Abstract
The rhesus macaque (Macaca mulatta) is the most utilized primate model in the biomedical and psychological sciences. Expressive behavior is of interest to scientists studying these animals, both as a direct variable (modeling neuropsychiatric disease, where expressivity is a primary deficit), as an indirect measure of health and welfare, and also in order to understand the evolution of communication. Here, intramuscular electrical stimulation of facial muscles was conducted in the rhesus macaque in order to document the relative contribution of each muscle to the range of facial movements and to compare the expressive function of homologous muscles in humans and macaques. Despite published accounts that monkeys possess less differentiated and less complex facial musculature, the majority of muscles previously identified in humans were stimulated successfully in the rhesus macaque and caused similar appearance changes to human facial movements. These observations suggest that the facial muscular apparatus of the monkey has extensive homology to the human face. The muscles of the human face, therefore, do not represent a significant evolutionary departure from that of monkey species. Thus, facial expressions can be compared between humans and rhesus macaques at the level of the facial musculature, facilitating the systematic investigation of comparative facial communication.
Keywords: Monkey, primate, FACS, facial expression, emotion, intramuscular electrical stimulation
Introduction
The rhesus macaque (Macaca mulatta) is the primary species used in clinical research to model different aspects of human neuropsychiatric disorders. Social communication with facial expression is shared by humans and non-human primates and is profoundly altered in many human disorders, such as Parkinson’s disease, schizophrenia, autism, and various manifestations of pain. Monkeys with MPTP-induced parkinsonism, for example, appear to have similarly inexpressive faces as human patients [1, 2, 3 and 4] and the efficacy of treatments that improve the clinical symptoms (e.g., deep brain stimulation) can be reflected in improvement in facial muscle tone and expressivity [5]. Thus, comparison between rhesus models and human patients could be highly useful in assessing treatment and progression of the disease. While global facial expressivity has been compared successfully between species (e.g. oro-facial dyskinesia [6]) in a similar manner to other motor symptoms of neuropsychiatric disease (levodopa-induced dyskinesia [7]; bradykinesis [8]; akinesia [9]), we do not have a system in macaques that allows us an objective description of facial movements in the normal or pathological case. Assessing facial expression in terms of homologous component muscle movements is necessary in order to understand the similarities between the human and macaque facial movement systems and to quantify deficits in facial movement in relation to its neuromuscular basis. In order to conduct such studies, it is essential to verify whether similar rhesus macaque and human facial movements share the same underlying muscle contractions.
The literature on the neuromuscular mechanisms that give rise to facial expressions in rhesus macaques is sparse. Early studies (e.g. [10]) claimed that rhesus macaques have less complex and more undifferentiated facial musculature than humans. Recent dissections of facial muscles, however, have found a great degree of similarity between humans and rhesus macaques [11]. These dissections were conducted using a unique method of removing the facial mask from the skull and dissecting this ‘inside-out’ preparation which retains many of the original structures [12]. This method was used successfully to identify the facial muscles in the chimpanzee (Pan troglodytes) and to compare the human and chimpanzee facial architecture with the human face [13]. Based on the known anatomy, the functional similarities were then compared using intramuscular electrical stimulation to document the changes that occur in each muscle [14].
A better understanding of chimpanzee facial movements facilitated the development of a coding system that allows systematic comparative analysis of facial expressions between humans and chimpanzees (Chimpanzee Facial Action Coding System: ChimpFACS [15]). ChimpFACS is an anatomically based system, where each specific muscle contraction is identified as a unit of movement, and is based on FACS (The Facial Action Coding System: [16]), which is the most commonly used objective and standardized coding system in human facial expression research. The development of a similar, objective coding system for the rhesus macaque requires measurements of facial movement in relation to the activation of each anatomically identified muscle. If, as the dissections suggest, rhesus macaques share a similar underlying musculature to both humans and chimpanzees, this technique can be extended to this monkey species to facilitate scientific comparison between facial expressions. Research using monkey models would be greatly aided by the development of a refined, accurate measurement tool for quantifying facial movement and, as this method would offer more detailed and accurate observations, this would also represent an important step in achieving the welfare goals of replacement, reduction and refinement in animal research [17]. Specifically, given that a FACS approach can greatly increase the quality of information gleaned from facial expressions, a small sample of rhesus macaque individuals could yield a rich data set, and animals that are already used in clinical trials could be additionally studied for facial expression deficits.
The objective of the present investigation was to document the facial appearance changes that occur when facial muscles contract in the rhesus macaque. Using intramuscular electrical stimulation techniques, we aimed to 1) record the surface changes during contraction of targeted facial muscles, and 2) compare these contractions to facial movements in humans and chimpanzees using FACS terminology.
Methods
Subject
Weak electrical stimulations of individual facial muscles were performed on one anesthetized adult male rhesus macaque aged 7 years. The testing session lasted approximately 1hr. All the procedures and the anesthesia (Ketamine 8mg/kg for pre-anesthesia and Propofol 200–600 ug/kg/minute as a continuous IV drip for anesthesia) were approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Arizona.
Procedures
The subject was positioned supine on a testing table with the head propped up to an angle of approximately 30° with respect to horizontal. The skin sites overlying various muscles were identified and cleansed with alcohol. Electrode placement was informed by recent dissections of rhesus macaques [11] combined with a review of published materials. The facial muscles of the rhesus macaque are depicted in Figure 1. Initially, electrical stimulation was attempted on the right side of the face, and if unsuccessful the left side was then attempted. A sterilized tungsten microelectrode (250 µm shaft diameter, ~ 2 µm tip diameter) was inserted through the skin and directed toward the target muscle. The microelectrode served as the active (cathode) electrode and a surface electrode (taped to the skin overlying the right clavicle) served as the return (anode) electrode.
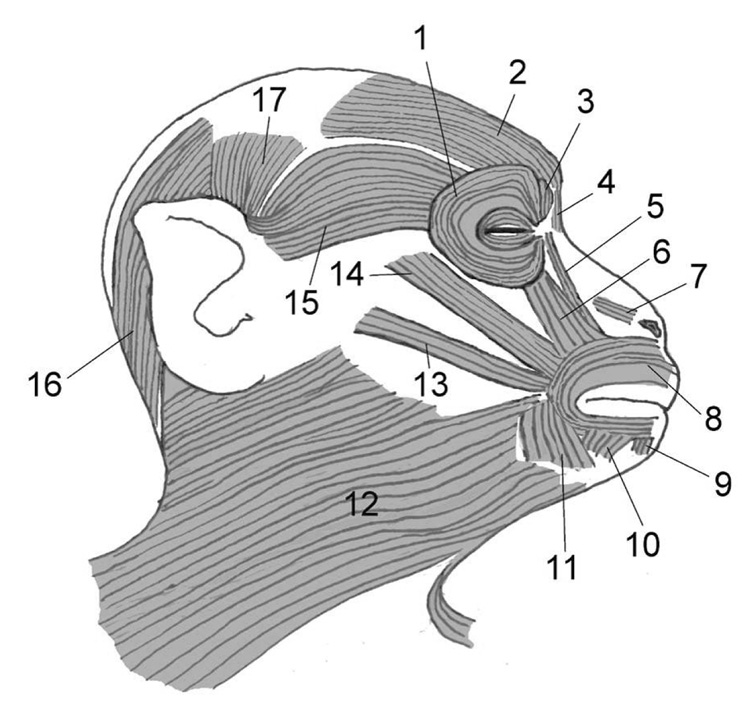
Figure 1.
Location of facial muscles in the rhesus macaque (Macaca mulatta) based on Burrows et al. [11]. Right side with abbreviations as follows: 1. orbicularis occuli m., 2. frontalis m., 3. corrugator supercilli m., 4. procerus m., 5. levator labii superioris alaeque nasi m., 6. levator labii superioris m., 7. nasalis m., 8. orbicularis oris m., 9. mentalis m., 10. depressor labii inferioris m., 11. depressor anguli oris m., 12. platysma m., 13. zygomaticus minor m., 14. zygomaticus major m., 15. orbitoauricularis m., 16. occipitalis m., 17. superior auricularis m. Note that the anterior and inferior auricularis mm. are not shown in this diagram as they are deep to the orbitoauricularis and occipitalis mm., respectively. While the zygomaticus minor m. was a variant, it is shown in this diagram in order to indicate its position when present.
Initially, low-intensity (1 – 4 mA) constant current pulses (0.5 ms in duration) were delivered by a stimulator and optically-isolated constant current unit (Grass Instruments S88 – West Warwick, Rhode Island) at a rate of 1 pulse/s. The position of the microelectrode was manually adjusted until a stimulation site was found that evoked motor responses in the target muscle. The muscle was then activated using a 2-s train of stimulus pulses at 30 pulses/s in order to evoke a sustained contraction of the target muscle. On repeated trials, the magnitude of the stimulus pulses was progressively increased from a level that barely elicited movement (< 1 mA) up through levels that evoked strong muscular contractions. The intramuscular stimulation procedures used in this experiment closely resembled the procedures used previously in humans and chimpanzees [14] to allow direct comparison among the three species.
Two digital video cameras were positioned at frontal and profile angles to capture the change in the shape of the face in response to the sustained stimulation for subsequent analysis. For a stimulus intensity that appeared to activate the muscle in isolation, three to five trials of sustained stimulation were elicited. If several attempts failed in stimulating movement in that area, the microelectrode was withdrawn and reinserted into a new site to test a different muscle, or attempted on the opposite side of the face.
Analysis
Footage was described using FACS terminology by BW (certified FACS and ChimpFACS coder) and then unlabelled and muted exemplar clips were extracted, reordered and sent to SJV (certified FACS and ChimpFACS coder) and MM (certified FACS coder) for additional FACS identification. Neither coder was present during the stimulation study (and so had not seen the stimulations before) and MM was naïve to the aims of the study. The coders were asked to label the clips with an action unit (AU) if the appearance changes met the minimum criteria for identification, and comment on any differences with human and/or chimpanzee AUs. Movements which did not have an equivalent human or chimpanzee AU were excluded from this analysis (i.e. ear movements). Statistical reliability was calculated for each movement using the following equation [18]:
This method gives an agreement for each movement between 0 and 1 (0 being no agreement, and 1 being absolute agreement). Table 1 shows the agreement reached for each stimulated movement. The mean agreement for the movements was 0.92, which is considered excellent [18].
Table 1.
FACS coding agreement among the three coders.
| Stimulated muscle | BW | SJV | MM | Agreement* |
|---|---|---|---|---|
| Levator labii superioris alaeque nasi | AU9 | AU9 | AU9 | 1 |
| Corrugator supercilli | AU4 | AU4 | AU4 | 1 |
| Levator labii superioris | AU10 | AU10 | AU10 | 1 |
| Depressor anguli oris | AU15 | AU15 | AU20 | 0.666666667 |
| Orbicularis oculi | AU6 | AU6 | AU6 | 1 |
| Mentalis | AU17 | AU17 | AU17 | 1 |
| Depressor labii inferioris | AU16 | AU16 | AU16 | 1 |
| Lateral frontalis | AU2 | AU2 | AU2 | 1 |
| Procerus | AU4 | AU4 | AU4 | 1 |
| Medial frontalis | AU1 | AU1 | AU1 | 1 |
| Nasalis | AU38 | AU38 | AU38 | 1 |
| Zygomatic major | AU12 | AU12 | AU12 | 1 |
| Orbicularis oris (inferior) | AU18 | AU18 | AU17+AU24 | 0.5 |
| Orbicularis oris (superior) | AU18 | AU18 | AU10+AU13+AU18 | 0.6 |
| Mean | 0.91 | |||
See text for details of agreement calculation.
Results and Discussion
The following section combines the results of the current study with comparison to human and chimpanzee facial movements (as documented in [14], FACS [16], and ChimpFACS [15]). Each muscle is first described in terms of gross anatomy [11], followed by a description of how the equivalent muscle moves in humans and chimpanzees. Finally, appearance change on stimulated contraction is described. Movements are classified as AUs if they met the minimum criteria for coding according to the human FACS, and this was agreed by all three coders. Any disagreement among coders is discussed in the text. All movements are best illustrated by the video clips (see supplemental material), but some figures are included as examples. Table 1 summarizes the function of facial muscles in rhesus macaques and how this compares to humans and chimpanzees.
Frontalis
The rhesus macaque frontalis is a flat, sheet-like muscle with no bony attachments. It originates from the anterior margin of the galea aponeurotica (see Figure 1 for muscle location).Medial fibers are continuous with the procerus and the thinner lateral fibers blend with orbitoauricularis and orbicularis oculi. In humans and chimpanzees [14], contraction of medial fibers causes AU1 (inner brow raiser) and contraction of lateral fibers causes AU2 (outer brow raiser), although in the chimpanzee these movements commonly occur together (AU1+2).
Medial (superior to glabella) and lateral (superior to mid-brow) sections were stimulated separately, which led to elevation of the medial and mid to lateral portions of the brow respectively (see supplemental material 01 and supplemental material 02). Both sites of stimulation resulted in small transverse wrinkles on the forehead, although these were minimal compared to those seen in humans (and to a lesser extent, chimpanzees) due to the extensive hair covering. Huber [10] considered the macaque frontalis to be part of a primitive muscle complex including the ear musculature (auricularis anterior et superior), and suggested that differentiation between these muscles has only occurred in humans due to growth of the cranial vault and greater selection for facial movement over ear movement. As with the chimpanzee stimulation [14], there was no evidence of ear movement during frontalis contraction in chimpanzees, indicating that the frontalis muscle can be functionally independent. Appearance changes on contraction of the rhesus frontalis muscle were sufficient to code AU1 and AU2.
Corrugator supercilli and procerus
In humans, these muscles are both associated with one specific movement – AU4 (brow lowerer). There is some debate, however, as to whether these muscles can operate independently. In FACS, independent action of corrugator supercilli is termed AU44 (eyebrow gatherer), and procerus is termed AU41 (glabella lowerer). In infant humans, the muscles are thought to produce distinctive facial movements, and are coded separately [19]. Thus, in BabyFACS, corrugator supercilli action is coded as AU3 (knitting of the brow due to medial contraction), and procerus action is coded as AU4 (knotting of the brow due to lowering of glabella). Both muscles are present in the rhesus macaque [11], and are structurally similar to humans.
Stimulation was attempted at the sites corresponding to each muscle respectively. The movements were very similar, however, and resulted in brow lowering. All coders agreed that both movements were equivalent to a component of AU4, but disagreed on which specific component (suggesting differences were minimal). Procerus action pulled the skin of the glabella inferiorly (see supplemental material 03). Corrugator stimulation at a more lateral site also caused brow lowering (supplemental material 04) with the addition of very slight corrugation (medial contraction). In FACS terminology this would be termed a trace movement. Both movements are similar to the independent movements described in FACS and BabyFACS. Observation of spontaneous movement is needed to clarify whether these muscles work in concert to produce a movement equivalent to AU4 in humans.
Orbicularis oculi
The orbicularis oculi surrounds the eye forming a sphincter muscle. The fibers below the eye are extensive and cover the origin of levator labii superioris, and above the eye fibers blend with frontalis. In humans, the inner fibers (palpebral portion) contract to cause tightening of the eyelids AU7 (lid tightener), and the outer fibers (orbital portion) contract causing the infraorbital triangle to raise (AU6: cheek raiser). AU6 has been successfully stimulated and observed in chimpanzees [14], but AU7 has not.
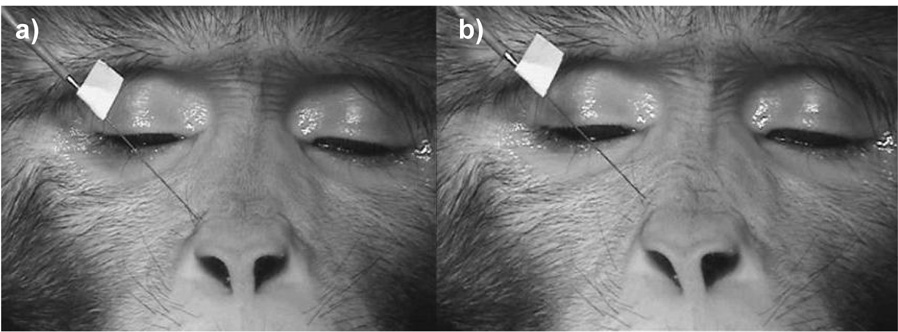
Contraction of the orbital section was achieved in the rhesus macaque by stimulating the muscle inferior and lateral to the eye (see supplemental material 05). The skin in this area was pushed medially and superiorly, causing the skin below the eye to bag and wrinkle. This movement is similar to the equivalent movement in humans, and thus is sufficient to code AU6. Superior sections of orbicularis oculi were possibly involved in some of the brow lowering movements achieved by stimulating procerus (supplemental material 03) and corrugator supercilli (supplemental material 04), although as these muscles are heavily intermingled it is difficult to separate them functionally. Stimulation of the palpebral portion was not attempted due to close proximity to the eye.
Levator labii superioris alaeque nasi
The rhesus macaque levator labii superioris alaeque nasi (llsan) arises from the upper part of the frontal part of the maxilla and inserts into the upper lip and upper fibers of the orbicularis oris muscle. Contraction of this muscle in both humans and chimpanzees elevates the upper lip and wrinkles the nose (AU9: nose wrinkler).
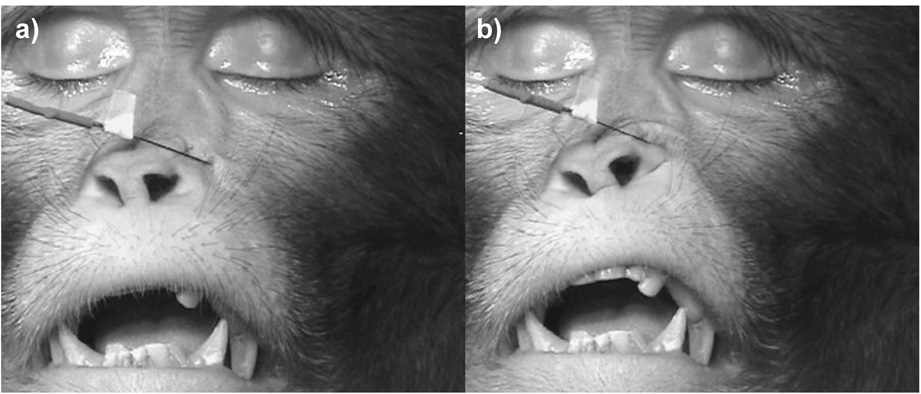
Stimulation (immediately lateral to the alar cartilage) resulted in wrinkling of the skin lateral and superior to the nose, moving the skin superiorly and elevating the upper lip very slightly (see Figure 2 and supplemental material 06). Contraction also caused the brows to depress subtly with marked transverse wrinkles in the glabella region (indicating that procerus may also have been recruited, which is intermingled with llsan). Appearance changes were sufficient to code AU9.
Figure 2.
Levator labii superioris alaeque nasi at rest (a) and contracting when stimulated (b).
Levator labii superioris
In rhesus macaques levator labii superioris originates from the maxilla and zygomatic arch and has a strong, direct connection to the upper fibers of orbicularis oris lateral to the alar cartilages of the nose. This muscle elevates the upper lip (AU10: upper lip raiser) in humans and chimpanzees.
Stimulation lateral to the nose caused the upper lip to elevate (see Figure 3 and supplemental material 07). The skin adjacent to the nose pouched and wrinkled, but did not cause the transverse wrinkles characteristic of AU9. Appearance changes were sufficient to code AU10.
Figure 3.
Levator labii superioris muscle at rest (a) and contracting when stimulated (b).
Zygomatic major
The rhesus macaque zygomatic major originates at the zygomatic arch and inserts onto the modiolus (muscular node at the corner of the mouth) and the upper fibers of orbicularis oris. Huber [10] noted that this muscle was in broad connection with the orbicularis oculi (he termed this a primitive condition), but more recent dissections have found it to be fully separated [11]. In humans and chimpanzees contraction of zygomatic major causes AU12 (lip corner puller), which raises the corner of the mouth superolaterally.
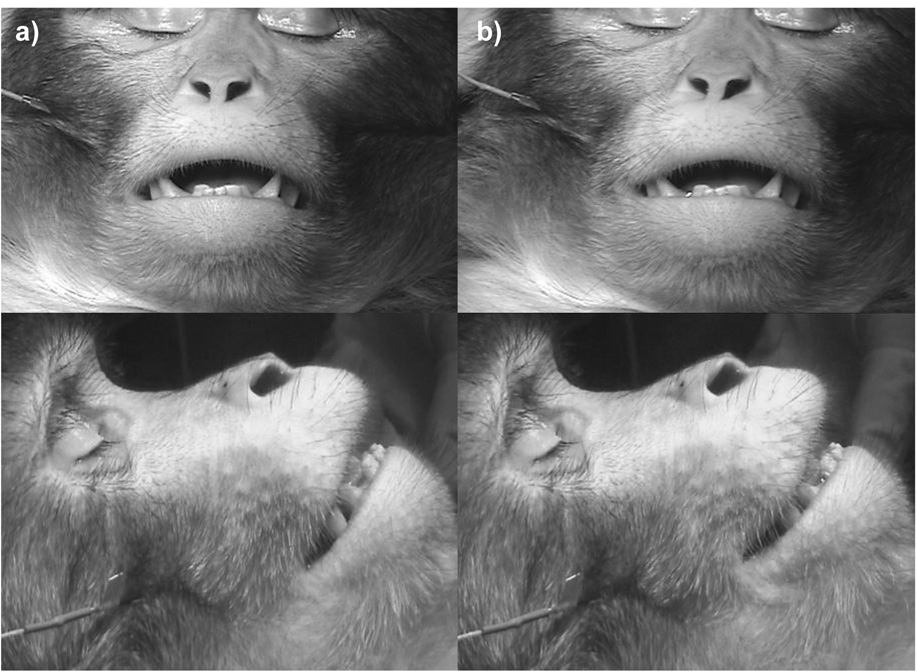
Stimulation caused the corner of the mouth to rise in a similar movement to humans and chimpanzees, and thus appearance changes were sufficient to code AU12 (see Figure 4, supplemental material 08 for front view and supplemental material 09 for profile). No movement of the orbicularis oculi was seen.
Figure 4.
Front and profile views of zygomatic major muscle at rest (a) and contracting when stimulated (b).
Nasalis
The nasalis is a series of transverse fibers stretching over the alar cartilages (nostril) down toward the levator labii superioris alaeque nasi. In humans, nasalis controls dilation and contraction of the nostril wings (AU38: nostril dilator; AU39: nostril compressor), and a portion also seems to act in association with levator superioris labii alaeque nasi in AU9 (nose wrinkler). When stimulated in humans, the skin of the bridge of the nose was wrinkled as if pinched, one of the movements seen in AU9 (nose wrinkle) [14]. This independent movement has not been stimulated or observed in chimpanzees, but also may be involved in the ChimpFACS AU9.
Similar to humans, stimulation of nasalis in the rhesus macaque caused tightening of the skin over the inferior part of the nose and flaring of the nostril (AU38: nostril dilator, see supplemental material 10).
Depressor anguli oris
The depressor anguli oris (also called triangularis) originates in the mandible (lateral to the mouth) and inserts onto the modiolus and lower fibers of the orbicularis oris muscle. In humans and chimpanzees contraction causes the lip to depress at the corner (AU15: lip corner depressor). Huber stated that although present in rhesus macaques, it overlaps the platysma and is not extended to the lower border of the mandible as in humans and chimpanzees. Presumably this arrangement would cause lip depression to be less pronounced. In contrast, recent dissections of rhesus macaques have observed it approaching the inferior border of the mandible [11].
Stimulation in the rhesus macaque was achieved at a position similar to that in both humans and chimpanzees (inferior and lateral to lip corner). Two coders agreed that appearance changes were sufficient to code AU15 (lip corner depressed on contraction, supplemental material 11) but one coder coded AU20 (see Table 1). These movements are often very similar in humans, but rhesus macaques do not possess the risorius muscle that underlies AU20 in humans [11].
Mentalis
The rhesus macaque mentalis originates on the mandible in the region of the incisor teeth and has strong, oblique fibers that flare laterally over the chin region. Huber [10] stated that (unlike humans) it remains in primitive connection with deep bundles of the buccinator. In contrast, Burrows et al. [11] found no connections with the buccinator. In humans and chimpanzees, the skin of the chin elevates on contraction, causing the lower lip to protrude, and (in humans) causes wrinkles and dimples to form in the skin of the chin boss (AU17: chin raiser).
Stimulation was achieved in the rhesus macaque, and the skin of the chin was raised superiorly (supplemental material 12). Bunching of the skin was visible, but wrinkles and dimpling were hard to discern due to hair covering. The lower lip did not protrude, although stronger contraction may have elicited this movement. No contraction of the buccinator (lateral to the mouth corners) was visible. The minimum criteria for coding AU17 were present.
Orbicularis oris
The rhesus orbicularis oris is a sphincter muscle that encircles the oral cavity with distinct upper and lower fibers. As in humans and chimpanzees it is also attached to muscles of the midface (zygomaticus major, zygomaticus minor, levator labii superioris, and llsan) and muscles of the lower face (depressor anguli oris, depressor labii inferioris, and brief attachments to the mentalis along with upper fibers of the platysma). In humans, a number of qualitatively different movements are produced from this muscle: funneling the lips (AU22: lip funneler), tightening the lips (AU23: lip tightener), pressing the lips together (AU24: lip presser) and rolling lips between teeth (AU28: lip suck). This muscle is also likely involved in AU18 (lip pucker), which may also recruit drawstring muscles above and below orbicularis oris (incisivii labii superioris and incisivii labii inferioris). In chimpanzees, AU22 was stimulated [14] and additionally AU24 had been identified in ChimpFACS [15], but no other movements have been distinguished.
In rhesus macaques, portions of the orbicularis oris were stimulated at localized sites (see supplemental material 13). Pursing and tightening of the lip margin and some funneling of the lips were achieved in the specific area of stimulation, but it was difficult to contract the full sphincter at once. Stronger stimulation caused fuller lip pursing where the lips protruded further, but due to the absence of everted lips at the margin (as is common in the human and chimpanzee AU22) this was more similar to AU18 than any other movement. The two coders who were aware of the aims of the study extrapolated from the localized areas to visualize a full contraction of the muscle, and concluded that appearance changes would be sufficient to code AU18. However, as MM coded purely on the appearance changes present in the localized stimulations, he identified additional movements from the stimulation videos, including AU18 (AU10, AU13, AU17, AU18 and AU24). Subsequent discussion resolved the disagreement and AU18 was agreed by all coders.
Depressor labii inferioris
The rhesus macaque depressor labii inferioris arises from the mandible and inserts onto the skin of the lower lip (blending with the paired muscle from the other side) and attaches to the lower fibers of orbicularis oris. Laterally, it is not differentiated from the platysma. When contracted in humans and chimpanzees, the lower lip depresses, displaying the lower teeth (AU16: lower lip depressor).
Stimulation resulted in qualitatively similar movement to both humans and chimpanzees (lower lip depression) and appearance changes were sufficient to code AU16 (supplemental material 14).
Muscles not located
Buccinator (AU14: dimpler), zygomatic minor (AU11: nasolabial furrow deepener) and risorius (AU20: lip stretcher) were not attempted. Buccinator lines the buccal pouch in rhesus macaques, and the main function seems to be masticatory. Zygomatic minor has been located in dissection, but is likely to be highly variable. As in chimpanzees, risorius is not present in the rhesus macaque [11]. Caninus (elevates the lips corners and puffs the cheeks in humans, AU13: cheek puffer) and depressor supercilli (pat of AU4: brow lowerer) were attempted but we were unable to locate and stimulate these muscles. This was not unexpected as recent dissections have not identified these muscles [11].
Additional muscles
The external ears are often used in combination with facial movements in rhesus macaques, and so coding systems of facial communication in rhesus macaques should take ear movements into account. Thus, some of the larger muscles controlling the ears were also stimulated to document appearance changes. While many of the anteriorly located muscles such as the tragicus and helices muscles were too small to have a reasonable chance of being located via stimulation, several larger ones, such as the orbito-auricularis and auricularis muscles, were able to be located. Orbito-auricularis contraction caused the ear to elevate superiorly and medially (supplemental material 15). The scalp retracted superiorly to an extent, although this may have been due to frontalis recruitment. Stimulation of the superior auricularis caused the ear to elevate superiorly (supplemental material 16), but without the medial contraction seen in orbito-auricularis. Stimulation of inferior auricularis caused the ear to flatten to the head and slight lateral retraction of the scalp (supplemental material 17). Although there were no equivalent human AUs to compare these movements to, the coders agreed that these movements were clear and distinct. A formal description of macaque facial action units should include a muscle-based description of ear movements as they are an essential component of social-emotional displays.
Summary
Figure 5 shows the potential facial movements in the rhesus macaque (as evidenced by stimulation) in comparison to humans, and represents a muscle movement map of the face. Of the 161 expressive facial muscles present in humans (14 of which have been stimulated using this method [14]), 13 (81%) were located and stimulated in a rhesus macaque. Eleven were stimulated successfully in chimpanzees [14].
Figure 5.
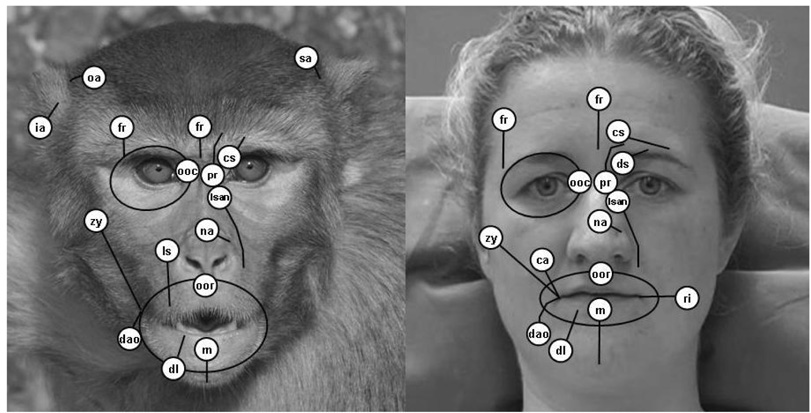
Muscle movement map in rhesus macaques (Macaca mulatta) and humans. White circles represent approximate muscle origins (excepting orbital muscles) and black lines show estimated length/orbit of the muscle. Contraction resulted in movement toward the origin (orbital muscles reduced aperture of orbit). Labels: fr = frontalis (medial and lateral portions), pr = procerus, cs = corrugator supercilli, ooc = orbicularis oculi, lsan = levator labii superioris alaeque nasi, lls = levator labii superioris, na = nasalis, zy = zygomatic major, ca = caninus, ri = risorius, oom = orbicularis oris, dao = depressor anguli oris, dl = depressor labii inferioris, m = mentalis, oa = orbito-auricularis, sa = superior auricularis, ia = inferior auricularis. Human diagram adapted from [14] (showing both stimulated movements as well as proposed movements from FACS and dissections) and rhesus macaque picture taken by Lisa Parr.
Conclusions
The appearance changes associated with each facial muscle movement have been documented and compared to both humans and chimpanzees. The findings have two main implications. First, the facial movements of the rhesus macaque evoked by intramuscular electrical stimulation are highly similar to the base units of movement (AUs) in humans. Thus, the facial muscles we see in humans appear to be little altered from the format we see in a monkey species, with the exception of the reduction in musculature around the external ear. Therefore, when tracing the evolution of facial expressions we do not have to look only to our closest relatives (chimpanzees) but also further a field in the primate order. The early literature documenting the facial muscles of monkeys painted a picture of a series of large, relatively undifferentiated set of muscles, capable primarily of gross, undifferentiated movements [10]. On the contrary, our results indicate that the muscles are highly differentiated and capable of subtle movement, and this conclusion is strongly supported by evidence from anatomical dissections of the facial muscles in rhesus macaques
Second, the similarity between rhesus macaques and humans demonstrates that a FACS-like approach is a viable method for comparing facial expression between the two species. The modification of FACS for use with chimpanzees (ChimpFACS: [15]) has offered considerable advantages to researchers interested in quantifying the expressions of chimpanzees. The ability to identify movements in terms of the underlying musculature has facilitated a description of the chimpanzee ethogram in terms of muscle action, which has in turn afforded a morphological comparison between the chimpanzee and human repertoire [20]. A similar comparison of homologous facial muscle movements that includes monkeys will aid cognitive and neurophysiological studies of primate communication. In clinical studies, the facial deficits of rhesus models for various human diseases can be compared to human patients at the level of the facial musculature. In cognitive studies, images of facial expressions can be standardized. Such images are often used as stimuli in experiments that assess the role of various brain regions in processing socio-emotional stimuli such as facial expressions [21 and 22], and although facial expressions can be selected for these tasks based on common classification categories (threat face, lipsmack etc.), without a detailed coding system it is unclear whether these labels do indeed represent uniform and distinct categories [20]. In addition, systematic evolutionary analyses of facial expressions among primate species and comparative analyses of evolutionary function [e.g. 23] will benefit greatly from an instrument that identifies facial movements in more detailed anatomical terms. Human facial expression research has used FACS methodology for decades, and as a result our understanding of facial expression as a dynamic, subtle and powerful mode of communication has increased dramatically. Using similar systems in rhesus macaques is likely to similarly increase our understanding of their facial communication.
While these data do not allow us to comment in detail on the species-typical displays of the rhesus macaque (they only inform us about the potential for movement), we can make some general statements. Rhesus macaques could (potentially) make many of the same facial expressions as humans and chimpanzees, and thus the phylogenetic precursors of human facial expressions may be reflected in the rhesus macaque repertoire. The most striking difference between the three species is the great potential for ear movement in the rhesus macaque, which is not seen in humans and chimpanzees. Huber suggested that there was selection for brow movements over ear movements throughout primate evolution, presumably because he also noted greater potential for ear movements in monkeys than apes [10]. The function of these differences and similarities can be readily investigated within a FACS framework.
An inevitable limitation of this research is the sample size: practical and ethical considerations restricted the study to one animal. Therefore, this study does not take into account individual variation within rhesus macaque facial muscles, which has been found in humans [24]. However, as the vast majority of muscles predicted to be present were found and stimulated in the position located in gross dissections, this can nevertheless be taken as an example of how these muscles do move when present. A further limitation is the possible bias introduced by using trained FACS coders to identify the muscle movement (AU), when they could potentially determine the target muscle from the electrode insertion point alone. In many cases the electrode was not easy to see and/or was positioned under the skin a significant distance from the insertion point, minimizing this bias, but the limitation must be taken into account.
In sum, the facial muscles of the rhesus macaque contract to produce qualitatively similar facial movements to those in humans and chimpanzees. Using these findings, techniques can be refined to increase the productivity of a multitude of research activities. In particular, scientists now have the framework to use these objectively measured changes in facial expressivity for tracking the onset, progression or efficiency of treatments for psychiatric or neurological disorders that affect the neural substrate of emotion, social behavior, or the neuromuscular apparatus of the face.
Supplementary Material
Table 2.
Observed function of facial muscles during intramuscular stimulation in the rhesus macaque (current study) compared to humans and chimpanzees [14].
| Muscle | Rhesus | Human | Chimpanzee |
|---|---|---|---|
| Frontalis (inner) | Elevates the medial portion of the brow (AU1: inner brow raiser) | Elevates the medial portion of the brow (AU1: inner brow raiser) | Elevates the medial portion of the brow (AU1: inner brow raiser) |
| Frontalis (outer) | Elevates the lateral portion of the brow (AU2: outer brow raiser) | Elevates the lateral portion of the brow (AU2: outer brow raiser) | Elevates the lateral portion of the brow (AU2: outer brow raiser) |
| Corrugator supercilli | Lowers the brow inferiorly with slight medial contraction (corrugation) (part of AU4: brow lowerer) | Draws the brow medially and superiorly (AU1+AU4: inner brow raiser + brow lowerer) | Not stimulated (but likely present) |
| Depressor supercilli | Not present | Depresses the medial portion of the brow (part of AU4: brow lowerer) | Not stimulated (but may be present) |
| Procerus | Depresses the medial portion of the brow (part of AU4: brow lowerer) | Depresses the medial portion of the brow (part of AU4: brow lowerer) | Depresses the medial portion of the brow (part of AU4: brow lowerer) |
| Orbicularis oculi | Inferior portion elevates infraorbital triangle (or equivalent area) superiorly and medialwards | Elevates the infraorbital triangle (cheek) superiorly and medialwards (AU6: cheek raiser) | Inferior portion elevates infraorbital triangle (or equivalent area) superiorly and medialwards |
| Nasalis | Wrinkles skin on bridge of nose (part of AU9) | Wrinkles skin on bridge of nose (part of AU9) | Not stimulated |
| Levator labii superioris alaeque nasi | Wrinkles the skin alongside the nose (AU9: nose wrinkler) | Wrinkles the skin alongside the nose (AU9: nose wrinkler) | Wrinkles the skin alongside the nose (AU9: nose wrinkler) |
| Levator labii superioris | Elevates upper lip (AU10: upper lip raiser) | Not stimulated (but present). | Elevates upper lip (AU10: upper lip raiser) |
| Zygomatic minor | Not stimulated (but may be present) | AU11 (nasolabial furrow deepener) | Not stimulated (but may be present) |
| Zygomatic major | Elevates lip corners superiorly and draws lip corners laterally, increasing angle of the mouth (AU12: lip corner puller) | Elevates lip corners superiorly and draws lip corners laterally, increasing angle of the mouth (AU12: lip corner puller) | Elevates lip corners superiorly and draws lip corners laterally, increasing angle of the mouth (AU12: lip corner puller) |
| Buccinator | Not stimulated (but present) | Not stimulated (but present) | Not stimulated (but present) |
| Depressor anguli oris | Depresses lip corners (AU15: lipcorner depressor) | Depresses lip corners (AU15: lip corner depressor) | Depresses lip corners (AU15: lip corner depressor) |
| Depressor labii inferioris | Depresses medial portion of lower lip (AU16: lower lip depressor) | Depresses medial portion of lower lip (AU16: lower lip depressor) | Depresses medial portion of lower lip (AU16: lower lip depressor) |
| Mentalis | Pushes skin of the chin boss superiorly (AU17: chin raiser) | Pushes skin of the chin boss superiorly (AU17: chin raiser) | Pushes skin of the chin boss superiorly (AU17: chin raiser) |
| Orbicularis oris | Reduces lip aperture and protrudes lips causing pursing (AU18: lip pucker) | Tightens lip margins (AU23: lip tightener). Other FACS movements not stimulated (AU18:lip pucker, AU22: lip funneler,AU24: lip presser; AU28: lip suck) | Reduces lip aperture and funnels/protrudes lips (AU22: lip funneller) |
Acknowledgements
This research was supported by grant NS-39489 from NIH/NINDS (to AJF) and MH070836 (to KMG). We would like to thank Prisca Zimmerman, Robert Gibboni III, and Clayton Mosher (University of Arizona), for help with the experiment, Marc Mehu for providing FACS coding and Paul Waby (University of Portsmouth) for technical assistance. We also thank Tim Smith for producing Figure 1, and Sarah-Jane Vick for providing extensive and invaluable comments on the manuscript.
Appendix 1
Glossary of terms
Alar
Relating to the nostrils
Anterior
At a position towards the front of another structure
Fascia
Fibrous tissue
Galea aponeurotica
Fibrous membrane underlying the scalp
Glabella
Space between the eyebrows
Inferior
At a position below another structure
Infraorbital furrow
Furrow below eye, from inner eye corner to cheekbone. See FACS.
Infraorbital triangle
Area above nasolabial furrow and below infraorbital furrow. See FACS.
Lateral
At a position farther from the midline of the body than another structure
Mandible
Lower jaw bone
Maxilla
Upper jaw bone
Medial
At a position closer to the midline of the body than another structure
Modiolus
Muscular node at the corner of the mouth
Nasolabial furrow
Furrow from nostril corner to its termination above, at or below the mouth corner. See FACS.
Nuchal
Relating to the back of the neck
Posterior
At a position behind (more dorsal than) another structure
Supercilliary arch
Eyebrow
Superior
At a position above another structure
Zygomatic arch
Cheekbone
Appendix 2
Supplemental Video Files
01 Medial frontalis
02 Lateral frontalis
03 Procerus
04 Corrugator supercilli
05 Orbicularis oculi
06 Levator labii superioris alaeque nasi
07 Levator labii superioris
08 Zygomatic major (frontal view)
09 Zygomatic major (profile view)
10 Nasalis
11 Depressor anguli oris
12 Mentalis
13 Orbicularis oris
14 Depressor labii inferioris
15 Orbito auricularis
16 Superior auricularis
17 Inferior auricularis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Counting medial and lateral frontalis as two muscles
References
- 1.Guridi J, Herrero MT, Luquin MR, Guillen J, Ruberg M, Laguna J, Vila M, Javoy-Agid F, Agid Y, Hirsch E, Obeso JA. Subthalamotomy in parkinsonian monkeys. Behavioural and biochemical analysis. Brain. 1996;119(Pt 5):1717–1727. doi: 10.1093/brain/119.5.1717. [DOI] [PubMed] [Google Scholar]
- 2.Imbert C, Bezard E, Guitraud S, Boraud T, Gross CE. Comparison of eight clinical rating scales used for the assessment of MPTP-induced parkinsonism in the Macaque monkey. J Neuro Meth. 2000;96(1):71–76. doi: 10.1016/s0165-0270(99)00184-3. [DOI] [PubMed] [Google Scholar]
- 3.Chassain C, Eschalier A, Durif F. Assessment of motor behavior using a video system and a clinical rating scale in parkinsonian monkeys lesioned by MPTP. J Neuro Meth. 2001;111(1):9–16. doi: 10.1016/s0165-0270(01)00425-3. [DOI] [PubMed] [Google Scholar]
- 4.Samadi P, Gregoire L, Rassoulpour A, Guidetti P, Izzo E, Schwarcz R, Bedard PJ. Effect of kynurenine 3-hydroxylase inhibition on the dyskinetic and antiparkinsonian responses to levodopa in Parkinsonian monkeys. Movement Disord. 2005;20(7):792–802. doi: 10.1002/mds.20596. [DOI] [PubMed] [Google Scholar]
- 5.Tir M, Devos D, Blond S, Touzet G, Reyns N, Duhamel A, Cottencin O, Dujardin K, Cassim F, Destee A, Defebvre L, Krystkowiak P. Exhaustive, one-year follow-up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of parkinsonian patients. Neurosurg. 2007;61(2):297–304. doi: 10.1227/01.NEU.0000285347.50028.B9. discussion 304–305. [DOI] [PubMed] [Google Scholar]
- 6.Andringa G, Stoof JC, Cools AR. Sub-chronic administration of the dopamine D-1 antagonist SKF 83959 in bilaterally MPTP-treated rhesus monkeys: stable therapeutic effects and wearing-off dyskinesia. Psychopharmacol. 1999;146(3):328–334. doi: 10.1007/s002130051124. [DOI] [PubMed] [Google Scholar]
- 7.Schneider JS, Gonczi H, Decamp E. Development of levodopa-induced dyskinesias in parkinsonian monkeys may depend upon rate of symptom onset and/or duration of symptoms. Brain Res. 2003;990(1–2):38–44. doi: 10.1016/s0006-8993(03)03382-1. [DOI] [PubMed] [Google Scholar]
- 8.Andringa G, Eshuis S, Perentes E, Maguire RP, Roth D, Ibrahim M, Leenders KL, Cools AR. TCH346 prevents motor symptoms and loss of striatal FDOPA uptake in bilaterally MPTP-treated primates. Neurobiol Disease. 2003;14(2):205–217. doi: 10.1016/s0969-9961(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZM, Andersen AH, Ai Y, Loveland A, Hardy PA, Gerhardt GA, Gash DM. Assessing nigrostriatal dysfunctions by pharmacological MRI in parkinsonian rhesus macaques. Neuroimage. 2006;33(2):636–643. doi: 10.1016/j.neuroimage.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Huber E. Evolution of Facial Musculature and Facial Expression. Oxford: Oxford University Press; 1931. [Google Scholar]
- 11.Burrows AM, Waller BM, Parr LA. Primate facial expression musculature: comparative anatomy and implications for the evolution of communication. Int J Primatol. 2006;27 Suppl. 1 abstract 112. [Google Scholar]
- 12.Burrows AM, Smith TD. Muscles of facial expression in Otolemur, with a comparison to Lemuroidea. Anat Rec Part A. 2003;274A:827–836. doi: 10.1002/ar.a.10093. [DOI] [PubMed] [Google Scholar]
- 13.Burrows AM, Waller BM, Parr LA, Bonar CJ. Muscles of facial expression in the chimpanzee (Pan troglodytes): Descriptive, comparative, and phylogenetic contexts. J Anat. 2006;208(2):153–168. doi: 10.1111/j.1469-7580.2006.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waller BM, Vick SJ, Parr LA, Bard KA, Smith Pasqualini MC, Gothard K, Fuglevand AJ. Intramuscular stimulation of facial muscles in humans and chimpanzees: Duchenne revisited. Emotion. 2006;6(3):367–382. doi: 10.1037/1528-3542.6.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vick SJ, Waller BM, Parr LA, Smith Pasaqualini MC, Bard KA. A cross-species comparison of facial morphology and movement in humans and chimpanzees using the facial action coding system (FACS) J Nonverb Behav. 2007;31:1–20. doi: 10.1007/s10919-006-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekman P, Friesen WV, Hager JC. Facial Action Coding System. Salt Lake City: Research Nexus; 2002. [Google Scholar]
- 17.Russell WMS, Burch RL. The Principles of Humane Experimental Technique. London: Methuen; 1959. [Google Scholar]
- 18.Ekman P, Friesen WV, Hager JC. Facial Action Coding System: The Investigators Guide. Salt Lake City: Research Nexus; 2002. [Google Scholar]
- 19.Oster H, Baby FACS. Unpublished monograph and coding manual. New York University; 2007. Facial Action Coding System for Infants and Young Children. [Google Scholar]
- 20.Parr LA, Waller BM, Vick SJ, Bard KA. Classifying chimpanzee facial expressions by muscle action. Emotion. 2007;7(1):172–181. doi: 10.1037/1528-3542.7.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophys. 2007;97(2):1671–2168. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial expression and gaze selective responses in the monkey amygdale. Curr Biol. 2007;17(9):766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 23.Waller BM, Dunbar RIM. Differential behavioural effects of Silent Bared Teeth Display and Relaxed Open Mouth Display’ in chimpanzees (Pan troglodytes) Ethology. 2005;111:129–142. [Google Scholar]
- 24.Waller BM, Cray JJ, Burrows AM. Selection for universal facial emotion. Emotion. doi: 10.1037/1528-3542.8.3.435. (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.