by anti-beta2-glycoprotein I antibodies. J Autoimmun. 1998;11:403–411. doi: 10.1006/jaut.1998.0224. [DOI] [PubMed] [Google Scholar]
- 48.Frisoni L, McPhie L, Colonna L, Sriram U, Monestier M, Gallucci S, et al. Nuclear autoantigen translocation and autoantibody opsonization lead to increased dendritic cell phagocytosis and presentation of nuclear antigens: a novel pathogenic pathway for autoimmunity? J Immunol. 2005;175:2692–2701. doi: 10.4049/jimmunol.175.4.2692. [DOI] [PubMed] [Google Scholar]
- 49.Tzeng TC, Suen JL, Chiang BL. Dendritic cells pulsed with apoptotic cells activate self-reactive T-cells of lupus mice both in vitro and in vivo. Rheumatology (Oxford) 2006;45:1230–1237. doi: 10.1093/rheumatology/kel106. [DOI] [PubMed] [Google Scholar]
- 50.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol. 2004;173:189–196. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 51.Bondanza A, Zimmermann VS, Dell’Antonio G, Cin ED, Balestrieri G, Tincani A, et al. Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum. 2004;50:1549–1560. doi: 10.1002/art.20187. [DOI] [PubMed] [Google Scholar]
- 52.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 53.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol. 2007;171:2–8. doi: 10.2353/ajpath.2007.070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barker RN, Erwig LP, Hill KS, Devine A, Pearce WP, Rees AJ. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin Exp Immunol. 2002;127:220–225. doi: 10.1046/j.1365-2249.2002.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voll RE, Roth EA, Girkontaite I, Fehr H, Herrmann M, Lorenz HM, et al. Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. 1997;40:2162–2171. doi: 10.1002/art.1780401210. [DOI] [PubMed] [Google Scholar]
- 58.Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, et al. Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol. 1997;159:5391–5399. [PubMed] [Google Scholar]
- 59.Grolleau A, Misek DE, Kuick R, Hanash S, Mule JJ. Inducible expression of macrophage receptor Marco by dendritic cells following phagocytic uptake of dead cells uncovered by oligonucleotide arrays. J Immunol. 2003;171:2879–2888. doi: 10.4049/jimmunol.171.6.2879. [DOI] [PubMed] [Google Scholar]
- 60.Xiao YQ, Someya K, Morita H, Takahashi K, Ohuchi K. Involvement of p38 MAPK and ERK/MAPK pathways in staurosporine-induced production of macrophage inflammatory protein-2 in rat peritoneal neutrophils. Biochim Biophys Acta. 1999;1450:155–163. doi: 10.1016/s0167-4889(99)00042-7. [DOI] [PubMed] [Google Scholar]
- 61.Cocca BA, Seal SN, D’Agnillo P, Mueller YM, Katsikis PD, Rauch J, et al. Structural basis for autoantibody recognition of phosphatidylserine-beta 2 glycoprotein I and apoptotic cells. Proc Natl Acad Sci U S A. 2001;98:13826–13831. doi: 10.1073/pnas.241510698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;199:265–285. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- 63.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 64.Donnelly S, Roake W, Brown S, Young P, Naik H, Wordsworth P, et al. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum. 2006;54:1543–1556. doi: 10.1002/art.21783. [DOI] [PubMed] [Google Scholar]
- 65.Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168:1779–1792. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalaaji M, Fenton KA, Mortensen ES, Olsen R, Sturfelt G, Alm P, et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 2007;71:664–672. doi: 10.1038/sj.ki.5002133. [DOI] [PubMed] [Google Scholar]
- 67.Izmirly PM, Rivera TL, Buyon JP. Neonatal lupus syndromes. Rheum Dis Clin North Am. 2007;33:267–285. vi. doi: 10.1016/j.rdc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Friedman DM, Rupel A, Buyon JP. Epidemiology, etiology, detection, and treatment of autoantibody-associated congenital heart block in neonatal lupus. Curr Rheumatol Rep. 2007;9:101–108. doi: 10.1007/s11926-007-0003-4. [DOI] [PubMed] [Google Scholar]
- 69.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–182. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 70.Neufing PJ, Clancy RM, Jackson MW, Tran HB, Buyon JP, Gordon TP. Exposure and binding of selected immunodominant La/SSB epitopes on human apoptotic cells. Arthritis Rheum. 2005;52:3934–3942. doi: 10.1002/art.21486. [DOI] [PubMed] [Google Scholar]
- 71.Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, et al. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- 72.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, et al. Biliary epithelial cells and primary biliary cirrhosis: The role of liver-infiltrating mononuclear cells. Hepatology. 2008 doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 74.Tran HB, Macardle PJ, Hiscock J, Cavill D, Bradley J, Buyon JP, et al. Anti-La/SSB antibodies transported across the placenta bind apoptotic cells in fetal organs targeted in neonatal lupus. Arthritis Rheum. 2002;46:1572–1579. doi: 10.1002/art.10316. [DOI] [PubMed] [Google Scholar]
- 75.Zykova SN, Seredkina NE, Rekvig OP. Glomerular targets for autoantibodies in lupus nephritis--an apoptotic origin. Ann N Y Acad Sci. 2007;1108:1–10. doi: 10.1196/annals.1422.001. [DOI] [PubMed] [Google Scholar]
- 76.Bendiksen S, Mortensen ES, Olsen R, Fenton KA, Kalaaji M, Jorgensen L, et al. Glomerular expression of large polyomavirus T antigen in binary tet-off regulated transgenic mice induces apoptosis, release of chromatin and initiates a lupus-like nephritis. Mol Immunol. 2008;45:728–739. doi: 10.1016/j.molimm.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Stassi G, De Maria R. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol. 2002;2:195–204. doi: 10.1038/nri750. [DOI] [PubMed] [Google Scholar]
- 78.Stassi G, Di Liberto D, Todaro M, Zeuner A, Ricci-Vitiani L, Stoppacciaro A, et al. Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol. 2000;1:483–488. doi: 10.1038/82725. [DOI] [PubMed] [Google Scholar]
- 79.Wang SH, Baker JR. The role of apoptosis in thyroid autoimmunity. Thyroid. 2007;17:975–979. doi: 10.1089/thy.2007.0208. [DOI] [PubMed] [Google Scholar]
- 80.Savinov AY, Tcherepanov A, Green EA, Flavell RA, Chervonsky AV. Contribution of Fas to diabetes development. Proc Natl Acad Sci U S A. 2003;100:628–632. doi: 10.1073/pnas.0237359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50(Suppl 1):S44–47. doi: 10.2337/diabetes.50.2007.s44. [DOI] [PubMed] [Google Scholar]
- 82.Pender MP. Treating autoimmune demyelination by augmenting lymphocyte apoptosis in the central nervous system. J Neuroimmunol. 2007;191:26–38. doi: 10.1016/j.jneuroim.2007.09.015. [DOI] [PubMed] [Google Scholar]