SUMMARY
p70 S6 kinase 1 (S6K) is a major downstream effector of the mammalian target of rapamycin (mTOR), primarily implicated in the control of protein synthesis, cell growth and proliferation. Here we demonstrate that specific bidirectional molecular targeting of mediobasal hypothalamic (MBH) S6K activity in rats is sufficient to significantly alter food intake, body weight, hypothalamic orexigenic neuropeptide expression, hypothalamic leptin sensitivity and the metabolic and feeding responses to a fast. In addition, adenoviral-mediated constitutive activation of MBH S6K improved cold tolerance and protected against high-fat diet induced overeating, fat deposition and insulin resistance. Our results provide direct evidence that MBH S6K activity bidirectionally drives behavioral and metabolic determinants of energy balance, and promote its assessment as a therapeutic target in metabolic diseases.
INTRODUCTION
In mammals, the mediobasal hypothalamus (MBH) is a primary center of convergence and integration of nutrient-related signals critical to the regulation of energy homeostasis (Schwartz et al., 2000). In response to nutrients such as glucose and amino acids, and the adiposity hormones insulin and leptin, the MBH engages both intracellular and extracellular signaling processes to coordinately drive behavioral and metabolic determinants of energy balance (Obici et al., 2002; Pocai et al., 2005b). Detailed knowledge of hypothalamic nutrient sensing pathways coupling hormonal signaling and nutrient detection in the control of food intake and energy homeostasis is essential to a better understanding of the mechanisms involved in the etiology of obesity and associated diseases such as type 2 diabetes.
S6K is a major effector of the ubiquitous evolutionarily-conserved and multi-tasking mTOR, known to integrate nutrient and insulin signals and control the initiation of protein synthesis, cell growth and proliferation in peripheral cells (Dennis et al., 2001; Fingar et al., 2002). S6K is also widely expressed in the central nervous system (Asaki et al., 2003) and recent data suggest a role for neuronal mTOR/S6K pathway in the regulation of energy balance both in the fruit fly Drosophila Melanogaster (Wu et al., 2005) and in rats (Cota et al., 2006; Um et al., 2004). In the hypothalamus, S6K colocalizes with orexigenic neuropeptide Y (NPY) and agouti-related protein (AgRP) neurons, as well as anorexigenic proopiomelanocortin (POMC) neurons, two sub-populations of neurons involved in the regulation of energy homeostasis (Cota et al., 2006). Hypothalamic S6K responds to intra-cerebroventricular hormonal and nutrient stimuli, and central blockade of mTOR signaling blunts the anorexigenic effects of these signals (Cota et al., 2006). These data leave unresolved: 1) the role of central S6K in driving multiple essential determinants of overall energy balance, and 2) the specific neuroanatomical loci critical for this control.
Here we show that MBH S6K plays a critical role in the regulation of food intake and energy balance in rats. Using adenoviruses expressing a constitutively active and a dominant negative mutant of S6K, we demonstrate that specific bidirectional alteration in MBH S6K activity is sufficient to significantly alter body weight, primary by changing food intake through altered MBH orexigenic neuropeptides NPY and AgRP expression. We also find that bidirectional alteration in MBH S6K activity induces dramatic changes in leptin sensitivity and alters the metabolic and feeding response to a fast, indicating that signaling through MBH S6K is required for an appropriate response to multiple indices of energy availability. Adenoviral-mediated constitutive activation of S6K also induced changes in energy expenditure, together with an improved ability to maintain body core temperature during a cold challenge. Finally, MBH S6K overactivation protects against high-fat diet induced overeating, fat deposition and insulin resistance. Our results provide direct evidence that MBH S6K activity bidirectionally drives behavioral and metabolic determinants of energy balance, and promote its assessment as a therapeutic target in metabolic diseases.
RESULTS
MBH S6K activity responds to physiological nutritional and hormonal signals
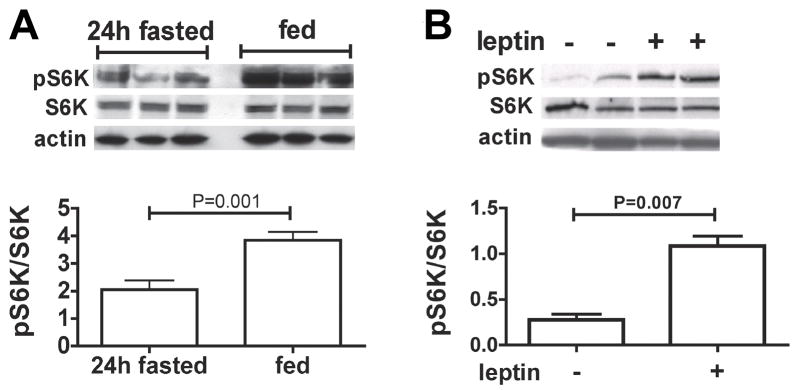
To begin to assess the physiological relevance of MBH S6K as an energy sensor, we first evaluated the degree to which food intake modulates MBH S6K activity in rats. The expression of the active form of S6K, phosphorylated at Thr389, was significantly increased within 3h of feeding following a 24 h fast (Fig. 1a). We then examined whether local hypothalamic administration of the adiposity hormone leptin would alter MBH S6K activity. Bilateral intra-MBH leptin administration (150 ng in 150 nl per side) induced a 4-fold increase in MBH Thr389 phosphorylation, within 30 min of leptin injection (Fig. 1b). Together these data indicate that MBH S6K rapidly responds to nutritional and hormonal signals important for the feedback control of energy intake.
Figure 1. Refeeding and leptin induce MBH S6K phosphorylation.
MBH S6K Thr389 phosphorylation (A) in 24h fasted (n=5) or 24h fasted and 3h refed (n=5) rats and (B) 30 min after bilateral MBH aCSF (n=4) or MBH leptin (n=4) injection (150 ng in 150 nl) in rats. All data are means ± SEM.
MBH S6K activity bidirectionally regulates food intake and body weight
We investigated the specific role of MBH S6K in the regulation of energy balance by targeting the arcuate nucleus of the hypothalamus with recombinant adenovirus expressing a constitutively active mutant of S6K (CA S6K), a dominant negative mutant of S6K (DN S6K) or LACZ (control) and evaluated the impact of these viral manipulations on several behavioral and metabolic effectors of energy balance. MBH CA S6K and DN S6K injections induced a 5 to 40 fold increase in S6K protein expression in the MBH compared to endogenous levels, without altering S6K protein expression in the paraventricular (PVN) or lateral hypothalamic nuclei (Supplementary Fig. 1). Recombinant adenovirus functional validity was confirmed in GT1–7 hypothalamic cell lines (Supplementary Fig. 2a and 2b), as well as in MBH extracts from injected animals. MBH expression of CA S6K and DN S6K mutants induced significant changes in S6K’s target ribosomal S6 protein activity, as assessed by its phosphorylation at Ser235/236 (Supplemental Fig. 2c), and CA S6K increased MBH S6K activity by 4.5 fold relative to LACZ controls (Supplemental Fig. 2d).
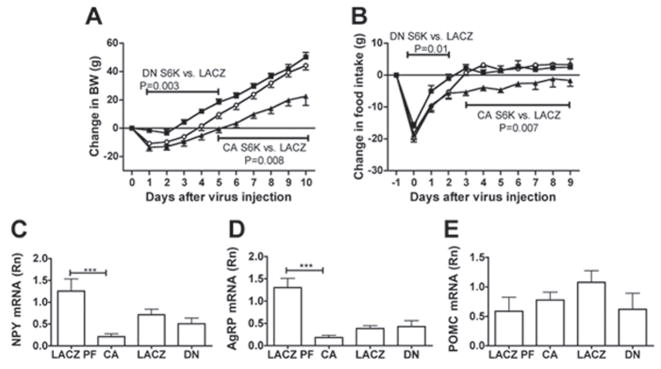
During the first 3 days following adenovirus injections, rats expressing DN S6K were protected against the acute weight loss and the decrease in food intake typically induced by brain parenchymal injections and observed in rats expressing either LACZ or CA S6K (Fig. 2a and 2b). Conversely, MBH expression of CA S6K significantly decreased food intake beginning on the 3rd day following the adenovirus injections, leading to a slower rate of weight gain when compared to the LACZ controls. These changes in food intake in both DN S6K and CA S6K were attributable to changes in the average meal size (Supplementary Fig. 3a), whereas daily meal number did not significantly differ among groups at any time point (Supplementary Fig. 3b). Cumulative food intake over 9 days was also decreased in rats expressing CA S6K compared to LACZ controls (P=0.004) and this was associated with decreases in MBH expression of orexigenic neuropeptides NPY and AgRP in CA S6K rats compared to LACZ pair-fed controls (Fig. 2c and 2d), whereas MBH POMC expression did not significantly differ between these groups (Fig. 2e). In DN S6K rats, MBH feeding neuropeptide expression did not significantly differ from LACZ controls. Body composition did not differ between groups (data not shown). Thus, bidirectional modulation of MBH S6K activity is sufficient to produce complementary bidirectional changes in food intake and body weight.
Figure 2. Bidirectional molecular targeting of MBH S6K activity is sufficient to alter food intake and body weight.
(A) changes in body weight and (B) changes in food intake after MBH injection of CA S6K (n=12, filled triangle), DN S6K (n=13, filled square) or LACZ (n=18, open circle). (C) MBH NPY mRNA, (D) MBH AgRP mRNA and (E) MBH POMC mRNA levels normalized to that of actin in CA S6K, DN S6K, LACZ and LACZ pairfed controls (n=6–8). Cumulative food intake over 9 days is 231.7 ± 9.4, 275.27 ± 6.65 and 269.1 ± 7.4 in CA S6K, DN S6K and LACZ controls, respectively. All data are means ± SEM.
MBH S6K activity bidirectionally affects energy expenditure and cold tolerance
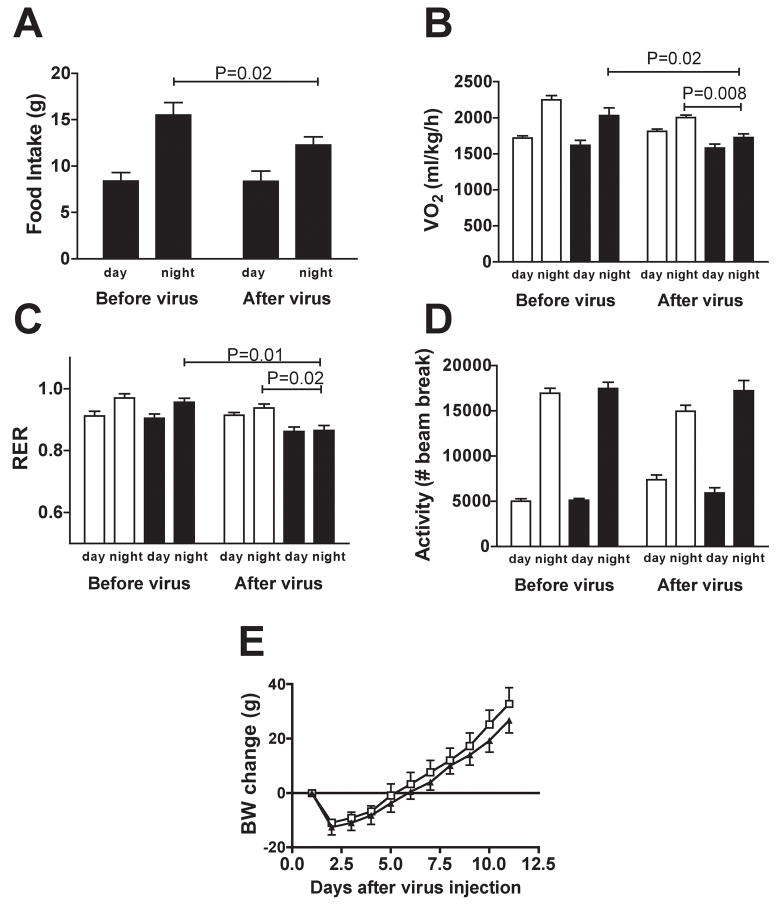
We next examined whether the decrease in food intake measured in CA S6K rats was associated with chronic changes in energy metabolism that could contribute to their reduced rate of body weight gain. CA S6K injection induced a specific decrease in nighttime food intake (Fig. 3a), accompanied by decreased nighttime oxygen consumption and metabolic rate (Fig. 3b, 3c). In contrast, no changes in oxygen consumption and metabolic rate were observed in LACZ pair-fed controls. CA S6K rats did not differ from LACZ PF controls in the day night distribution or amount of locomotor activity (Fig 3d). These results, together with the absence of a difference in post-injection body weight gain in CA S6K rats compared to LACZ pair-fed controls (Fig. 3e), support the interpretation that increased MBH S6K activity reduced body weight gain primarily by reducing ad libitum food intake.
Figure 3. Constitutive activation of MBH S6K decreases energy expenditure.
(A) food intake, (B) oxygen consumption, (C) respiratory quotient and (D) physical activity before (average of d-3 to d-1) and after (average of d5 to d7) MBH adenoviral injection in CA S6K (filled bar, n=4) or LACZ pair-fed controls (open bar, n=4). (E) Change in body weight in CA S6K (n=12, filled triangle) and LACZ pair-fed (open square, n=9). All data are means ± SEM.
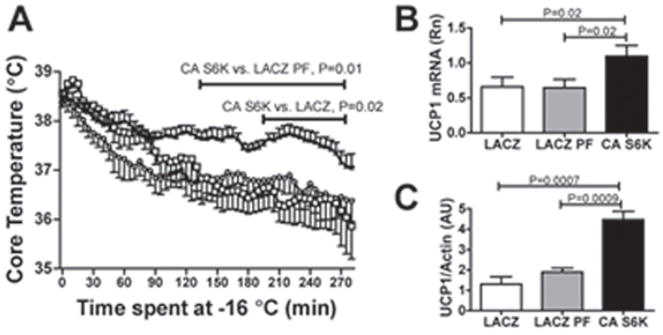
We further tested the effect of MBH S6K overactivation on energy metabolism in the context of a metabolic challenge by assessing the ability of MBH CA S6K, LACZ, and LACZ pair-fed rats to maintain body core temperature during cold exposure. CA S6K rats maintained their core temperature at significantly higher levels during the challenge compared to LACZ and LACZ pair-fed controls (Fig. 4a). Consistent with this difference, mRNA levels and protein expression for the thermogenic uncoupling protein 1 in intrascapular brown adipose tissue were increased in CA S6K rats relative to LACZ and LACZ pair-fed controls (Fig. 4b and 4c). In contrast, cold tolerance in DN S6K expressing rats tended to be lower than in LACZ controls (Supplementary Fig. 4A). Body core temperature after 5h at −16°C was lower in DN S6K rats (P=0.05) (Supplementary Fig. 4B) and accordingly, brown adipose tissue UCP1 protein level was lower in DN S6K rats than in LACZ controls (Supplementary Fig. 4D), with no change in brown adipose tissue UCP1 mRNA level (Supplementary Fig. 4C). Thus, bidirectional modulation of MBH S6K activity is sufficient to produce complementary bidirectional changes in adaptive thermogenesis and sympathetic drive to intrascapular brown fat during a cold challenge.
Figure 4. Constitutive activation of MBH S6K increases thermogenesis during a cold challenge.
(A) core temperature during a 5h cold challenge at −16 °C 7 days after adenoviral injection in CA S6K (filled triangle, n=6), LACZ (open circle, n=4) or LACZ pair-fed controls (open square, =4). Intrascapular brown adipose tissue UCP1 (B) mRNA level normalized to actin and (C) protein level normalized to actin in LACZ (n=7), LACZ pair-fed (n=7) and CA S6K rats (n=9). All data are means ± SEM.
MBH S6K affects the energetic response to a fast and MBH leptin sensitivity
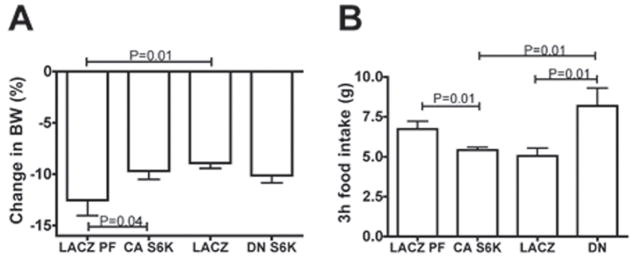
To extend our analysis of the relationship between MBH S6K activity and nutrient availability, we assessed whether bidirectional alteration of MBH S6K activity would affect the feeding and metabolic responses to a 24h fast. Rats expressing CA S6K were partially protected against the 24h fasting-induced body weight loss that was observed in LACZ pair-fed controls, whereas DN S6K rats tended to lose more body weight than ad libitum fed LACZ controls (P=0.08) (Fig. 5a). Moreover, 3h food intake following the 24h fast was significantly lower in CA S6K rats than in LACZ pair-fed controls and conversely, significantly higher in DN S6K rats than in ad libitum fed LACZ controls (Fig. 5b).
Figure 5. Bidirectional alteration of MBH S6K activity modulates the metabolic and feeding response to a 24h fast.
(A) changes in body weight after a 24h fast in LACZ (n=10), LACZ pair-fed (n=10), CA S6K (n=10) and DN S6K (n=10) rats. (B) 3h food intake following a 24h fast in LACZ (n=5), LACZ pair-fed (n=5), CA S6K (n=5) and DN S6K (n=5) rats. All data are means ± SEM.
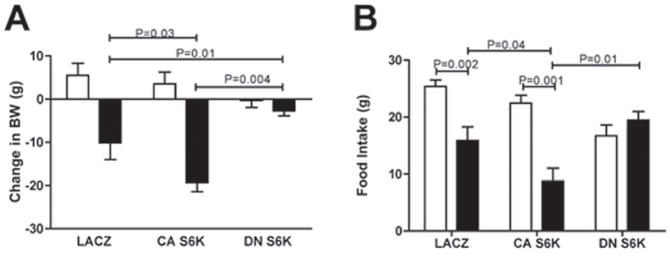
Because MBH S6K affected adaptive energy intake and metabolic responses to reduced nutrient availability during a fast, we next investigated whether MBH S6K activity mediates feeding and metabolic responses to MBH administration of the adiposity hormone leptin. In rats expressing CA S6K, leptin-induced reductions in body weight and food intake were significantly more pronounced than in LACZ controls, whereas in rats expressing DN S6K, MBH leptin had no effect on either measure (Fig. 6a and 6b). These effects of CA S6K and DN S6K virus on leptin sensitivity could not be explained by alterations in leptin-induced STAT3 activation, because MBH leptin induced a similar increase in STAT3 Tyr705 phosphorylation in all groups (Supplementary fig. 5) indicating that S6K functions downstream of or in a parallel pathway to STAT3. These data demonstrate that MBH S6K activation is required for MBH leptin’s effects on energy balance.
Figure 6. Bidirectional molecular targeting of MBH S6K activity is sufficient to alter hypothalamic leptin sensitivity.
(A) 24h change in body weight and (B) 24h food intake after MBH artificial-cerebrospinal fluid (open bar) vs. leptin (150 ng in 150 nl per side, filled bar) injections in LACZ (n=9), CA S6K (n=6) and DN S6K rats (n=5). All data are means ± SEM.
MBH S6K constitutive activation protects against high-fat diet induced disorders
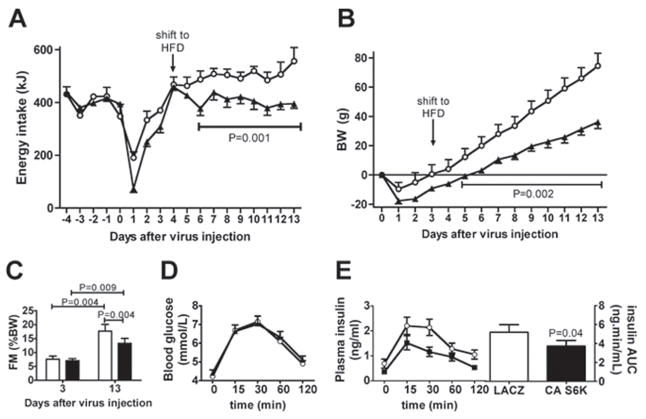
Finally, we examined the effects of MBH S6K activation under conditions that stimulate feeding during exposure to a palatable high-fat diet (HFD). Unlike LACZ controls, CA S6K rats failed to exhibit any sustained overeating beginning two days after a shift to HFD (Fig. 7a). Accordingly, body weight gain was significantly lower in CA S6K rats than in LACZ controls (Fig. 7b). In addition, CA S6K rats were partially protected against the 2.3 fold increase in fat mass observed in LACZ controls 10 days after the shift (Fig. 7c). In the context of a global assessment of the onset of a metabolic syndrome after the shift to the HFD, we also measured oral glucose tolerance in this cohort of animals. During an oral glucose tolerance test, CA S6K rats had lower plasma insulin levels than LACZ controls, suggesting that MBH S6K overactivation protects against high-fat diet induced insulin resistance (Fig. 7d and 7e). Taken together, these data support the interpretation that hypothalamic S6K overactivation protects against the development of multiple features of the metabolic syndrome in high-fat fed rats.
Figure 7. Constitutive activation of MBH S6K protects against adverse effects of a HFD.
(A) Energy intake and (B) changes in body weight after MBH injection of CA S6K (n=6, filled triangle) or LACZ (n=6, open circle) and shift to a HFD. (C) fat mass (FM) in CA S6K (n=6, filled bar) or LACZ (n=6, open bar) rats before (day 3) and 10 days after (day 13) a shift to a HFD. (D), blood glucose and (E), plasma insulin during an oral glucose tolerance test (1g/kg) in LACZ (open circles, n=6) and CA S6K (filled squares, n=6) rats 10 days after a shift to a HFD. All data are means ± SEM.
DISCUSSION
Our data provide several converging lines of evidence underscoring the importance of endogenous MBH S6K in energy homeostasis: (i) MBH S6K is activated by multiple physiological signals of nutrient availability; (ii) bidirectional alteration of MBH S6K activity is sufficient to elicit complementary bidirectional changes in body weight, food intake and cold tolerance; (iii) MBH S6K upregulation is consistently associated with an increased sensitivity to anorexigenic stimuli (refeeding, leptin) and a decreased sensitivity to orexigenic stimuli (fasting, HFD), while the reciprocal pattern of responses occurs during MBH S6K downregulation. Together, these findings demonstrate that MBH S6K is a physiologically relevant nutrient sensor and a critical mediator required for appropriate feeding and metabolic responses that maintain energy balance.
The rapid ability of DN S6K to promote food intake and body weight gain in the days immediately following MBH injection strongly argues in favor of a role for endogenous MBH S6K in the regulation of energy balance. These effects disappear after 5 days, most likely due to the competitive nature of the downregulation that could engage compensatory mechanisms to ensure the maintenance of energy homeostasis. In contrast, within 1 day of the injection recovery time, MBH CA S6K-induced anorexia and reduced body weight gain is sustained during normal chow and high fat diet maintenance, indicating that any effective counterregulatory response to CA S6K expression is prevented. These results may appear discordant with the phenotype of S6K−/− mice (Um et al., 2004), which are protected against age- and diet-induced obesity, but this apparent difference most likely reflects the direct effects of S6K deficiency on energy expenditure in peripheral tissues (Kahn and Myers, 2006). These apparently divergent responses of the periphery and the central nervous system to suppression of S6K signaling reflect their differential roles in maintaining adequate responses to nutrient surfeit or nutrient deficit, as has been reported for other signals (Caspi et al., 2007).
Whole body knockdown of S6K is also associated with increased insulin sensitivity (Um et al., 2004), a finding consistent with results reporting feedback inhibition exerted by S6K on insulin signaling through activation of IRS1 serine phosphorylation (Tremblay et al., 2007). Recent data indicate that prolonged hypothalamic activation of S6K also leads to decreased insulin signaling in the MBH (Ono et al., 2008). These data support the prediction that in rats expressing CA S6K, decreased MBH anorexigenic insulin/PI 3 kinase signaling would induce hyperphagia and increased weight gain (Niswender et al., 2003), in contrast to what we report in the current work. This contrast suggests that MBH signaling pathways regulating glucose homeostasis and food intake do not entirely overlap and in fact diverge, at least at the level of S6K. Alternatively, activation of S6K by the insulin/PI 3 kinase pathway may even contribute to insulin’s anorexigenic effects, as suggested in the fruit fly Drosophila (Wu et al., 2005).
The primary feeding phenotypes associated with bidirectional alterations in MBH S6K activity represent novel central counterparts to S6K’s well-known ability to trigger peripheral anabolic processes (Fingar et al., 2002). Here we demonstrate that direct MBH targeting of S6K alters feeding behavior, extending the role of S6K as a nutrient sensor to include effects on whole body energy availability. These changes in feeding behavior are manifested by specific bidirectional consequences of MBH S6K alteration on meal size, a critical index of satiety processes, thereby positioning MBH S6K activation as part of the neural basis of satiation (Azzara et al., 2002).
The present finding that bidirectional changes in MBH S6K activity are associated with bidirectional changes in MBH leptin sensitivity represent a direct and specific demonstration that MBH S6K is a critical target of leptin in the regulation of energy balance, as previously suggested (Cota et al., 2006). In agreement with our result, recent findings indicate that the consumption of a high-fat diet induces a decrease in hypothalamic S6K signaling, both in the basal state and after an intra-cerebroventricular leptin challenge (Cota et al., 2008), supporting the idea that reduced hypothalamic S6K signalling contributes to the development of leptin resistance in diet-induced obesity. Downregulation of NPY and AgRP expression in rats expressing CA S6K suggests these two peptides and their PVN targets as effectors of the feeding and metabolic responses to MBH S6K activation. NPY deficiency is associated with a decreased sensitivity to a fast and to a high-fat diet (Patel et al., 2006; Segal-Lieberman et al., 2003), as we found in rats expressing CA S6K. Impairment of MBH NPY release decreases food intake (Burlet et al., 1995), whereas PVN NPY administration stimulates feeding (Stanley and Leibowitz, 1985), decreases sympathetic outflow (van Dijk et al., 1994) and intra-cerebroventricular NPY decreases brown adipose tissue thermogenesis (Billington et al., 1991). These results are consistent with those observed after alterations in MBH S6K activity. Moreover, MBH leptin administration modulates this same cluster of markers, affecting NPY synthesis (Nogueiras et al., 2008), sympathetic outflow (Rahmouni and Morgan, 2007), and altering food intake by specific effects on meal size (Moran et al., 2006; Morton et al., 2005). Accordingly, several lines of evidence demonstrate that reduced NPY release from the MBH to the PVN mediate leptin’s hypophagic and thermogenic actions (Kotz et al., 1998; Wang et al., 1997). Thus, similarly to leptin, the observed effects of altered MBH S6K activity on food intake and thermogenesis could be mediated by changes in MBH NPY release to the PVN.
Taken together, our findings establish a physiological role for MBH S6K, an evolutionary conserved nutrient sensing enzyme, in the regulation of energy balance in mammals. Hormonal and nutritional signals inform the brain about whole body energy status by altering the activity of a subset of neurons in the MBH that in turn engage appropriate responses to regulate feeding and energy expenditure (Schwartz et al., 2000). Our data demonstrate that multiple indices of energy supply converge on MBH S6K, and that signaling through MBH S6K is required for appropriate feeding and metabolic responses to variations in nutrient availability. We propose that MBH S6K drives the regulation of food intake and brown fat thermogenesis by regulating the expression of AgRP and NPY. The present findings that MBH S6K upregulation provides sustained protection against the development of several markers of the metabolic syndrome make it a compelling therapeutic target for intervention in the treatment of obesity and related metabolic disorders. Future progress will be driven by understanding how the S6K pathway functions as part of an integrated hypothalamic nutrient sensing network.
EXPERIMENTAL PROCEDURES
Animals and study design
Experiments were performed on 10-week-old male Sprague Dawley rats (Charles River Laboratories) housed in individual cages and maintained in a temperature-controlled room under a standard light/dark cycle with ad libitum access to water and standard chow unless specifically indicated. Using stereotaxic surgery performed under ketamine/xylazine anaesthesia, rats were bilaterally injected with adenovirus (2 × 106 pfu in 2 μl/side over a 20-min period) expressing a constitutively active mutant of S6K (CA S6K), a dominant negative mutant of S6K (DN S6K) or LACZ in the MBH (coordinates from bregma: A/P –3.0 mm, D/V –10.4 mm) and in some cases a chronic bilateral intrahypothalamic cannula (Plastics One Inc.) was implanted. Body weight, feeding behavior, energy expenditure and physical activity were monitored from 4 days before to 7–10 days after virus administration as described below. Sensitivity to a fast was determined by measuring 24h fast-induced body weight loss and 3h food intake during refeeding. In the high-fat diet shift experiment, rats were shifted from a standard chow diet to a high-fat diet 3 days after MBH virus injection; shift-induced hyperphagia, weight gain and fat deposition were monitored for 10 days and glucose tolerance was assessed with a 1g.kg−1 BW oral glucose challenge. Body composition was determined by magnetic resonance spectroscopy using an ECHO MRS instrument (Echo Medical Systems). All rats were sacrificed by decapitation and hypothalamic nuclei were dissected as previously described (Pocai et al., 2005a). Successful adenovirus administration in the MBH was confirmed by immunoblot analysis with S6K antibody. All experimental protocols were approved by the Institute for Animal Studies of the Albert Einstein College of Medicine.
Feeding studies
One week prior to adenovirus injection, rats (n=70) were adapted to individual feeding chambers (Med Associates) equipped with 45-mg pellets dispensers and fed ad libitum with a standard chow diet (Bioserv 45-mg precision pellets F05524, 15.8 kJ/g) or a high-fat diet (TestDiet 45-mg pellets 1812366, 18.4 kJ/g). One group of rats treated with the LACZ virus (n=9) was pair-fed to the CA S6K group. Food intake was monitored continuously from 4 days before to 10–14 days after virus administration and body weight was assessed daily. Meal patterns were determined as previously described (Azzara et al., 2002).
Indirect Calorimetry
One week prior to adenoviral injection, rats (n=8) were adapted to individual metabolic chambers. Metabolic measurements (oxygen consumption, carbon dioxide production, food intake and locomotor activity) were obtained continuously using a CLAMS (Columbus Instruments) open-circuit indirect calorimetry system from 4 days before to 7 days after virus administration.
Cold Challenge
Prior to adenovirus injection, rats (n=16) were implanted with intraperitoneal radiofrequency impedance temperature probes (MiniMitter) under isoflurane anaesthesia and allowed to a 1 week recovery. Seven days after virus administration, rats were exposed to a 5h cold challenge at −16°C during which body core temperature was recorded continuously using MiniMitter ER-4000 receivers.
MBH Leptin sensitivity
One week after adenoviral injection, hypothalamic leptin sensitivity was assessed by measuring 24h food intake and body weight change following a local leptin injection in the MBH (recombinant rat leptin, R&D Systems, 150 ng in 150 nl per side). MBH leptin-induced STAT3 activation was assessed seven days after adenovirus injection in rats equipped with a bilateral cannula targeting the MBH. Rats received an MBH injection of aCSF or leptin (150 ng in 150 nl per side over 5 min) and MBH were collected 30 min later and processed as described below.
Subcloning and adenovirus preparation
Rat p70 S6 kinase 1 (shorter form of Rps6kb1) cDNA was subcloned using RT-PCR from rat liver. N-terminal Flag-tag and mutations in S6K at F5A, K100Q, T389E and RSPRR to ASPAA (AA410–414) were respectively introduced using PCR-based mutagenesis, with confirmation of whole sequences. Adenovirus for S6K mutants were generated using the Adeno-X expression system version 1 (BD Clontech), with a substitution of the promoter in the shuttle vector from CMV to CAG (Ono et al., 2003). LACZ adenovirus was prepared as previously described (Ono et al., 2003). Adenovirus were amplified in 293 cells and purified with an Adenopure kit (Puresyn Inc.). Adenoviral titers were measured by endpoint dilution assay with 1:3 serial dilutions on a 96-well plate of HEK293 cells.
Adenovirus functional validation in vitro
Hypothalamic GT1–7 cells (gift from Dr. Pamela Mellon) were infected with CA S6K, DN S6K or LACZ adenovirus. 48h later, cells were serum starved overnight and stimulated with 0, 1, 10 or 100 nM insulin for 30 min, then harvested and processed as described below.
S6K activity assay
The p70 S6 kinase assay kit (Upstate) was used with minor modifications. Briefly, 200 μg of tissue lysate was incubated with 3 μg of anti-S6K antibody and 30 μL of Protein-A Sepharose beads in modified buffer A (50 mM Tris pH=7.5, 1 mM EDTA, 1 mM EGTA, 2 mM sodium orthovanadate, 0.1 % 2-mercaptoethanol, 1 % Triton X-100, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 0.2 mM PMSF, Complete inhibitor cocktail (Roche) and agitated for 2 h at 4 °C, then washed 3 times with the same buffer and twice with Assay Dilution Buffer, then incubated with synthetic substrate peptide (KKRNRTLTK) and [α-32P]ATP at 30 °C for 10 min with continuous agitation. After a brief spin down, supernatants were spotted onto W81 filter paper, washed with diluted phosphoric acid and counted with a scintillation counter.
Real-time PCR
Total RNA was isolated from frozen MBH wedges (3–5 mg) and brown adipose tissue (100 mg) using RNeasy Mini Kit (Quiagen) according to the manufacturer’s instructions. Extracted RNA was quantified using a NanoDrop ND-1000 (Nanodrop) and RNA integrity was confirmed with Ethidium Bromide staining. Following treatment with DNase I (Invitrogen), purified RNA was used as template for first-strand cDNA synthesis using SuperScript III (Invitrogen). Quantitative real-time RT-PCR was run using LC-Fast Start DNA SYBR Green I chemistry (Roche Diagnostics) on a LightCycler 2.0 platform (Roche Diagnostics). Samples contained 2 μl Fast Start DNA Master SYBR Green I, 4mM MgCl2 and 0.5 μM of each primer in a 20 μl final volume. Forward and reverse primer pairs were as listed (Supplementary Table 1). Samples of RNA in which the reverse transcriptase was omitted and samples without cDNA were included as negative controls. For each sample, the crossing point (CP) was determined at a constant fluorescence threshold; for each target gene, PCR efficiency (E) was calculated using a series of 10-fold dilutions of cDNA. Relative quantification of each transcript in comparison to β-actin was determined using the following formula (Pfaffl, 2001):
Signaling studies and Western Blot analysis
MBH wedges, brown adipose tissue and GT1–7 cells were homogenized in 50 mM Tris, 1 mM EGTA, 1 mM EDTA, 50 mM sodium fluoride, 10 mM β-glycerophosphate, 20 mM sodium pyrophosphate, 2 mM orthovanadate, 2 mM PMSF, 1% Triton and Complete phosphatase inhibitor cocktail (Roche). Protein concentration was measured with a BCA protein quantification kit (Pierce Biotechnology). Protein extracts were run on Criterion gels (Bio-Rad) and blotted onto nitrocellulose membranes (Millipore). After blocking for 1h at room temperature, immunoblots were incubated overnight at 4°C in primary antibodies against phospho-p70 S6 kinase (Thr389), phospho-STAT3 (Tyr705), STAT3, phospho-S6 ribosomal protein (Ser235/236), phospho-IRS1 (Ser636/639) (Cell Signaling Technology), p70 S6 Kinase (Upstate Biotechnology), UCP1 (alpha-Diagnostics), Flag (Sigma) or β-actin (Santa Cruz Biotechnology Inc.). Blots were then incubated for 1 hour in fluorescent (Alexa Fluor 680–conjugated anti-mouse IgG, Invitrogen or IR Dye 800–conjugated goat anti-rabbit IgG, Rockland Immunochemicals) or HRP-linked (anti-rabbit or anti-mouse HRP-linked IgG, Cell Signaling Technology) secondary antibodies and proteins were detected using either the fluorescence-based Odyssey Infrared Imaging System (LI-COR Biosciences) or enhanced chemiluminescence (ECL Plus, Amersham). Quantification was performed with the gel analyze tool of the Odyssey software (http://www.licor.com/bio/odyssey/index.jsp) or the NIH Image/J software (http://rsbweb.nih.gov/ij/), respectively.
Analytical procedures
Blood glucose levels were determined using a Glucometer (Precision Xtra, MediSense) and plasma insulin levels using ELISA (Linco Rat Insulin Kit).
Statistical Analysis
All data, presented as means ± SEM, have been analyzed using GraphPad Prism 5. For all statistical tests, an α risk of 5% was used. All kinetics were analyzed using a mixed model for repeated measurements. Multiple comparisons were tested with an ANOVA and adjusted with Tukey post-test. Single comparisons were made using one-tail Student T-tests.
Supplementary Material
Supplemental Data include one table and 5 figures.
Acknowledgments
We thank Xiaosong Li for technical assistance. This work was supported by NIH DK 066618, New York Obesity Research Center DK026687, the Skirball Institute for Nutrient Sensing and the French Society for the Study of Nutrition. G.J.S and C.B conceived the experiment; C.B. and H.O. carried it out; C.B. designed and carried out the data analysis; C.B. and G.J.S co-wrote the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T. Localization of translational components at the ultramicroscopic level at postsynaptic sites of the rat brain. Brain Res. 2003;972:168–176. doi: 10.1016/s0006-8993(03)02523-x. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. doi: 10.1016/s0031-9384(02)00883-1. [DOI] [PubMed] [Google Scholar]
- Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260:R321–327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- Burlet A, Grouzmann E, Musse N, Fernette B, Nicolas JP, Burlet C. The immunological impairment of arcuate neuropeptide Y neurons by ricin A chain produces persistent decrease of food intake and body weight. Neuroscience. 1995;66:151–159. doi: 10.1016/0306-4522(94)00573-n. [DOI] [PubMed] [Google Scholar]
- Caspi L, Wang PY, Lam TK. A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab. 2007;6:99–104. doi: 10.1016/j.cmet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28:7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Myers MG., Jr mTOR tells the brain that the body is hungry. Nat Med. 2006;12:615–617. doi: 10.1038/nm0606-615. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Briggs JE, Pomonis JD, Grace MK, Levine AS, Billington CJ. Neural site of leptin influence on neuropeptide Y signaling pathways altering feeding and uncoupling protein. Am J Physiol. 1998;275:R478–484. doi: 10.1152/ajpregu.1998.275.2.R478. [DOI] [PubMed] [Google Scholar]
- Moran TH, Aja S, Ladenheim EE. Leptin modulation of peripheral controls of meal size. Physiol Behav. 2006;89:511–516. doi: 10.1016/j.physbeh.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Lopez M, Lage R, Perez-Tilve D, Pfluger P, Mendieta-Zeron H, Sakkou M, Wiedmer P, Benoit S, Datta R, Dong JZ, Culler M, Sleeman M, Vidal-Puig A, Horvath T, Treier M, Dieguez C, Tschop MH. Bsx, a Novel Hypothalamic Factor linking Feeding with Locomotor Activity, is Regulated by Energy Availability. Endocrinology. 2008 doi: 10.1210/en.2007-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM, Schwartz GJ, Rossetti L. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Viana AY, Fukushima Y, Abe M, Shojima N, Kikuchi M, Yamada N, Oka Y, Asano T. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes. 2003;52:2905–2913. doi: 10.2337/diabetes.52.12.2905. [DOI] [PubMed] [Google Scholar]
- Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, Ahima RS. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes. 2006;55:3091–3098. doi: 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K (ATP) channels control hepatic glucose production. Nature. 2005a;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005b;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G, Trombly DJ, Juthani V, Wang X, Maratos-Flier E. NPY ablation in C57BL/6 mice leads to mild obesity and to an impaired refeeding response to fasting. Am J Physiol Endocrinol Metab. 2003;284:E1131–1139. doi: 10.1152/ajpendo.00491.2002. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci U S A. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- van Dijk G, Bottone AE, Strubbe JH, Steffens AB. Hormonal and metabolic effects of paraventricular hypothalamic administration of neuropeptide Y during rest and feeding. Brain Res. 1994;660:96–103. doi: 10.1016/0006-8993(94)90843-5. [DOI] [PubMed] [Google Scholar]
- Wang Q, Bing C, Al-Barazanji K, Mossakowaska DE, Wang XM, McBay DL, Neville WA, Taddayon M, Pickavance L, Dryden S, Thomas ME, McHale MT, Gloyer IS, Wilson S, Buckingham R, Arch JR, Trayhurn P, Williams G. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes. 1997;46:335–341. doi: 10.2337/diab.46.3.335. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A. 2005;102:13289–13294. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data include one table and 5 figures.