Abstract
This study aimed to continue our characterization of finger strength and multi-finger interactions across the lifespan to include those in their sixties and older. Building on our previous study of children, we examined young and elderly adults during isometric finger flexion and extension tasks. Sixteen young and sixteen elderly, gender-matched subjects produced maximum force using either a single finger or all four fingers in flexion and extension. The maximum voluntary finger force (MVF), the percentage contributions of individual finger forces to the sum of individual finger forces during four-finger MVF task (force sharing), and the non-task finger forces during a task finger MVF task (force enslaving), were computed as dependent variables. Force enslaving during finger extension was greater than during flexion in both young and elderly groups. The flexion-extension difference was greater in the elderly than the young adult group. The greater independency in flexion may result from more frequent use of finger flexion in everyday manipulation tasks. The non-task fingers closer to a task finger produced greater enslaving force than non-task fingers farther from the task finger. The force sharing pattern was not different between age groups. Our findings suggest that finger strength decreases over the aging process, finger independency for flexion increases throughout development, and force sharing pattern remains constant across the lifespan.
Keywords: finger, force, extension, aging, development, enslaving, sharing
1. Introduction
Manipulative motor performances are associated with neuromuscular changes across the lifespan. These changes are often reflected by optimal or suboptimal development in finger strength, independent actions of fingers, and coordination of multiple fingers. During childhood, hypertrophy of muscle fibers (Lexell, Sjostrom, Nordlund, & Taylor, 1992; Sjostrom, Lexell, & Downham, 1992) and maturation of neuronal connections and pathways (Caramia, Desiato, Cicinelli, Iani, & Rossini, 1993; Gibbs, Harrison, & Stephens, 1997; Muller & Homberg, 1992; Muller, Ebner, & Homberg, 1994) have been considered to be responsible for the developmental increases in maximum force production and submaximal force control (Deutsch & Newell, 2001; Potter, Kent, Lindstrom, & Lazarus, 2006; Shim, Lay, Zatsiorsky, & Latash, 2004; Smits-Engelsman, Westenberg, & Duysens, 2003). On the other hand, previous studies have suggested that the impairments in manipulation strength and dexterity, often observed in the elderly, are attributed to neuromuscular changes, such as a decrease in the number of motor neurons, an increase in motor neuron size, changes in motor unit discharge patterns, and changes in contractile properties (Botterman, Iwamoto, & Gonyea, 1986; Doherty & Brown, 1997; Duchateau & Hainaut, 1990; Galganski, Fuglevand, & Enoka, 1993; Kamen & Roy, 2000; Kornatz, Christou, & Enoka, 2005; Tracy, Maluf, Stephenson, Hunter, & Enoka, 2005; Vaillancourt, Larsson, & Newell, 2003).
Impairments of hand digit control and coordination in late adulthood can be attributed to the changes in both peripheral properties of neuromuscular system and central organization of descending commands to finger muscles (Larsson & Ansved, 1995; Shim et al., 2004; Shinohara, Latash, & Zatsiorsky, 2003a; Vaillancourt et al., 2003). Therefore, clumsy finger actions could result from inaccurate control of finger actions in both flexion and extension. For example, fast key stroking and releasing in keyboarding is achieved by sequential actions of flexion and extension of individual digits.
Although previous studies have reported age-related changes in finger strength, independent finger control, and synergic finger interactions (Deutsch & Newell, 2001; Oliveira, Shim, Loss, Petersen, & Clark, 2006; Potter et al., 2006; Shim et al., 2004; Shinohara, Li, Kang, Zatsiorsky, & Latash, 2003b; Smits-Engelsman et al., 2003), to our knowledge, no studies have used the same experimental paradigm to examine maximum voluntary force (MVF) and finger interaction indices to describe the changes across the lifespan. Particularly, age-related changes of finger strength and multi-finger interactions during finger extension actions are lacking in the current literature.
Recently, we investigated age-related changes in finger strength and interaction in children. We found that finger strength and independency increases from 6 to 10 years of age and the rate of finger strength development with respect to the children’s age was greater in flexion as compared to extension. While the force sharing pattern during four-finger maximum force production did not change with age, finger strength and independency were greater in flexion than in extension for all children(Shim et al., 2007).
The current study is a follow-up to our previous study on children’s finger strength and multi-finger interactions. Here we extend the age-related characterization of finger strength and finger independency to those in their sixties and older. We specifically investigate age-related changes in MVF and finger interaction indices in adults and the elderly using the same paradigm. We test three hypotheses. (1) Our recent study on finger flexion and extension tasks in children showed greater finger force enslaving (unintended finger force production by non-task fingers) in extension and a slower decreasing rate of finger enslaving with age in extension as compared to flexion (Shim et al., 2007). If the greater changes in the finger enslaving with age for flexion is due to the functional demand and frequent use of flexor muscles in everyday manipulation tasks (e.g., grasping), we would expect the finger enslaving difference between flexion and extension to be greater in the elderly compared to the young adults. (2) Additionally, if the force sharing pattern during four-finger MVF does not change across development, as previously suggested (Shim et al., 2007), we expect that finger force sharing pattern (the contributions of each finger force to the total force during four-finger force production) would be the same between young adults and elderly adult. (3) Previous studies on finger flexion tasks showed that finger enslaving is greater in non-task fingers that are closer to the task fingers, which has been known as the “proximity” hypothesis (Zatsiorsky, Li, & Latash, 1998; Zatsiorsky, Li, & Latash, 2000). If this phenomenon is due to common muscles that have different insertions in multiple fingers (Malerich, Baird, McMaster, & Erickson, 1987), greater overlapping of adjacent digit representations in the hand area of the primary motor cortex, synchronous firing of cortical cells, and a common neuronal input to multiple muscles (Bremner, Baker, & Stephens, 1991; Fetz & Cheney, 1980; Malerich et al., 1987; Matsumura, Chen, Sawaguchi, Kubota, & Fetz, 1996), we would expect to find similar trends for finger extension tasks.
2. Method
2.1 Participants
Sixteen young and sixteen elderly, gender-matched adults participated as subjects in this study. All subjects were healthy and right-handed, according to their preferential use of the hand during daily activities, such as writing, drawing, and eating. None of the subjects had a history of neuropathy or trauma to the upper limbs. The ages and physical characteristics of the subjects are shown in Table 1. The hand lengths were measured between the distal crease of the wrist and the middle finger tip when subjects positioned the palm side of their right hand and lower arm on a table with all finger joints extended. The hand width was measured between the radial side of the index finger metacarpal joint and the ulnar side of the little finger metacarpal joint. Both young and elderly subjects were recruited from the university community. All gave informed consent based on the procedures approved by the University of Maryland’s Institutional Review Board (IRB).
Table 1.
Subject age, hand lengths, and hand widths.
| Age (yrs) | Hand length (cm) | Hand width (cm) | |
|---|---|---|---|
| Young males (n=8) | 22.0 ± 1.5 | 19.7 ± 1.7 | 8.6 ± 0.5 |
| Elderly males (n=8) | 69.3 ± 5.0 | 19.9 ± 1.6 | 9.0 ± 0.6 |
| Young female (n=8) | 21.8 ± 3.1 | 18.0 ± 1.2 | 7.7 ± 0.6 |
| Elderly females (n=8) | 65.8 ± 4.8 | 17.6 ± 1.4 | 7.8 ± 0.2 |
Mean±S.E.
2.2. Apparatus
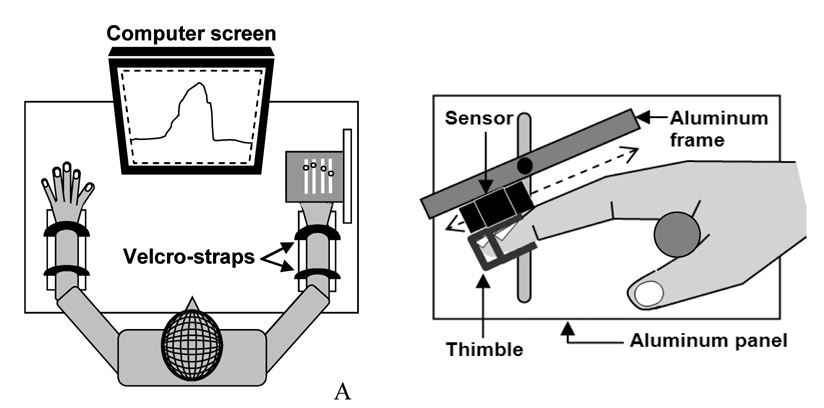
The experimental setup included four two-directional (tension and compression) force sensors (black rectangles in Fig. 1A) for four fingers (2nd–5th digits) with amplifiers (Models 208 M182 and 484B, Piezotronics, Inc.). The sensors were mounted on a customized aluminum frame (14.0 × 9.0 × 1.0 cm) along four slits which allowed adjustments of the sensor positions along the long axis of fingers depending on the individual hand and finger sizes. Adjacent slits were separated medio-laterally by 2 cm (Fig. 1B). The frame was attached to a large vertical aluminum panel (21.0 × 16.0 × 2.0 cm) with a vertical slit (14.0 cm), which allowed the frame two degrees-of-freedom: one for vertical translation and the other for rotation about Z-axis. C-shaped aluminum thimbles were attached on the bottom of each sensor. The frame was tilted at 25° with respect to the antero-posterior axis such that all finger joints (distal inter-phalangeal, proximal inter-phalangeal, and metacarpo-phalangeal joints) were slightly flexed when the distal phalanges were positioned inside the thimbles. After the position adjustments of the sensors and the frame, the sensors were mechanically fixed to the frame and the frame was fixed to the panel using a nut-bolt structure.
Fig. 1.
Experimental setting: (A) the wrists and the forearms of the subject were rested in a wrist-forearm brace and held by Velcro straps. The subject sat in a chair and watched the computer screen to perform a task. (B) The experimental settings for the right hand: the two-directional (tension and compression) sensors (black rectangles) were attached to an aluminum frame and the C-shaped thimbles were attached to the bottom of the sensors. The subject inserted the distal phalange of each finger in the thimbles while holding a cylindrical handle (gray circle). The sensor positions were adjustable along the aluminum frame.
Signals from the sensors were conditioned, amplified, and digitized at 1000 Hz with a 16-bit A/D board (PCI 6034E, National Instruments Corp.) and a custom software program made in LabVIEW (LabVIEW 7.1, National Instruments Corp.). A desktop computer (Dimension 4700, Dell Inc.) with a 19” monitor was used for data acquisition. The task finger force was displayed on the monitor screen online.
2.3 Procedure
All subjects sat in a chair facing a computer screen with the shoulder abducted 35° in the frontal plane and elbow flexed 45° in the sagittal plane such that the forearm was parallel to the frame (Fig. 1B). The forearm rested on the customized wrist-forearm brace (comprised of a piece of foam that was attached to a semi-circular plastic cylinder) fixed to a wooden panel (29.8 × 8.8 × 3.6cm). Velcro straps were used to avoid forearm and wrist movements. The subjects were asked to rest the distal phalange of each finger in a thimble such that all joints were slightly flexed and formed a dome shape with the hand. In order to remove the gravitational effects of the fingers and possible assistance to flexion or extension due to passive stretching of the intrinsic and extrinsic muscles, the force signals for the initial 0.5 seconds were averaged for each finger and subtracted from the later signals. Only the force signals after subtraction were shown on the computer monitor as real-time feedback.
Subjects performed ten trials in total: one trial for each task finger (I, M, R, and L for single-finger tasks and IMRL together for a four-finger task) in two finger force directions (flexion and extension). The order of the trials was pseudo-randomized and balanced across subjects. During each trial, all fingers were in the thimbles, and subjects were asked to produce maximum isometric force with the task finger(s) in flexion or extension over a 3-s interval while watching the force feedback of the task finger(s) on the computer screen. The experimenter watched the subjects’ right hand carefully for any superfluous joint movements. Any trials with visible non-task related finger or wrist joint movements were rejected (<2%) and performed again by the subjects. The subjects were instructed to concentrate on the task finger and not to pay attention to non-task fingers. The task finger force produced was displayed on-line on the computer screen in front of the subject.
2.4 Data processing
The MVF values were determined as the maximum forces produced by the task finger(s). The Force enslaving (FE) values were calculated as the average non-task finger forces for the task fingers. These values were averaged across all fingers to calculate the overall finger inter-dependency indices FE (Eqs. 1).
| (1) |
where i ≠ j, n = 4, where is the maximum force produced by the finger, i, and Fij is the force produced by the non-task finger, i, during the j finger maximum force task.
Note that FE for each finger represents the averaged percent force of non-task fingers for the same trial with respect to the task finger MVF. Some previous studies employed finger independency indices (Hager-Ross & Schieber, 2000; Li, Dun, Harkness, & Brininger, 2004) rather than finger inter-dependency. However, this study used the finger inter-dependency index (i.e., FE) to compare the current study with other previous studies employing finger FE values in young and elderly adults (Shinohara et al., 2003b; Shinohara et al., 2003a; Zatsiorsky et al., 2000). Force deficit (FD) values for each finger were calculated by the difference between single-finger MVF and the force of the same finger at four-finger MVF task. This value was normalized by the single-finger MVF and averaged over fingers to calculate FD (Shim et al., 2007; Zatsiorsky et al., 1998). Force sharing (FS) values for each finger were calculated as the percent contributions of each finger force to the sum of the finger forces during the four-finger MVF task.
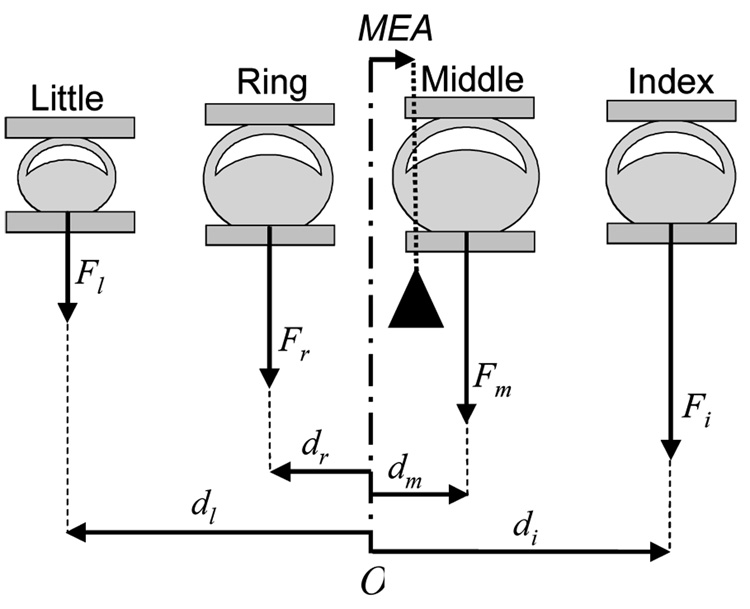
In order to test the proximity hypothesis (i.e., greater finger enslaving of non-task fingers whose proximity of greater to task fingers during task finger flexion) (Zatsiorsky et al., 1998; Zatsiorsky et al., 2000) in finger extension, we calculated the average value of non-task finger forces across the fingers next to (F1), second next to (F2), and third next to (F3) the task fingers (Eqs. 2). The moment equilibrium axis (MEA), the medio-lateral position of a hypothetical antero-posterior axis about which the resultant moment of finger pressing forces is in static equilibrium (Eq. 3 & Fig. 2), was also calculated in order to test the proximity hypothesis.
| (2) |
when k = 1, k= 2, and k = 3, j respectively represent the non-task fingers next, second next to, and third next to the task fingers. m is the number of non-task fingers.
| (3) |
, where Fi, Fm, Fr, and Fl are index, middle, ring, and little finger forces and di, dm, dr, and dl are the moment arms of the forces from the midpoint (O) between the ring and middle finger sensors in Fig. 2., q represents the individual fingers: q = {index,middle,ring,little}.
Fig. 2.
The index, middle, ring, and little finger forces (Fi, Fm, Fr, and Fl) and the moment arms (di, dm, dr, and dl) of the forces from the midpoint (O) between the ring and middle finger sensors. The black triangle represents a hypothetical fulcrum about which the moments of the individual finger forces are in static equilibrium. MEA represents the horizontal position of the fulcrum with respect to O.
2.5 Statistics
Standard descriptive statistics and mixed-effects ANOVAs with the factors of AGE (young adults and elderly adults), GENDER (males and females), DIRECTION (flexion and extension), and PROXIMITY (F1, F2, and F3) were used to analyze MVF, FE, FD and FS. Although the gender influence was not the focus of this paper, data were analyzed for GENDER to compare our results with previous studies (Shinohara et al., 2003a; Shinohara et al., 2003b). A MANOVA was used for statistical analysis of FS values. Since the sum of individual finger force sharing is always 100%, the sharing values of only middle, ring, and little fingers were used for the MANOVA (Danion, Latash, Li, & Zatsiorsky, 2001). The Bonferroni corrections were used for significance adjustments for multiple comparisons. The level of significance was set at p=.05.
3. Results
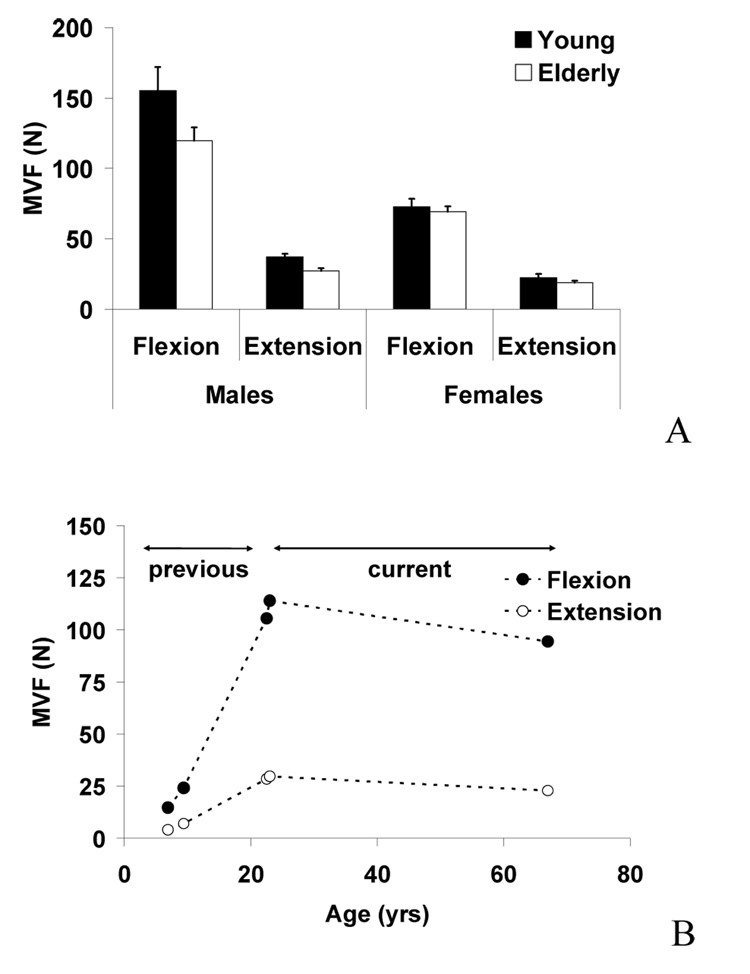
During the four-finger MVF tasks, the young adult subjects produced greater MVF compared to the elderly subjects (on average 148%), Fig. 3A. The male subjects produced greater MVF than the female subjects (on average 139%). The flexion tasks showed greater MVF values than the extension tasks (on average 149%). The differences in the MVF values between the males and females were greater in flexion (on average 160%) than in extension (on average 145%). These findings were supported by three-way mixed-effects ANOVA with the factors of AGE, GENDER, and DIRECTION, which showed significant factor effects of AGE [F(1,28)=6.3, p<.05], GENDER [F(1,28)=55.9, p<.001], DIRECTION [F(1,28)=278.3, p<.001] and significant DIRECTION × GENDER interaction [F(1,28)=278.3, p<.001]. Another notable trend was that the differences in the MVF values between males and females was greater in the young adults (on average 51%) than the elderly adults (on average 45%) although the statistical significance was slightly below the level of significance [F(1,28)=3.4, p=.076]. Figure 3B shows the MVF values from our previous study on children and young adults (Shim et al., 2007) together with our current results (young and elderly adults). As shown in the figure 3B, the finger strength increased from children to young adults and decreased from young to elderly adults.
Fig. 3.
(A) Maximum voluntary force (MVF) values during four-finger flexion and extension force production tasks in young males, young females, elderly males, and elderly females. Averaged across subjects data are shown with standard error bars. (B) MVF values averaged across genders during four-finger flexion and extension tasks from our previous study (Shim et al., 2007) and the current study.
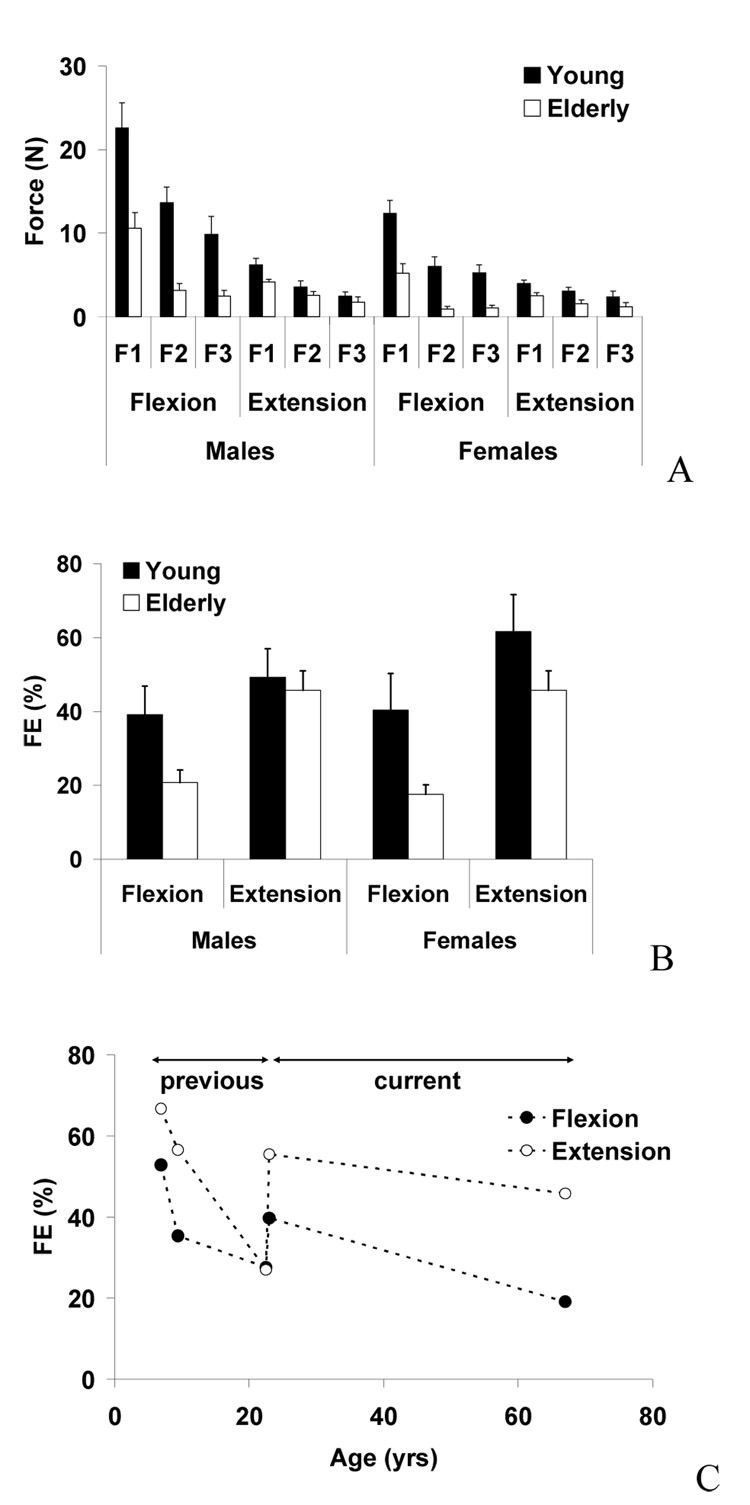
When FE values were averaged across all fingers (Eqs. 1), as shown in Fig. 4A, the young adult group showed the greater FE values than the elderly group (on average 167%). The differences were more evident in flexion than in extension and in females than in males, Fig. 4B. These findings were supported by three-way mixed-effects ANOVA with the factors of AGE, GENDER, and DIRECTION, which showed the significant AGE [F(1,28)=33.2, p<.001] and GENDER [F(1,28)=7.9, p<.005] factor effects and the significant AGE × DIRECTION [F(1,28)=14.0, p<.005] and AGE × GENDER [F(1,28)=278.3, p<.001]. The force enslaving values were 40% and 54% in young adults and 20% and 46% in elderly adults, for flexion and extension, respectively. We plotted our previous FE values in children and young adults (Shim et al., 2007) with our current young adults and elderly subjects. As shown in Figure 4C, a discrepancy was found in FE values of young adults between our previous study and the current study [flexion (p= 0.08); extension (p<0.01).
Fig. 4.
(A). Force enslaving (FE) values during finger flexion and extension force production tasks in young males, young females, elderly males, and elderly females. F1, F2, and F3 represent the average forces across the fingers next to, second next, and third next to the task fingers, respectively. The averaged across subjects data are shown with standard error bars. (B) FE values during finger flexion and extension force production tasks in young males, young females, elderly males, and elderly females. Averaged across subjects data are shown with standard error bars. (C) FE values averaged across genders during flexion and extension tasks from our previous study (Shim et al., 2007) and the current study.
The difference between the individual MVF finger forces during the single-finger task and the forces of the same fingers during the four-finger MVF tasks allow us to compute the deficits of individual finger MVF during the four-finger tasks. The FD values did not differ between young male adults (22.1% ± 5.5% flexion; 21.1% ± 10.5% extension) and the elderly (30.7% ± 6.2 % flexion; 10.5% ± 13.1% extension) neither between young female young adults (30.5% ± 7.2% flexion; 25.6% ± 8.7 % extension) and the elderly (23.6% ± 5.6 % flexion; 8.98% ± 13.2% extension). Gender differences were also not found. These results were supported by two-way repeated-measures ANOVA which showed no age differences between the FD values and no significant effects of GENDER, DIRECTION, or a GENDER × DIRECTION interaction.
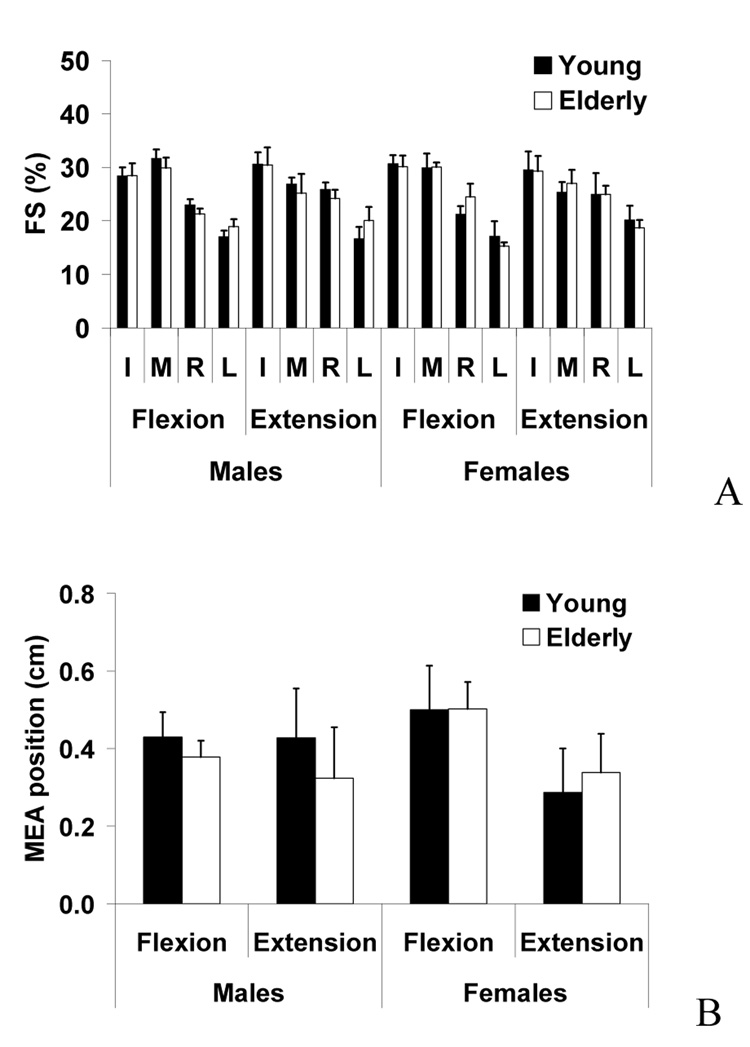
When subjects performed the four-finger tasks, all four fingers produced forces, and the individual finger forces were calculated as the percentages of the four-finger total force (FS). The FS values of individual fingers showed no significant changes between young and elderly adults (Fig. 5A). In general, the index and middle finger FS values (I: 29% and M: 28% on average) were larger than the values of the ring and little finger (R: 24% and L: 18% on average). The middle finger FS was greater in flexion (30%) than extension (26%) whereas the ring finger FS was greater in extension (25%) than flexion (22%). The FS values for flexion and extension were similar for the index and little fingers. These findings were supported by a MANOVA showing a significant effect of DIRECTION [M: F(1,48)=6.8, p=.012; R: F(1,48)=3.8, p=.057; L: F(1,48)=1.3, p=.26], but no other significant factor or interaction effects.
Fig. 5.
(A) Force sharing (FS) of index (I), middle (M), ring (R), and little (L) fingers during four-finger maximum flexion and extension force production tasks. Averaged group data are shown with standard error bars. (B) The medio-lateral positions of moment equilibrium axis (MEA) during finger flexion and extension force production tasks in young males, young females, elderly males, and elderly females. Averaged group data are shown with standard error bars.
MEA values showed no significant factor or interaction effects by three-way mixed-effects ANOVA with the factors of AGE, GENDER, and DIRECTION (Fig. 5B). The proximity hypothesis was tested by calculating the average forces of non-task finger forces across the fingers next to (F1), second next to (F2), and third next to (F3) the task fingers. The non-task finger force magnitudes were in the order of F1 (on average 8.5 N), F2 (4.3 N), and F3 (3.3 N). These findings were supported by four-way mixed-effects ANOVA, which showed significant factor effects of PROXIMITY [F(1,28)=126.0, p<.001], DIRECTION [F(1,28)=53.6, p<.001], AGE [F(1,28)=15.1, p<.005], GENDER [F(1,28)=55.9, p<.001], and a significant PROXIMITY × DIRECTION interaction [F(1,28)=4.1, p<.05].
4. Discussion
The results consistently showed that finger MVF values were greater in flexion than extension. This trend is similar to our previous study on children (Shim et al., 2007) which showed that MVF values were about four times greater in flexion than extension, even at different metacarpophalangeal joint angles (e.g., 20° and 80° metacarpophalangeal (MCP) joint flexion). The greater MVF values in flexion than extension found in the current study can be attributed to the difference in muscular strength between flexors and extensors rather than the muscle force-length relationship (Ralston, 1953). These differences in muscle strength between the flexors and extensors may be partially due to the larger cross-sectional area of flexors than extensors in the forearm (e.g., extrinsic flexor and extensor muscles) (Lieber, Fazeli, & Botte, 1990; Lieber, Jacobson, Fazeli, Abrams, & Botte, 1992). Not surprisingly, the maximum muscular strength indicated by the MVF values continuously increased from children between 6 and 11 years (50.3 N in flexion and 17.2 N in extension) to young adults (Shim et al., 2007) and decreased from young adults (106.6 N in flexion and 28.4 N in extension) to elderly adults (92.7 N in flexion and 22.6 N in extension). Our study investigated only one MCP joint angle, and it is currently unknown how the MVF and finger interaction indices would change due to the finger joint angle changes. Additionally, the greater MVF values found in male subjects confirm previous findings of gender effects during finger MVC tasks (Shinohara et al., 2003b).
Although increased finger independency is considered to be desirable for skillful hands manipulation, it has been documented that humans are not capable of independent control of individual digits. We can neither move a single digit without changing the positions of the others (Hager-Ross & Schieber, 2000; Li et al., 2004; Schieber & Santello, 2004) nor produce one digit force without producing forces with the other digits (Li, Latash, & Zatsiorsky, 1998; Reilly & Hammond, 2000). There are both peripheral and central factors contributing to this observed incapacity for independent digit control. The peripheral factors include anatomical connections of hand and forearm [e.g., digit connections by web space soft tissue and insertions of a flexor digitorum profundus into multiple digits (Hager-Ross & Schieber, 2000; Malerich et al., 1987)]. Central factors, on the other hand, include interdependent digit control by the CNS due to overlapping digit representation in the hand area of the primary motor cortex, synchronous firing of cortical cells, and a common neuronal input to multiple muscles (Bremner et al., 1991; Fetz & Cheney, 1980; Matsumura et al., 1996; Schieber, 2001)
Force enslaving is a measure of finger inter-dependency that is opposite to finger independency. The level of finger independency has been considered a critical aspect of finger movement control (Li et al., 2004; Schieber & Santello, 2004; Shim et al., 2007). For example, we would make frequent mistakes in typing wrong keys during keyboarding or piano playing if our fingers were not able to move independently (Engel, Flanders, & Soechting, 1997; Fish & Soechting, 1992; Haueisen & Knosche, 2001; Schmuckler & Bosman, 1997). In order to avoid these mistakes, “additional commands” of the CNS should be delivered to non-intended finger muscles to not move these fingers, which may cause inefficient use of the neuromuscular system. Thus, the smaller finger independency during extension found in this study may contribute to the less effective performance of finger extension tasks as compared to flexion tasks (Carson, 1996; Carson & Riek, 1998). For example, Carson (1996; 1998) asked subjects to coordinate maximum angular displacement of the index finger metacarpophalangeal joint in flexion and extension with an auditory metronome. It was found that synchronization of finger movements with the metronome signal in extension was more variable than an equivalent task of finger flexion.
We previously showed greater finger force enslaving and a slower decreasing rate of finger enslaving with children’s age in extension than flexion. The force enslaving values in children between 6 and 10 years of age were about 43% and 61% for flexion and extension, respectively (Shim et al., 2007). We have interpreted that the greater changes in finger enslaving with children’s age for flexion is due to the functional demand and frequent use of flexor muscles in everyday manipulation tasks (e.g., grasping). Based on this speculation, we formulated our first hypothesis that the finger enslaving difference between flexion and extension would become greater in elderly persons as compared to young persons.
Previous studies suggested that the finger independency is critical not only in playing musical instruments but also in everyday manipulation tasks (Leijnse, Walbeehm, Sonneveld, Hovius, & Kauer, 1997; Schieber, 1991; Shinohara et al., 2003b; Shinohara et al., 2003a; Zatsiorsky et al., 1998). The continuous decreases in force enslaving from children to elderly adults reflect lifelong development of finger independency. The decrease in force enslaving values from young to elderly adults found in our current study, as well as previous studies, (Shinohara et al., 2003a; Shinohara et al., 2003b) may be counterintuitive because one would expect to find an age-related increase in force enslaving in elderly persons considering their impaired finger dexterity (Contreras-Vidal, Teulings, Stelmach, & Adler, 2002; Giampaoli et al., 1999; Hackel, Wolfe, Bang, & Canfield, 1992; Hughes et al., 1997; Latash, Shim, Gao, & Zatsiorsky, 2004).
Although finger independency has been considered as a desirable motor capability for manipulation tasks, the functional implication of finger independency in manipulative tasks is not as clear as it appears to be. Previous reports have shown that loss in hand dexterity with aging may be more related to the synergic actions of multiple fingers than finger independency (Shim et al., 2004; Shinohara et al., 2003a; Shinohara et al., 2003b). Although the ability to control accurate finger force can be improved with specialized training (Carson, 2006; Ranganathan, Siemionow, Sahgal, Liu, & Yue, 2001), elderly subjects have shown less accurate time profiles to produce multi-finger force and/or moments (Shim et al., 2004; Shinohara et al., 2003b). The relationship between enslaving and hand dexterity is not straightforward and remains a challenge for further investigations. During static prismatic grasping (i.e. grasping an object with the thumb opposing other fingers), for example, enslaving forces from the other fingers caused by a task finger can help stabilize the resultant torque acting on the object (Shim, Latash, & Zatsiorsky, 2005; Shim, Park, Zatsiorsky, & Latash, 2006). In this sense, the decrease in force enslaving in elderly adults may have a negative impact on the stability of prismatic grasping tasks.
The changes in the neuromuscular system due to experiences in everyday finger actions appear to be an important aspect to be considered for the force enslaving differences between flexion and extension. Our previous study reported that the slope of age-related finger force enslaving changes were greater for flexion than extension in children (Shim et al., 2007). From this result, we expected to find a greater finger force enslaving in extension than flexion in elderly adults. Assuming that the greater force enslaving rate of development in flexion is based on frequent use of flexion in everyday activities, we also expected greater changes of finger enslaving in flexion than extension from young adults to elderly adults. Furthermore, the differences found between flexion and extension could be due the influence of inherent characteristics between the agonist muscles (flexors and extensors). It is possible that, despite the effects of everyday experience, there could be some neural/anatomical differences that may limit the improvement in enslaving in the extensor muscle(s) compared with the flexor(s) muscle. Our results showed that the changes in finger enslaving from young adults to elderly adults were obviously greater in flexion than extension, corroborating our first hypothesis. The gender differences found in finger enslaving from our study support previous findings (Shinohara et al., 2003b).
When the current FE values were compared to our previous study (Shim et al., 2007) for young adults, a discrepancy was found. A possible explanation from such discrepancy is the difference between the metacarpophalangeal joint angles used in both studies (previous: 25° and current: 20°). The angle of flexion of the metacarpophalangeal joint seems to have effects on the level of FE. We previously showed that with a decrease in the metacarpophalangeal joint (from 80° to 30°), force enslaving was smaller for extension and greater for flexion (Shim et al., 2007). However, in this study we showed that FE values increased from 20° to 25°. The torque-angle and torque-muscle length relationships could have possibly affected the MVF production and FE values. Although to our knowledge no previous studies have fully described the relationship between the finger muscle length and its effects on MVF production and FE, the preliminary results from a developing experiment in our laboratory is showing that the torque–angle curve is nonlinear. The FE values increases during smaller angles until certain angle and decreases at larger angles of the metacarpophalangeal joint. In addition, different subject groups who participated in two different studies may have played a role in such discrepancy.
For the extension condition, if only the previous adult’s values are taken into account to analyze the age-related differences, a decrease in force independency from young adults to old adults could be noted. If that is the case, a reverse interpretation of the results is also possible: force independency of finger extension increases throughout development. Unfortunately, no previous studies have explored age-related changes on finger extension tasks, which limit our discussion and do not lead us a conclusive interpretation. On the other hand, such differences require future investigations on development of finger independence.
Overall, FD values did not show significant age and direction differences in present study. This result is somewhat different from findings of previous studies (Shinohara et al., 2003a & 2003b). Shinohara et al. found statistically significant effects of age (i.e., increased force deficits with aging). During the flexion condition, the male subjects’ FD values showed a similar trend to the Shinohara’s previous findings. The difference is currently unclear in terms of its sources. However, the differences may be due to different experimental settings used in our study. Shinohara used an experimental setup which required subjects to stabilize horizontal movements of the fingers in the loops attached at the end of long wires while our study used mechanically fixed finger thimbles.
Despite the complex changes of finger interactions due to musculotendinous and neuromuscular changes spanning the development from children to elderly adults, our previous findings (Shim et al., 2007) and the results of this study showed that the force sharing values for each finger during four finger MVF tasks are constant across development (index: ~30%, middle: ~30%, ring: ~22%, and little: ~18%). In other words, the relative contributions of each finger force to the total force during four-finger force production remained constant although the absolute MVF values of all fingers increase from children to young adults (Shim et al., 2007) and decreased from young adults to elderly adults. This constant force sharing confirms our second hypothesis and also supports the premise that the neuromuscular system for fingers from the age of 6 onwards minimizes the change in the resultant torque about the longitudinal axis. In future studies, we propose to examine if this consistency in force sharing is seen as a property of infant grasping.
The principle of minimization of secondary moments about the longitudinal functional axis of the hand was previously suggested as an organizational principle of the CNS defined sharing patterns among the fingers (Li et al., 1998; Li et al., 2004). When the position of the hypothetical moment equilibrium axis (i.e., MEA) was calculated for young and elderly groups, the position was constant regardless of age group. The constant MEA position has a functional implication in grasping as the constant finger force sharing patterns can help the CNS control the resultant torque on an object in prismatic grasping. For example, if the same sharing pattern for all-digit object grasping is preserved throughout development, the CNS would not need to change the relative position of the thumb opposition to other fingers or to adjust individual finger forces during grasping to generate the same resultant moment on a hand-held object.
Previous studies on finger flexion tasks have shown that finger enslaving increases when the task finger is more proximal to a non-task finger [i.e., proximity hypothesis (Zatsiorsky et al., 1998; Zatsiorsky et al., 2000)]. The greater enslaving in closer non-task fingers may be caused by both central (Schieber & Hibbard, 1993)and peripheral factors. For example, close finger representations of adjacent fingers in the motor cortex (38) and a large force transfer from a task finger muscle compartment to non-task finger muscle compartments (Leijnse, 1998) can cause the ranked order of enslaving level. If this interpretation holds true for finger flexion tasks, we would expect to find similar trends for finger extension tasks. In this sense, our third hypothesis was elaborated in order to test if the finger force enslaving would be greater in non-task fingers that are closer to than to those farther from the task fingers.
Our current analyses confirms the proximity hypothesis in finger extension by showing increased enslaving forces in non-task fingers with an increase in the distances between the task and non-task fingers. Furthermore, a greater finger force enslaving in extension than flexion was found in the current study. Our current experimental design is limited in quantifying the contributions of peripheral and central factors to the finger enslaving. However, a new experiment involving passive and active finger movements similar to a previous study (Hager-Ross & Schieber, 2000) may provide insights about contributions of the peripheral and central factors to the ordered level of enslaving as well as the enslaving difference between flexion and extension.
5. Conclusion
In summary, the results of our study indicate that finger enslaving during isometric finger force production in extension is greater than in flexion for both young and elderly adults, and the flexion-extension difference is greater in elderly adults. The force sharing pattern is constant across multiple age groups, and non-task fingers that are closer to a task finger produce greater enslaving force. The convergence of our current findings with our previous results on age-related changes in children (Shim et al., 2007) allows us to point out that developmentally, strength of the digits increases throughout childhood, reaching maximum levels around age 22. Over the aging process, however, the finger strength decreases significantly. Our findings suggest that we continuously develop our ability to move our fingers independently even into our advancing years. Overall, at the behavioral level of analyses, our studies suggest changes in the finger force production strategies as a function of age, particularly due the decreased levels of digit-dependency. However, further investigation is needed to clarify what is developing and what are the underlying mechanisms of finger independency.
Acknowledgements
National Institute of Health Grant R01HD42527 (to J. E. Clark). We would also like to thank Minjung Woo and Bradley King for helping with data collection and review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Botterman BR, Iwamoto GA, Gonyea WJ. Gradation of isometric tension by different activation rates in motor units of cat flexor carpi radialis muscle. Journal of Neurophysiology. 1986;56:494–506. doi: 10.1152/jn.1986.56.2.494. [DOI] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Effect of task on the degree of synchronization of intrinsic hand muscle motor units in man. Journal of Neurophysiology. 1991;66:2072–2083. doi: 10.1152/jn.1991.66.6.2072. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Desiato MT, Cicinelli P, Iani C, Rossini PM. Latency jump of "relaxed" versus "contracted" motor evoked potentials as a marker of cortico-spinal maturation. Electroencephalography and Clinical Neurophysiology. 1993;89:61–66. doi: 10.1016/0168-5597(93)90086-5. [DOI] [PubMed] [Google Scholar]
- Carson RG. Neuromuscular-skeletal constraints upon the dynamics of perception-action coupling. Experimental Brain Research. 1996;110:99–110. doi: 10.1007/BF00241379. [DOI] [PubMed] [Google Scholar]
- Carson RG. Changes in muscle coordination with training. Journal of Applied Physiology. 2006;101:1506–1513. doi: 10.1152/japplphysiol.00544.2006. [DOI] [PubMed] [Google Scholar]
- Carson RG, Riek S. The influence of joint position on the dynamics of perception-action coupling. Experimental Brain Research. 1998;121:103–114. doi: 10.1007/s002210050442. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Teulings HL, Stelmach GE, Adler CH. Adaptation to changes in vertical display gain during handwriting in Parkinson's disease patients, elderly and young controls. Parkinsonism & Related Disorders. 2002;9:77–84. doi: 10.1016/s1353-8020(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Danion F, Latash ML, Li ZM, Zatsiorsky VM. The effect of a fatiguing exercise by the index finger on single- and multi-finger force production tasks. Experimental Brain Research. 2001;138:322–329. doi: 10.1007/s002210100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch KM, Newell KM. Age differences in noise and variability of isometric force production. Journal of Experimental Child Psychology. 2001;80:392–408. doi: 10.1006/jecp.2001.2642. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Brown WF. Age-related changes in the twitch contractile properties of human thenar motor units. Journal of Applied Physiology. 1997;82:93–101. doi: 10.1152/jappl.1997.82.1.93. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. Journal of Physiology. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel KC, Flanders M, Soechting JF. Anticipatory and sequential motor control in piano playing. Experimental Brain Research. 1997;113:189–199. doi: 10.1007/BF02450317. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. Journal of Neurophysiology. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Fish J, Soechting JF. Synergistic finger movements in a skilled motor task. Experimental Brain Research. 1992;91:327–334. doi: 10.1007/BF00231666. [DOI] [PubMed] [Google Scholar]
- Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. Journal of Neurophysiology. 1993;69:2108–2115. doi: 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]
- Giampaoli S, Ferrucci L, Cecchi F, Lo NC, Poce A, Dima F, Santaquilani A, Vescio MF, Menotti A. Hand-grip strength predicts incident disability in non-disabled older men. Age and Ageing. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Cross-correlation analysis of motor unit activity recorded from two separate thumb muscles during development in man. Journal of Physiology. 1997;499(Pt 1):255–266. doi: 10.1113/jphysiol.1997.sp021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Physical Therapy. 1992;72:373–377. doi: 10.1093/ptj/72.5.373. [DOI] [PubMed] [Google Scholar]
- Hager-Ross C, Schieber MH. Quantifying the independence of human finger movements: comparisons of digits, hands, and movement frequencies. Journal of Neuroscience. 2000;20:8542–8550. doi: 10.1523/JNEUROSCI.20-22-08542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haueisen J, Knosche TR. Involuntary motor activity in pianists evoked by music perception. Journal of Cognitive Neuroscience. 2001;13:786–792. doi: 10.1162/08989290152541449. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gibbs J, Dunlop D, Edelman P, Singer R, Chang RW. Predictors of decline in manual performance in older adults. Journal of the American Geriatrics Society. 1997;45:905–910. doi: 10.1111/j.1532-5415.1997.tb02957.x. [DOI] [PubMed] [Google Scholar]
- Kamen G, Roy A. Motor unit synchronization in young and elderly adults. European Journal of Applied Physiology. 2000;81:403–410. doi: 10.1007/s004210050061. [DOI] [PubMed] [Google Scholar]
- Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. Journal of Applied Physiology. 2005;98:2072–2080. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Progress in Neurobiology. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- Latash ML, Shim JK, Gao F, Zatsiorsky VM. Rotational equilibrium during multi-digit pressing and prehension. Motor Control. 2004;8:392–404. doi: 10.1123/mcj.8.4.392. [DOI] [PubMed] [Google Scholar]
- Leijnse JN. A method and device for measuring force transfers between the deep flexors in the musician's hand. Journal of Biomechanics. 1998;31:773–779. doi: 10.1016/s0021-9290(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Leijnse JN, Walbeehm ET, Sonneveld GJ, Hovius SE, Kauer JM. Connections between the tendons of the musculus flexor digitorum profundus involving the synovial sheaths in the carpal tunnel. Acta Anatomica (Basel) 1997;160:112–122. doi: 10.1159/000148003. [DOI] [PubMed] [Google Scholar]
- Lexell J, Sjostrom M, Nordlund AS, Taylor CC. Growth and Development of Human Muscle - A Quantitative Morphological-Study of Whole Vastus Lateralis from Childhood to Adult Age. Muscle & Nerve. 1992;15:404–409. doi: 10.1002/mus.880150323. [DOI] [PubMed] [Google Scholar]
- Li ZM, Dun S, Harkness DA, Brininger TL. Motion enslaving among multiple fingers of the human hand. Motor Control. 2004;8:1–15. doi: 10.1123/mcj.8.1.1. [DOI] [PubMed] [Google Scholar]
- Li ZM, Latash ML, Zatsiorsky VM. Force sharing among fingers as a model of the redundancy problem. Experimental Brain Research. 1998;119:276–286. doi: 10.1007/s002210050343. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. Journal of Hand Surgery [Am.] 1990;15:244–250. doi: 10.1016/0363-5023(90)90103-x. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Jacobson MD, Fazeli BM, Abrams RA, Botte MJ. Architecture of selected muscles of the arm and forearm: anatomy and implications for tendon transfer. Journal of.Hand Surgery [Am.] 1992;17:787–798. doi: 10.1016/0363-5023(92)90444-t. [DOI] [PubMed] [Google Scholar]
- Malerich MM, Baird RA, McMaster W, Erickson JM. Permissible limits of flexor digitorum profundus tendon advancement--an anatomic study. Journal of Hand Surgery [Am.] 1987;12:30–33. doi: 10.1016/s0363-5023(87)80156-9. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Chen D, Sawaguchi T, Kubota K, Fetz EE. Synaptic interactions between primate precentral cortex neurons revealed by spike-triggered averaging of intracellular membrane potentials in vivo. Journal of Neuroscience. 1996;16:7757–7767. doi: 10.1523/JNEUROSCI.16-23-07757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K, Ebner B, Homberg V. Maturation of fastest afferent and efferent central and peripheral pathways: no evidence for a constancy of central conduction delays. Neuroscience Letters. 1994;166:9–12. doi: 10.1016/0304-3940(94)90828-1. [DOI] [PubMed] [Google Scholar]
- Muller K, Homberg V. Development of speed of repetitive movements in children is determined by structural changes in corticospinal efferents. Neuroscience Letters. 1992;144:57–60. doi: 10.1016/0304-3940(92)90715-j. [DOI] [PubMed] [Google Scholar]
- Oliveira MA, Shim JK, Loss JF, Petersen RD, Clark JE. Effect of kinetic redundancy on hand digit control in children with DCD. Neuroscience Letters. 2006;410:42–46. doi: 10.1016/j.neulet.2006.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter NL, Kent RD, Lindstrom MJ, Lazarus JA. Power and precision grip force control in three-to-five-year-old children: velocity control precedes amplitude control in development. Experimental Brain Research. 2006:1–15. doi: 10.1007/s00221-005-0322-5. [DOI] [PubMed] [Google Scholar]
- Ralston HJ. Mechanics of voluntary muscle. American Journal of Physical Medicine. 1953;32:166–184. [PubMed] [Google Scholar]
- Ranganathan VK, Siemionow V, Sahgal V, Liu JZ, Yue GH. Skilled finger movement exercise improves hand function. Journals of Gerontology Series A: Biological and Medical. 2001;56:M518–M522. doi: 10.1093/gerona/56.8.m518. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Hammond GR. Independence of force production by digits of the human hand. Neuroscience Letters. 2000;290:53–56. doi: 10.1016/s0304-3940(00)01328-8. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Individuated finger movements of rhesus monkeys: a means of quantifying the independence of the digits. Journal of Neurophysiology. 1991;65:1381–1391. doi: 10.1152/jn.1991.65.6.1381. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. Journal of Neurophysiology. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science. 1993;261:489–492. doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. Journal of Applied Physiology. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Schmuckler MA, Bosman EL. Interkey timing in piano performance and typing. Canadian Journal of Experimental Psychology. 1997;51:99–111. doi: 10.1037/1196-1961.51.2.99. [DOI] [PubMed] [Google Scholar]
- Shim JK, Latash ML, Zatsiorsky VM. Prehension synergies: trial-to-trial variability and principle of superposition during static prehension in three dimensions. Journal of Neurophysiology. 2005;93:3649–3658. doi: 10.1152/jn.01262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Lay BS, Zatsiorsky VM, Latash ML. Age-related changes in finger coordination in static prehension tasks. Journal of Applied Physiology. 2004;97:213–224. doi: 10.1152/japplphysiol.00045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Oliveira MA, Hsu J, Huang J, Park J, Clark JE. Hand digit control in children: age-related changes in hand digit force interactions during maximum flexion and extension force production tasks. Experimental Brain Research. 2007;176:374–386. doi: 10.1007/s00221-006-0629-x. [DOI] [PubMed] [Google Scholar]
- Shim JK, Park J, Zatsiorsky VM, Latash ML. Adjustments of prehension synergies in response to self-triggered and experimenter-triggered load and torque perturbations. Experimental Brain Research. 2006;175:641–653. doi: 10.1007/s00221-006-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. Journal of Applied Physiology. 2003a;95:1361–1369. doi: 10.1152/japplphysiol.00070.2003. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. Journal of Applied Physiology. 2003b;94:259–270. doi: 10.1152/japplphysiol.00643.2002. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Lexell J, Downham DY. Differences in Fiber Number and Fiber Type Proportion Within Fascicles - A Quantitative Morphological-Study of Whole Vastus Lateralis Muscle from Childhood to Old-Age. Anatomical Record. 1992;234:183–189. doi: 10.1002/ar.1092340205. [DOI] [PubMed] [Google Scholar]
- Smits-Engelsman BC, Westenberg Y, Duysens J. Development of isometric force and force control in children. Brain Research. Cognitive Brain Research. 2003;17:68–74. doi: 10.1016/s0926-6410(03)00081-8. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Maluf KS, Stephenson JL, Hunter SK, Enoka RM. Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle and Nerve. 2005;32:533–540. doi: 10.1002/mus.20392. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiology of Aging. 2003;24:25–35. doi: 10.1016/s0197-4580(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Coordinated force production in multi-finger tasks: finger interaction and neural network modeling. Biological Cybernetics. 1998;79:139–150. doi: 10.1007/s004220050466. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Experimental Brain Research. 2000;131:187–195. doi: 10.1007/s002219900261. [DOI] [PubMed] [Google Scholar]