Abstract
Numerous neurosecretory cells are known to secrete more than one peptide, in both vertebrates and invertebrates. These co-expressed neuropeptides often originate from differential cleavage of a single large precursor, and are then usually sorted in the regulated pathway into different secretory vesicle classes to allow separable release dynamics. Here, we use immuno-gold electron microscopy to show that two very different neuropeptides (the nonapeptide crustacean cardioactive peptide (CCAP) and the 30 kDa heterodimeric bursicon) are co-packaged within the same dense core vesicles in neurosecretory neurons in the abdominal ganglia of Periplaneta americana. We suggest that this co-packaging serves a physiological function in which CCAP accelerates the distribution of bursicon to the epidermis after ecdysis to regulate sclerotization of the newly formed cuticle.
Keywords: neuropeptide, bursicon, crustacean cardioactive peptide, electron microscopy, immuno-gold labeling, insect, cockroach
Introduction
Neuropeptides orchestrate numerous complex behaviors in insects. The process of ecdysis, in which insects shed their old cuticle and replace it with a new one, is beautifully controlled by the regulated release of six different bioactive peptides [14]. Two of these peptides, crustacean cardioactive peptide (CCAP) and bursicon, are involved in regulating the last phases of ecdysis, i.e. the motor pattern for shedding the remains of the old cuticle [24], and for controlling the hardening of the new soft cuticle after shedding [13], respectively. Bursicon is present in all ganglia of the ventral nervous system of all insects investigated [3], expressed in large lateral neurosecretory cells (Cell 27). The axons of these cells in the abdominal ganglia project to the alary muscles of the heart, but also possess many en passant varicosities suggesting that bursicon is hormonally distributed throughout the entire body. Previous immunocytochemistry has shown that CCAP is expressed in intrinsic interneurons (IN 704 cells) paired with Cell 27. IN 704 cells most likely drive the ecdysis motor pattern [24]. However, CCAP is also co-expressed with bursicon in Cell 27. CCAP is known to increase heart activity [25, 17, 4, 18, 8], and thus, is likely to enhance the delivery of bursicon to its target sites.
In this study, we use immuno-gold electron microscopy to determine the subcellular localization of CCAP and bursicon in Cell 27 in the abdominal ganglia of P. americana. The submicroscopic architecture of Cell 27 was first described by Adams and O’Shea [1] for P. americana and G. bimaculatus; named by them “lateral white” (LW) neurons. We show that CCAP and bursicon are not only co-expressed in the same Cell 27 neurosecretory soma and processes, but importantly that the two neuropeptides are co-packaged within the same secretory vesicles. The initial hypothesis of vesicular co-packaging originated from the observation that CCAP/bursicon immunolabeling (as seen by confocal imaging) is not evenly distributed but appears in granules double-labeled for CCAP and bursicon [20; supporting information]. This study confirms the vesicular co-packaging hypothesis. The co-release of CCAP and bursicon provides a critical mechanism for enabling the joint action of the two neuropeptides in coordinating the late stages of the ecdysis behavior program.
Methods
Animals
Adult and last larval stages of P. americana from our laboratory culture where cold anesthetized at −20°C for 4 mins. Animals were dissected in ice-cold physiological saline. The abdominal part of the ventral nervous system was exposed and the single ganglia separated. The preparation was washed briefly in phosphate buffer (PB, pH 7.4) before fixing.
Electron Microscopy
Analyses of Cell 27 neuron ultrastructure were performed using standard TEM techniques, as reported previously [22, 21]. Abdominal ganglia were fixed in 2% glutaraldehyde for at least 1 hr. The specimens were then washed for 10 mins in phosphate-buffered sucrose, transferred into 1% OsO4 in distilled water for 1 hr and then washed three times in distilled water. Preparation were stained in 1% aqueous uranyl acetate for 1 hr and then washed three times in distilled water. Tissues were dehydrated using a series of ethanol (30–100%), followed by propylene oxide for 30 mins as a transitional solvent. Propylene oxide was gradually replaced with propylene oxide/Araldite (1:1, then 1:3) until specimens were completely infiltrated with 100% araldite (Electron Microscopy Sciences; Hartford, PA) embedding media. Ultrathin sections of 60nm were obtained using a Leica Ultracut UCT 54 ultramicrotome. The sections were transferred onto formvar-coated slot grids and examined using a Phillips CM10 TEM, operating at 80 kV. Digital images were taken with a 2-megapixel AMT CCD (charge-coupled-device) side-mounted camera.
Immunocytochemistry
Abdominal ganglia were fixed in 4% PFA/0.5% glutaraldehyde in phosphate-buffered saline (PBS, pH 7.4) for 2 hrs, washed in PBS and dehydrated as above. Sections of 100nm were collected on grids. All immunostaining incubation and washing procedures were done in 40µl drops on parafilm in a moist chamber and on a magnetic stirrer to induce movements of the grids on the drops of incubation solution in the following sequence: block in PBS + 1% BSA for 1 hr; incubation with anti-CCAP antibody (rabbit; 1:5000) and anti-PBURS antibody (mouse; 1:1000) in PBS for 1 hr; wash in PBS for 45 mins; wash in TRIS-HCl (pH 8.0) for 15 mins; block with TRIS + 1% BSA for 45 mins; secondary antibody incubation (anti-mouse and anti-rabbit, both coupled to gold particles of indicated sizes and diluted 1:25) for 1 hr in TRIS + 1% BSA; wash in TRIS buffer and a rinse in distilled water for 15 mins; and finally dry blotting of grids. For counter-staining, sections were fixed in 0.5% glutaraldehyde in PBS for 5 mins, rinsed in PBS for 2 mins and then washed four times in distilled water (2 mins each). Grids were exposed to uranyl acetate for 5 mins and lead citrate for 2 mins. Slides were imaged as for regular electron microscopy above. Presentation images were processed in Adobe Photoshop. For the counts of labeled or non-labeled vesicles shown in (Fig. 2) vesicles containing one gold granule of either size were evaluated as labeled.
Antibody specificities
Specificity tests for anti-CCAP and anti-PBURS antisera were carried out using both immunocytochemistry and Western blotting [16, 3]. Preabsorption of the anti-CCAP antiserum (dilution 1:1000) with 40 μmolL−1 synthetic CCAP peptide (Sigma) eliminated all specific labeling in CCAP immunoreactive neurons. In addition, the anti-PBURS antibodies recognize the recombinant heterodimer [20], and recognize a single ~17 kDa band (size of PBURS homodimer) in Western blots on SDS polyacrylamid gels loaded with homogenates of ventral nerve cord homogenates [3], i.e. recognize the monomer PBURS. .
Results
P. americana is a favored insect model for the study of bursicon because the nervous system expresses a high amount of the neuropeptide, in larval stages as well as in adults [15]. Bursicon is co-expressed with CCAP in the population of large, lateral neurosecretory Cell 27 in all ganglia of the ventral nervous system [12, 13]. Bioactive bursicon is a ~30 kDa heterodimer of two cystine knot proteins highly conserved among insects and crustaceans [13]. Antibodies directed against each of the two monomers (called BURS and PBURS: [20]) co-label Cell 27 in all species investigated so far [20, 19, 3, 13]. For double-detection of bursicon and CCAP, a mouse antibody against the PBURS bursicon monomer was used. For detection of CCAP, a rabbit antiserum generated by H. Agricola, University of Jena, Germany was used.
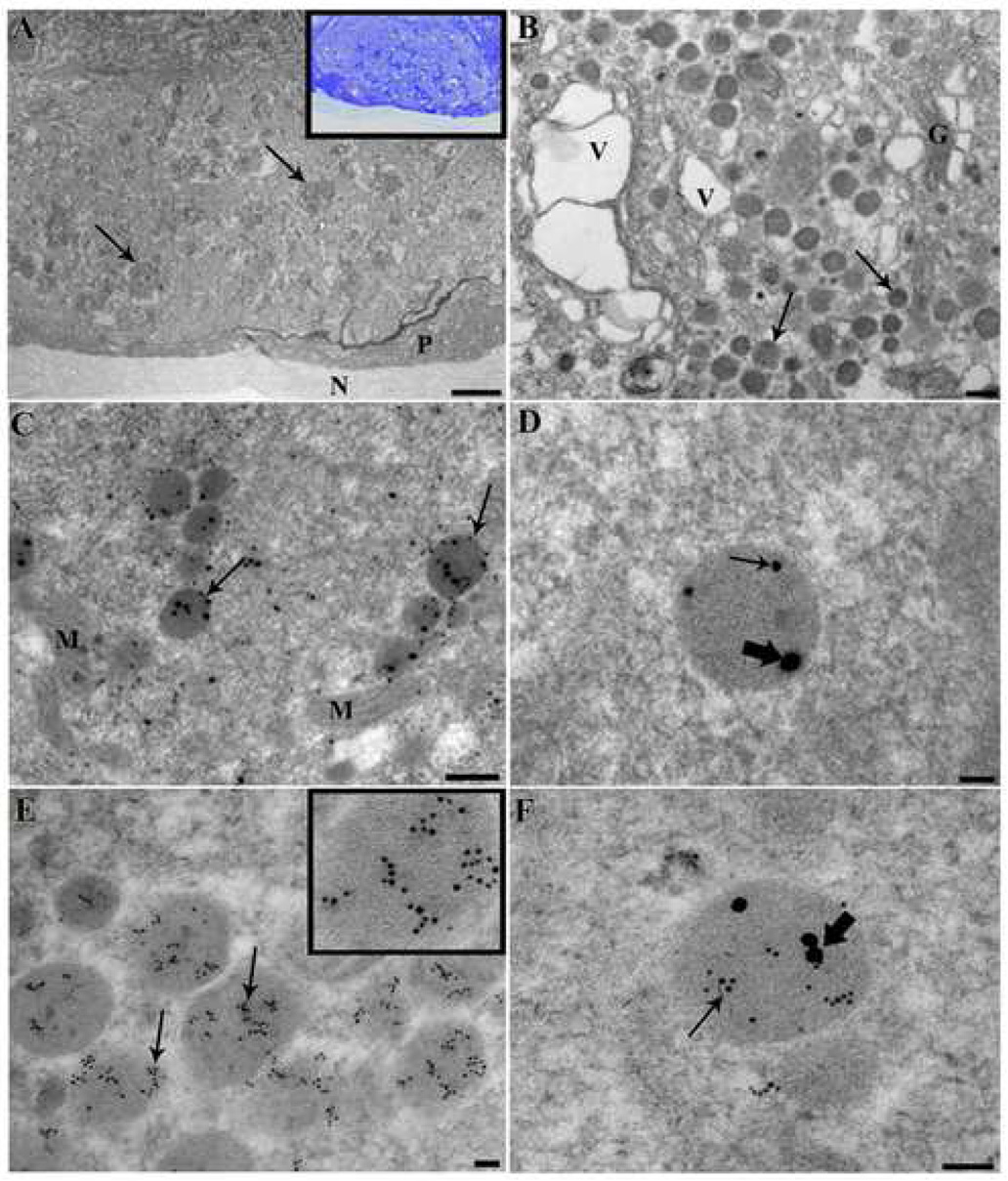
In semi-thin sections of abdominal ganglia stained with toluidine blue (Fig. 1A, inset) Cell 27 neurons are easily identifiable by their large size (~70×30 µm) and lateral position directly below the perineurium. With osmium used as a secondary fixative, ultra-thin sections (60 nm) showed dense bodies along with numerous mitochondria and an abundance of small cisternae, with dense-cored vesicles occurring in clusters (Fig. 1A, B), as also described in [1]. The clusters of dense-cored vesicles most likely correspond to the bursicon/CCAP labeled granules (cf. Introduction) seen with confocal microscopy, since the resolution of convential microscopy does no allow resolution of single vesicles. The double labeling of granules thus indicates a mixed population of CCAP and bursicon containing vesicles in theses clusters.
Figure 1.
A–F: Electron micrographs of CCAP and PBURS immunoreactivity in Cell 27 of P. americana. A: Low magnification electron micrograph showing whole neuron processed by conventional electron microscopy, arrows point to clusters of electron dense vesicles; perineurium (P), neurilemma (N). The inset shows the same cell at the light microscope level, stained with toluidine blue. B: High magnification of Cell 27 cytoplasm. Arrows point to both single small and large electron dense vesicles. This image also shows multiple large vacuoles (V) and Golgi (G). C: Immunogold co-labeling of CCAP (large particles, 25 nm gold) and PBURS (small particles, 15nm gold). Arrows point to electron dense vesicles that are clearly co-labeled; mitochondria(M). D: High magnification view of co-labeled vesicle. Thick arrows point to 25nm gold conjugated to CCAP, and thin arrow points to 15nm gold conjugated to PBURS. E: Immunogold labeling of 6 nm gold granules conjugated to CCAP. Inset shows a magnification of the vesicle in the lower right. . F: High magnification of a co-labeled vesicle with 15 nm gold conjugated to PBURS (thick arrow) and 6 nm gold conjugated to CCAP (thin arrow). Scale bars: A: 50 microns; B, C: 250nm; D, E and F: 50nm.
For double labeling, 100 nm sections of Cells 27 were probed with anti-CCAP and anti-PBURS antisera, with secondary antibodies coupled to gold particles of 25 nm for CCAP and 15 nm for PBURS. In most sections, gold particles of both sizes could be seen clearly within many vesicles (Fig. 1 C, D). In addition, however, some vesicles contained either one or the other gold label, and an abundance of vesicles were not labeled with either probe. Sections were similarly incubated with secondary antisera coupled to 6 nm gold particles to detect CCAP and 15 nm particles to detect PBURS. Many more vesicles are labeled with these smaller gold particles (Fig. 1 E), suggesting improved penetration. However, the same general picture was manifest, i.e. co-labeling of CCAP and bursicon gold particles in many of the same vesicles (Fig. 1 F). As above, non-labeled vesicles were also apparent, suggesting that other vesicle classes are present that contain neither mature CCAP or BURSICON neuropeptides.
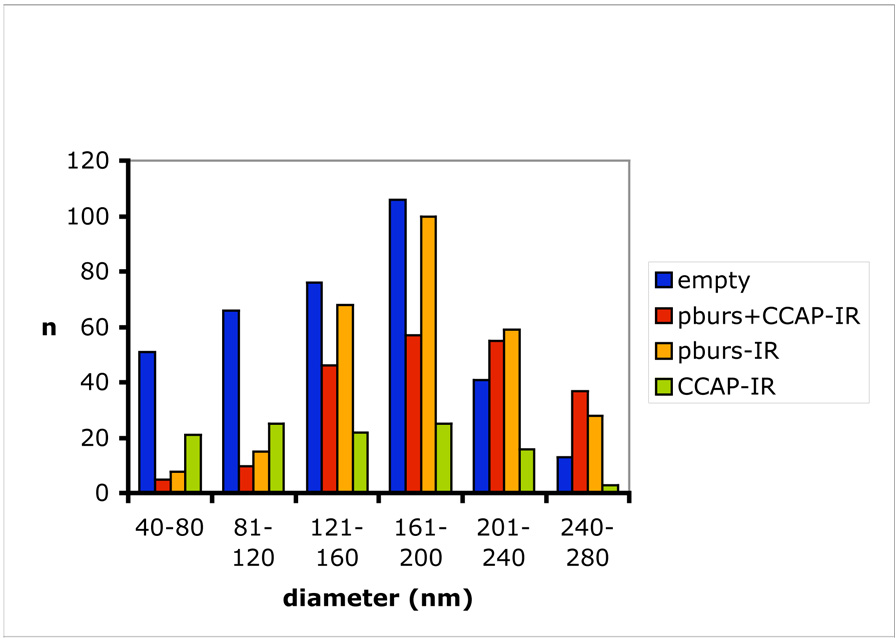
In 25 sections of one Cell 27, 953 vesicles were quantified for immuno-gold labeling at a magnification of 25,000X; PBURS labeled with 15 nm gold particles and CCAP labeled with 25 nm particles (Fig. 2). Of these total vesicles, 211 were clearly co-labeled for both CCAP and PBURS (22%), whereas 278 vesicles labeled only for PBURS (29%; 15 nm particles), 113 vesicles labeled only for CCAP (12%; 25 nm particles), and 351 vesicles were not labeled by either probe (37%; no gold particles). The single peptide labeling of vesicles warrants at least two possible interpretations. First, in many section planes, only the epitope of one or the other peptide might be available for antibody binding in epoxy embedded material. The peptides might be segregated within the dense cored vesicle so that only one peptide is detected in a given section plane. Second, and more probably, both single packaging and co-packaging of the two peptides occurs concurrently. This presumably allows specific release of each single peptide, for isolated action, or the co-release of both peptides, for coordinated action. The large number of non-labeled vesicles (37% of the total) makes it unlikely that gold labeling of bursicon or CCAP was missed in all of them. It rather indicates that other peptide species besides CCAP and bursicon are likely expressed by these same neurons and independently packaged to enable release specificity (but see also Discussion).
Figure 2.
Immuno-gold labeling of dense cored vesicles in a single Cell 27 in the abdominal ganglia of P. americana. A total of 953 vesicles were evaluated at 25,000X magnification for 15 nm gold particles detecting PBURS (beige), 25 nm gold particles detecting CCAP (green), co-labeling with both gold particles (red) or no gold granules (blue).
Discussion
We report here the co-packaging of two distinctly different peptides, CCAP and bursicon, within a single vesicle in a specialized neurosecretory cell (Cell 27) in P. americana. In insects of several different orders, i.e. Manduca sexta, Drosophila, Locusta migratoria and Teleogryllus commodus (Gryllus bimaculatus), and also crustaceans (Carcinus maenas), similar neurosecretory cells with conserved morphology and co-expression of CCAP and bursicon are known [12, 19, 3, 13, 27]. CCAP (PFCNAFTGCamide) is highly conserved among insects and crustaceans. In M. sexta it is processed from a single precursor protein of 125 amino acids [18]. Bursicon is a 30 kDa heterodimer of two monomers of 140 and 120 amino acids, respectively, in Drosophila. [6, 20]. The bioactive heterodimer dimerizes spontaneously if both monomers are present [20]. Thus, the processing of CCAP and bursicon is quite different, yet the mature peptides are co-packaged within the same secretory vesicle.
Peptide sorting in the regulated secretory pathway involves processing through the transgolgi network. Peptides may be cleaved from a common precursor and sorted into different vesicle classes [23], as shown in Aplysia bag cells [9] and for somatomammotrophs prolaction and growth hormone [10]. On the other hand, peptide co-packaging has also been observed. For example, adipokinetic hormone (AKH) I, II and III are found in the same vesicles of secretory cells in the corpus cardiacum of Schistocerca gregaria [7], although they are cleaved from three different preprohormones [11]. Sossin et al. [23] suggest that co-packaging may arise from fusion of two segregated vesicles, with peptides displaying segregation within the vesicle. In the current study, however, co-packaged CCAP and bursicon do not clearly show subvesicular segregation (cf. Figs. 1 C, D and F). Nevertheless, vesicles containing only one of each peptide clearly co-exist alongside co-packaged vesicles, supporting the fact that populations of single peptide vesicles exist that may subsequently fuse to form co-packaged vesicles.
In addition to the CCAP and bursicon vesicle pools, more than a third of vesicles in Cell 27 were not labeled in our study; the single largest population of dense cored vesicles (cf. Fig. 2). Taking into consideration possible limitations of the immunogold labeling technique, and the probability that the anti-CCAP antiserum does not detect the immature CCAP precursor protein, this finding nevertheless strongly suggests additional classes of peptidergic vesicles within Cell 27. In Drosophila 3rd larval instar, a subset of Cell 27 homologs show immunoreactivity to antisera against myoinhibitory peptides (MIPs), in addition to CCAP and bursicon [14]. Similarly, M. sexta Cell 27 may express other cardioacceleratory peptides (CAPs) in addition to CCAP [2, 26]. Further immunocytochemical studies are currently underway to investigate the possible peptide diversity within this cell. These results suggest that the C27 neurosecretory cell type has multifunctional capacities to release CCAP and bursicon individually, both peptides together from the same vesicle, as well as other peptide classes that may mediate related or distinct signaling activities.
We suggest that co-packaging of CCAP and bursicon has evolved because of functional constraints during ecdysis. The new soft cuticle in arthropods (insects and crustaceans) needs to be hardened quickly after ecdysis, because the animal is highly vulnerable and exposed to predation and desiccation during the critical transitional period. CCAP triggers increased heart rate (cf. Introduction), thereby increasing the rate of bursicon distribution throughout the body and delivery to the epidermis to trigger cuticle sclerotization [13]. One role of bursicon is activation of epidermal tyrosine hydroxylase (TH) via phosphorylation [5]. TH converts tyrosine to DOPA, a rate-limiting amine in the sclerotization pathway. We postulate that co-packaging of CCAP and bursicon, as the basis of coordinated co-release, synergistically improves the effectiveness of peptide activity and hence rapid conclusion of the vulnerable period of ecdysis.
Acknowledgements
The anti-CCAP antiserum used in this study was a gift of H. Agricola, University of Jena, Germany. This work was supported by NIH grant GM54544 to K.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams ME, O’Shea M. Vacuolation of an identified peptidergic (proctolin-containing) neuron. Brain Res. 1981;230:439–444. doi: 10.1016/0006-8993(81)90430-3. [DOI] [PubMed] [Google Scholar]
- 2.Broadie KS, Sylwester AW, Bate M, Tublitz NJ. Immunological, biochemical and physiological analyses of cardioacceleratory peptide (CAP2) activity in the embryo of the tobacco hawkmoth Manduca sexta. Development. 1990;108:59–71. doi: 10.1242/dev.108.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Dai L, Dewey EM, Zitnan D, Luo CW, Honegger HW, Adams ME. Identification, developmental expression, and functions of bursicon in the tobacco hawkmoth, Manduca sexta. J. Comp Neurol. 2008;506:759–774. doi: 10.1002/cne.21575. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Dulcis D, Hildebrand JG. Innervation of the heart and aorta of Manduca sexta. J Comp Neurol. 2001;440:245–260. doi: 10.1002/cne.1383. [DOI] [PubMed] [Google Scholar]
- 5.Davis MM, O”Keefe SL, Primrose DA, Hodgetts RB. A neuropeptide hormone cascade controls the precise onset of post-eclosion cuticular tanning in Drosophila melanogaster. Development. 2007;134:4395–4404. doi: 10.1242/dev.009902. [DOI] [PubMed] [Google Scholar]
- 6.Dewey EM, McNabb SL, Ewer J, Kuo GR, Takanishi CL, Truman JW, Honegger HW. Identification of the gene encoding bursicon, an insect neuropeptide responsible for cuticle sclerotization and wing spreading. Curr Biol. 2004;14:1208–1213. doi: 10.1016/j.cub.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 7.Diederen JHB, Maas HA, Pel HJ, Schooneveld H, Jansen WF, Vullings HGB. Co-localization of the adipokinetic hormones I and II in the same glandular cells and in the same secretory granules of corpus cardiacum of Locusta migratoria and Schistocerca gregaria An immuno-electron-microscopic study. 1987;249:379–389. [Google Scholar]
- 8.Dulcis D, Levine RB, Ewer J. Role of the neuropeptide CCAP in Drosophila cardiac function. J Neurobiol. 2005;64:259–274. doi: 10.1002/neu.20136. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JM, Sossin W, Newcomb R, Scheller RH. Multiple neuropeptides derived from a common precursor are differentially packaged and transported. Cell. 1988;54:813–822. doi: 10.1016/s0092-8674(88)91131-2. [DOI] [PubMed] [Google Scholar]
- 10.Fumagalli G, Zanini A. In cow anterior pituitary, growth hormone and prolactin can be packaged into separate granules of the same cell. J Cell Biol. 1985;100:2029–2024. doi: 10.1083/jcb.100.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haarthoorn LF, Diederen JHB, Oudejans RCHM, Van der Horst DJ. Differential location of peptide hormones in the secretory pathway of insect adipokinetic cells. Cell Tissue Res. 1999;298:361–369. doi: 10.1007/s004419900094. [DOI] [PubMed] [Google Scholar]
- 12.Honegger HW, Market D, Pierce LA, Dewey EM, Kostron B, Wilson M, Choi D, Klukas KA, Mesce KA. Cellular localization of bursicon using antisera against partial peptide sequences of this insect cuticle-sclerotizing neurohormone. J Comp Neurol. 2002;452:163–177. doi: 10.1002/cne.10357. [DOI] [PubMed] [Google Scholar]
- 13.Honegger H-W, Dewey EM, Ewer J. Bursicon, the tanning hormone of insects: Recent advances following the discovery of its molecular identity. J Comp Physiol A. 2008;194:989–1005. doi: 10.1007/s00359-008-0386-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Kostron B, Marquardt K, Kaltenhauser U, Honegger H. Bursicon, the cuticle sclerotizing hormone-comparison of its molecular mass in different insects. J Insect Physiol. 1995;41:1045–1053. [Google Scholar]
- 16.Kostron B, Kaltenhauser U, Seibel B, Bräunig P, Honegger HW. Localization of bursicon in CCAP-immunoreactive cells in the thoracic ganglia of the cricket Gryllus bimaculatus. J Exp Biol. 1996;199:367–377. doi: 10.1242/jeb.199.2.367. [DOI] [PubMed] [Google Scholar]
- 17.Lehman HK, Murgiuc CM, Miller TA, Lee TD, Hildebrand JG. Crustacean Cardioactive peptide in the Sphinx Moth Manduca sexta. Peptides. 1993;14:735–741. doi: 10.1016/0196-9781(93)90106-q. [DOI] [PubMed] [Google Scholar]
- 18.Loi PK, Emmal SA, Park Y, Tublitz NJ. Identification, sequence and expression of crustacean cardioactive peptide (CCAP) gene in the moth Manduca sexta. J Exp Biol. 2001;204:2803–2816. doi: 10.1242/jeb.204.16.2803. [DOI] [PubMed] [Google Scholar]
- 19.Luan H, Lemon WC, Peabody NC, Pohl JB, Zelensky PK, Wang D, Nitabach MN, Holmes TC, White BH. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo CW, Dewey EM, Sudo S, Ewer J, Hsu SY, Honegger HW, Hsueh AJ. Bursicon, the insect cuticle-hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2. Proc Natl Acad Sci U S A. 2005;102:2820–2825. doi: 10.1073/pnas.0409916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan L, Zhang YQ, Woodruff K, Broadie K. The Drosophila Fragile X Gene Negatively Regulates Neuronal Elaboration and Synaptic Differentiation. Curr Biol. 2004;14:1863–1870. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 22.Rohrbough J, Rushton E, Woodruff E, III, Fergestad T, Vigneswaran K, Broadie K. Presynaptic Establishment of the Synaptic Cleft Extracellular Matrix is Required for Post-Synaptic Differentiation. Genes & Dev. 2007;21:2607–2628. doi: 10.1101/gad.1574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sossin WS, Fisher JM, Scheller RH. Cellular and molecular biology of neuropeptide processing and packaging. Neuron. 1989:1407–1417. doi: 10.1016/0896-6273(89)90186-4. [DOI] [PubMed] [Google Scholar]
- 24.Truman JW. Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam Horm. 2005;73:1–30. doi: 10.1016/S0083-6729(05)73001-6. [DOI] [PubMed] [Google Scholar]
- 25.Tublitz NJ, Truman JW. Insect cardioactive peptides. II. Neurohormonal control of heart activity by two cardioacceleratory peptides in the tobacco hawkmoth, Manduca sexta. J. Exp. Biol. 1985;114:381–395. doi: 10.1242/jeb.114.1.381. [DOI] [PubMed] [Google Scholar]
- 26.Tublitz NJ, Sylwester AW. Postembryonic alteration of transmitter phenotype in individually identified peptidergic neurons. J Neurosci. 1990;10:161–168. doi: 10.1523/JNEUROSCI.10-01-00161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcockson DC, Webster SG. Identification and developmental expression of mRNAs encoding putative insect cuticle hardening hormone, bursicon in the green shore crab Carcinus maenas. Gen. Comp. Endocrrinol. 2008;156:113–125. doi: 10.1016/j.ygcen.2007.12.003. [DOI] [PubMed] [Google Scholar]