Abstract
Background
There is extensive variation in gene expression among individuals within and between populations. Accurate measures of the variation in mRNA expression using microarrays can be confounded by technical variation, which includes variation in RNA isolation procedures, day of hybridization and methods used to amplify and dye label RNA for hybridization.
Methodology/Principal Findings
In this manuscript we analyze the relationship between the amount of mRNA and the fluorescent signal from the microarray hybridizations demonstrating that for a wide-range of mRNA concentrations the fluorescent signal is a linear function of the amount of mRNA. Additionally, the separate isolation, labeling or hybridization of RNA does not add significant amounts of variation in microarray measures of gene expression. However, single or double rounds of amplification for labeling do have small but significant affects on 10% of genes, but this source of technical variation is easy to avoid. To examine both technical and stochastic biological variation, mRNA expression was measured from the same five individuals over a six-week time course.
Conclusion
There were few, if any, meaningful differences in gene expression among time points. Thus, microarray measures using standard laboratory procedures can be precise and quantitative and are not subject to significant random biological noise.
Introduction
Microarrays simultaneously quantify several hundred to thousands of genes on a single glass slide and their use has greatly expanded the breadth of quantified gene expression [1]–[10]. Yet the preparation of RNA affects the precision of microarray measures and therefore the ability to accurately quantify the content of an RNA sample [11]. Additionally, differences in microarray platforms, laboratory procedures and post-quantification analyses affect the precision among arrays [12]–[15]. Thus, technical variation can substantially affect the interpretation of microarrays.
For the teleost fish Fundulus heteroclitus variation among individuals in mRNA expression is extensive: >60% of genes have significant differences in expression among individuals within a population [1], [9], [16], [17]. Many of these differences in gene expression are associated with variation in cardiac metabolism [9]. However, the accuracy and biological relevance of these differences in expression depends on the technical variation inherent to microarray processing [1].
Accurate microarray quantification is best realized when there is a linear relationship between fluorescence and RNA concentration. This linear relationship fails when the dynamic range of microarrays are exceeded. For any microarray, there are two parameters that define its dynamic range: the range of fluorescence that can be measured and the range of RNA concentrations that can bind to a specific array feature. These two components of the dynamic range reflect the two types of saturation that can occur on a microarray: photomultiplier tube (PMT) saturation and biological saturation. A linear relationship between fluorescence and RNA concentration can only occur if the cDNA on the microarray captures proportional amounts of RNA and if the PMT is not saturated.
The PMT measures the number of photons from the fluorescently labeled RNA that are excited by the lasers. PMT saturation is a result of the photomultiplier tube becoming oversaturated due to an overabundance of converted electrons by the analog to digital (A/D) converter. The A/D converter can only convert the PMT signal into a value less than or equal to 216-1 or 65,535 and thus any fluorescent photons captured at this value of 65,535 are not discernable [18]. This type of saturation can be avoided by reducing the PMT voltage and laser power. Alternatively, the specific activity of the mRNA (number of fluorescent molecules per message) can be reduced. However, the reduction of the PMT voltage, power of the lasers, or reduced labeling, does not address the question of whether or not a particular cDNA on a microarray is biologically saturated.
Biological saturation occurs when the amount of mRNA that can hybridize to the DNA on a microarray reaches a maximum binding capacity of the printed DNA. If biological saturation is reached, then the amount of a mRNA will be underestimated and differences among arrays or experiments can not be appropriately determined. To avoid biological saturation, the amount of target RNA must be present in quantities less than the amount that the cDNA on the microarray slide can bind. To determine the range and linear response of increasing amounts of mRNA, we hybridized a 500-fold concentration range of labeled RNA from cardiac tissue to the F. heteroclitus 384 cDNA metabolic microarray.
Sources of technical variation, other than PMT and biological saturation, come from methods used to fluorescently label the mRNA, the day on which the RNA is processed and varying amounts of available tissue [19], [20]. One of the most common approaches to fluorescently label mRNA for microarray studies is to amplify the RNA by synthesizing cDNA with a T7 RNA polymerase binding site. RNA is then synthesized in vivo by using the T7 RNA polymerase to incorporate amino allyls followed by covalent binding of fluorescent molecules to the incorporated amino allyls [21]. For small amounts of starting mRNA, the synthesis of RNA using T7 can be repeated to double the amplification. To understand the effect of a single round versus a double round of linear amplification we compared the quantification of RNA using both methods.
The day and process used to isolate mRNA are two additional sources of technical variation. Variation in the preparation of mRNA could alter its quality affecting how well the RNA amplifies, is fluorescently labeled, and the signal observed on the microarray. The day on which a tissue is sampled is not strictly technical but can introduce a second type of variation: biological variation. That is, isolating tissues on different days could introduce technical variation because of the precision of dissection and the quality of tissue or RNA preparation. However, because tissues are sampled on different days, the organisms may be biologically different (under more or less stress, healthier, or just one day older). To examine technical variation due only to RNA isolation, a single blood sample was divided into four, RNA was separately isolated from each sample and, gene expression was quantified. Biological variation was examined in a separate experiment where five fish were bled every two weeks for a total of six weeks in order to collect four separate samples from each individual. Gene expression was quantified for these four temporally separate samples.
These experiments indicate that for a wide range of experimental conditions, microarray experiments using the Fundulus array are both accurate and precise.
Materials and Methods
Organism
Fundulus heteroclitus were caught from wild populations in Wiscasset, Maine, USA (43°57′41″N, 69°42′45″W) by minnow trap. Fish were transported to the Rosenstiel School of Marine and Atmospheric Science at the University of Miami and acclimated to 20°C and 15ppt for approximately 6 months.
Blood Sampling
Fundulus heteroclitus (N = 20) were anesthetized with MS222 (0.1 g·l−1) and given tags with subdermal latex markers. Whole blood samples from each fish were taken every two weeks by caudal puncture using a 50 µl Hamilton syringe rinsed with heparinzed saline (50 i.u. ·ml−1). Samples were immediately frozen in liquid N2 and stored at −80°C. Only individuals that had all four serial samples taken (N = 5) were used in the present study.
RNA isolation and amino allyl labeling
Total RNA was isolated using 4.5 M guanidinium thiocyanate, 2% N-lauroylsarcosine, 50 mM EDTA, 25 mM Tris-HCl, 0.1 M β-Mercaptoethanol and 2% Antifoam A. The extracted RNA was further purified using a Qiagen RNeasy Mini kit in accordance with the manufacturer's protocols. The quantity and quality of the RNA was determined using a spectrophotometer (Nanodrop, ND-1000 V3.2.1) and a bioanalyzer (Agilent 2100). RNA was then converted into amino allyl labeled RNA (aRNA) using the Ambion Amino Allyl MessageAmp II aRNA Amplification kit. This method converts poly-A RNA into cDNA with a T7 RNA polymerase binding site; T7 is then used to synthesize new strands of RNA (in vitro transcription)[22]. During this in vitro transcription of aRNA, an amino allyl UTP (aaUTP) is incorporated into the elongating strand. aaUTP incorporation allows for the coupling of Cy3 or Cy5 dyes (GE biosciences) onto aRNA for microarray hybridization.
Dye labeled aRNA aliquots for each hybridization (typically 30 pmol each of Cy3 and Cy5) were vacuum dried together and resuspended in 15 µl hybridization buffer (final concentration of each labeled sample = 2 pmol/µl). Hybridization buffer consisted of 5× SSPE, 1% SDS, 50% formamide, 1 mg/ml polyA, 1 mg/ml sheared herring sperm carrier DNA, and 1 mg/ml BSA. Slides were washed in sodium borohydride solution in order to reduce autofluorescence. Following rinsing, slides were boiled for 2 minutes and spin-dried in a centrifuge at 800 rpm for 3 minutes. Samples (15 µl) were heated to 90°C for 2 minutes, quick cooled to 42°C, applied to the slide (hybridization zone area was 350 mm2), and covered with a cover slip. Slides were placed in an airtight chamber humidified with paper soaked in 5× SSPE and incubated 24–48 hours at 42°C.
Microarrays
mRNA expression was measured using microarrays where each array had four spatially separated replicates per gene. The 384 F. heteroclitus cDNA microarrays were printed using 55 control genes and 329 cDNAs which encode essential proteins for cellular metabolism (Table 1). The annotation of genes and related pathways used FunnyBase [23] and these were manually compared to KEGG pathway designations. Because many genes belong to more than one pathway, central metabolic pathways were preferentially used if the gene coded for a protein that was a catabolic or anabolic enzyme (versus acting in a signaling pathway that affected metabolism). Controls include DNA spots labeled with Cy5 (positive control for position and gridding) and Ctenophore cDNA as negative controls.
Table 1. 384 Array Metabolic Pathways.
| Amino acid metabolism | 28 |
| ATP synthesis | 27 |
| Blood group glycolipid biosynthesis | 3 |
| Channel | 3 |
| Citrate cycle (TCA cycle) | 24 |
| Fatty acid metabolism/transport | 36 |
| Fructose and mannose metabolism | 4 |
| Galactose metabolism | 2 |
| Glutamate metabolism | 7 |
| Glutathione metabolism | 10 |
| Glycerolipid metabolism | 7 |
| Glycolysis/Gluconeogenesis | 27 |
| Inositol phosphate metabolism | 14 |
| Ox-Phos-ATPsyn | 64 |
| Pentose phosphate pathway | 6 |
| Purine & Pyrimidine metabolism | 9 |
| Pyruvate metabolism | 2 |
| Signaling pathway | 10 |
| Starch and sucrose metabolism | 2 |
| Sterol biosynthesis | 8 |
| Synthesis and degrad. of ketone bodies | 4 |
| Tetrachloroethene degradation | 3 |
| Secondary | 27 |
| TOTAL METABOLIC GENES | 329 |
Microarrays were created by printing cDNAs amplified with amine-linked primers onto 3-D Link Activated slides (Surmodics Inc., Eden Prairie, MN) at the University of Miami's microarray facility. All printed cDNAs were re-sequenced from the same source used for printing. The microarray slides were scanned using ScanArray Express. The raw TIFF-image data was quantified using Imagene (v5).
All experiments used a loop design for hybridization of dye labeled aRNA [24], [25]. In a loop design [24], [25] each individual is labeled with Cy3 and Cy5. Each dye labeled sample is then hybridized on different arrays with another individual [26]. Thus, each individual is hybridized to two arrays with four replicates per array for a total of eight technical replicates per individual. This experimental design is a more efficient use of resources, providing more data per array and is thus statistically more powerful than a reference design.
To test for the relationship between fluorescence and the quantity of RNA, five concentrations of fluorescently labeled RNA were used: 1.2 to 700 pmol of Cy3 or Cy5 labeled mRNA where pmol are for the amount of incorporated dye (Table 2). A 15 µl hybridization using the 384 cDNA array corresponds to 0.09 to 47 µM of Cy dye. Cy5 dye labeled RNA was used at concentrations 18% less than Cy3 because the Cy5 dye is a more efficient fluorophore (greater fluorescence per photon) than the Cy3 dye. The average of eight fluorescence values for each gene was normalized to the original concentration of RNA added.
Table 2. Concentrations of Cy3 and Cy5 dye labeled RNA used for hybridization.
| 50× | 10× | 5× | 1× | .1× | |
| Cy 3 | 700 pmol | 140 pmol | 70 pmol | 14 pmol | 1.4 pmol |
| Cy 5 | 583.3 pmol | 116.6 pmol | 58.3 pmol | 11.6 pmol | 1.2 pmol |
Criteria for Inclusion
For a gene to be included in an analysis, the average signal among all arrays and dyes had to exceed background but not exceed 95% of PMT saturation (65,535). Background signal was determined as the amount of fluorescence in negative control array elements. Not all genes met these criteria and therefore were not included in the analysis.
Statistics
To adjust for systematic variation, gene expression values were first sum normalized, log2 transformed, and then loess normalized using Microarray Data Analysis System Software (MIDAS) [12], [27] and SAS JMP Genomics v.6.0.2. For every gene, eight fluorescence values were captured; four Cy3 values and four Cy5 values. Analysis of variance (ANOVA) was performed using SAS JMP Genomics v.6.0.2. To look for differences between single and double rounds of amplification the following ANOVA model was applied: yijkl = μ+Ai+Dj+k+Rl+εijkl where μ is the sample mean, Ai is the effect of the i th array (i = 1–18), Dj is the effect of the j th dye (Cy3 or Cy5), Tk is the effect of the number of rounds of amplification (single or double, k = 2), Rl is the effect of the day on which samples were prepared (l = 3), and epsilon is stochastic effects. The number of rounds of amplification (single or double) and channel variables were treated as fixed effects and array, and day on which samples were prepared were treated as random effects. Statistical analyses of replicate blood samples or repetitive measures of the same five individuals were applied to a separate ANOVA for each individual. The ANOVA model for this comparison was as follows: ymnp = μ+Am+Dn+Tp+εmnp where μ is the sample mean, Am is the effect of the m th array (m = 1–4 for both replicate and repetitive samples), Dn is the effect of the n th dye (Cy3 or Cy5), Tp is the treatment effect and epsilon is stochastic effects. Sample, representative of either one of four temporal samples from an individual or one of four replicate blood samples, and channel were treated as fixed effects. Array was treated as a random effect. Significant differences were evaluated with a p-value cut-off of 0.01.
Results
Biosaturation
The concentration of fluorescently labeled RNA (0.09 to 47 µM of Cy dye) represents 0.1×, 1×, 5×, 10×, 50× the concentration of RNA typically used on F. heteroclitus cDNA microarrays [9], [26], [28]–[30] (Table 2, MIAME GSE12858). Among the 329 metabolic genes on the array, 212 of these genes met our criteria of being less than 95% of the PMT saturation and more than two standard deviations above the negative controls (Ctenophore cDNA with no similarity to vertebrate genes).
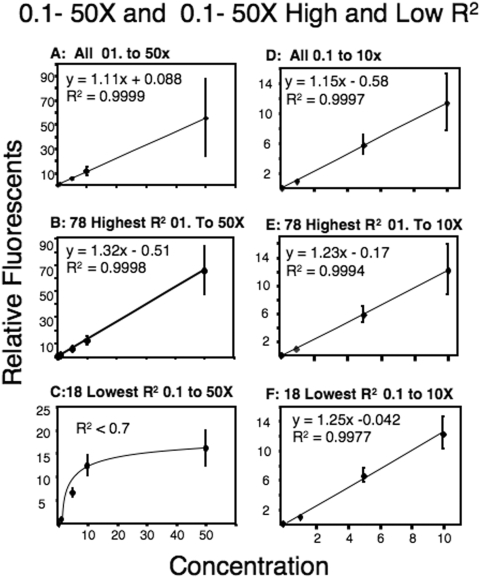
The linear relationship between the amount of RNA and relative fluorescence is shown in Figure 1. To remove the gene specific differences in expression, the fluorescence at each concentration was divided by the mean fluorescence for that specific gene (Fig. 1). The linear relationship between the amount of total fluorescent RNA added and the measures of gene specific fluorescence was determined for each gene. Most genes (176/212 or 83%) had an R2>95% and 78 genes had a nearly perfect R2 (>0.995; Fig. 1B; Table 3). Examining the 18 genes with the lowest R2 values (less than 0.8) revealed a non-linear relationship that can be explained by an apparent saturation at the 50× concentration of RNA (Fig. 1C). The relationship disappears if the fluorescence values for the 50× concentrations of RNA are removed and the 0.1 to 10× are plotted (Fig 1D–F). In the 100-fold range (0.1 to 10×) only three genes (1.4%) had R2 values less than 0.8 (Table 3). Examination of the higher concentrations (1.0 to 50×) revealed 19 genes (9%) with R2 less than 0.8 (Table 3). These data suggest that for most genes there is a linear relationship for a 500-fold range of RNA, however some cDNAs on the microarray will reach biological saturation at the highest RNA concentration.
Figure 1. Linear relationship of RNA concentration to relative fluorescence.
Graphs show linear relationship between concentrations of RNA (0.1–50×, A–C, and 0.1–10×, D–F) and relative fluorescence. Relative fluorescence is a normalized measure of fluorescence divided by the gene specific mean. 1× RNA is equal to 0.9 pmol/µl. Shown are the RNA concentrations versus fluorescence for 0.1 to 50× (A–C) and for 0.1× to 10× (D–F); for all genes (A and D), for the 78 genes with the highest R2 values (B and E), and for the 18 with lowest R2 values (C and F).
Table 3. Number of genes and corresponding R2 for various ranges of RNA concentrations.
| R2 | 0.1–50× | 0.1–10× | 1.0–50× |
| >0.9 | 176 | 199 | 178 |
| <0.8 | 18 | 3 | 19 |
Variation in RNA preparation
To determine how RNA preparation affects variation, cardiac RNA from three individuals were combined, and then evenly divided and amino allyl and dye labeled on three separate days using single and double rounds of amplification (MIAME, GSE12858). Only 110 genes met our criteria for inclusion because many genes were below the low cut-off (Ctenophore negative control cDNAs). In this experiment fewer genes met our criteria of above background and below saturation due to sample RNA being divided for separate labeling using either single and double rounds of amplification. An ANOVA was performed to measure differences between single and double rounds of amino allyl labeled RNA amplification. Twelve of the 110 genes (11%) used in this analysis were significantly different between single and double rounds of amplification at p<0.01. The majority of genes (59%) had a higher fluorescence signal when only one round of amplification was performed.
Consistency of Quantitative Determination
In teleost fish, red blood cell (RBCs) nuclei are transcriptionally active [31], [32], and these cells can be sampled without sacrificing the fish. Thus to assess the consistency of microarray determinations, two experiments were performed on blood gene expression: 1) to examine technical variation a single sample of blood was divided into four samples; RNA isolations, amino allyl and dye labeling, hybridization and quantitative analyses were performed on each sample and 2) to examine biological variation, RNA isolated from blood from the five individuals were each sampled four times over a 6 week period (two weeks between samples; MIAME, TBA).
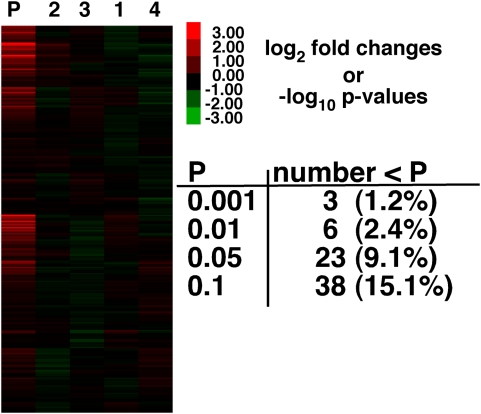
A one-way ANOVA was used to test for the technical variation in gene expression between the four RNA samples isolated from a single blood sample (Fig. 2). Among all 252 genes (eight replicates per gene per sample) only 6 genes were significantly different for the four isolates at a critical p-value of 0.01. Three false-positives are expected at a p-value of 0.01 and thus with only 6 significant differences (Fig. 2) there is little evidence that separate RNA isolation, labeling and hybridization has much affect on measures of gene expression. The lack of differences is not due to high technical variation: CV (standard deviation/mean) among the eight replicates was 4% and, only three genes had a CV of >10%. Nor was it due to the low p-value of 0.01 versus 0.05 (Fig. 2); the number of significant differences simply reflects the p-values.
Figure 2. Gene expression for Single Blood isolate.
Heat map for single blood isolate that was divided into four. RNA was purified, labeled and hybridized separately for each sample. Red is greater and green is less than the average gene specific fluorescence. First column (P) is the p-value from a one-way ANOVA. Only 6 genes (2.3%) out of 252 are significant at a critical p-value of 0.01. P-values (−log10) shown in the heat map are from an ANOVA for significant differences among samples using the 8 replicates for each separate RNA isolation. Color bar gives fold difference for log2 gene expression (e.g., 2 = 4×) and negative log10 p-value (e.g., 2 = p-value of 0.01).
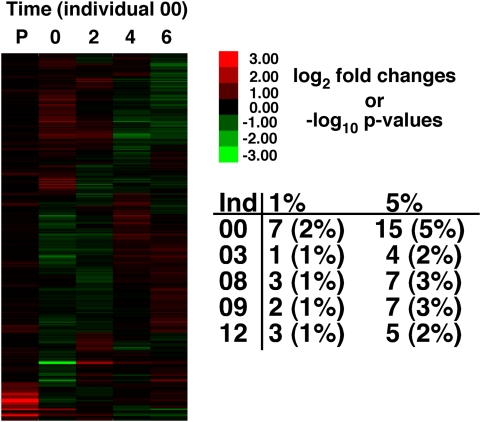
Random biological variation can contribute to differences in expression. We tested for random biological variation by bleeding the same five individuals four times with two weeks between bleedings (Fig. 3, MIAME, TBA). For each of the 304 genes that met our criteria, an ANOVA tested for differences in expression among the four different time periods for each individual (four sample periods with eight replicates per gene per sample period). Among the four temporal samples, there were between one and seven genes that had a significant difference in expression at a p-value of 0.01 (Fig. 3). Only one individual had more than the expected number of false positives at the critical p-value: individual-00 had 7 (2%) significant genes at p-value 0.01 for 304 genes.
Figure 3. Individuals sampled over time.
Heat map for one individual (00) sampled 4 times over a total of 6 weeks. Numbers above the heat map are time points (0, 2, 4 & 6 weeks) and the “P” is for p-value (−log10). P-values are from the ANOVA that tested for differences among separate blood isolations within an individual (4 isolations and 8 replicates per isolation). For gene expression, red is greater and green is lower expression than the mean expression for each gene. Table provides number of significant genes and percent (rounded up) out of the total of 304. Color bar gives fold difference for log2 gene expression (e.g., 2 = 4×) and negative log10 p-value (e.g., 2 = p-value of 0.01).
Discussion
Understanding sources of variation in gene expression is important for determining the biological importance of measured differences in mRNA expression. The analyses of technical variation in the metabolic F. heteroclitus cDNA microarray suggest that measures of gene expression using the F. heteroclitus 384 cDNA microarray are quantitative and precise. This conclusion is based on the observation that there is a linear increase in fluorescence with increasing mRNA (Fig. 1), and that there is little additional variation due to RNA processing (Fig. 2) or the day on which RNA is isolated (Fig. 3).
There is a linear increase in fluorescence with increasing mRNA for 98.5% of genes between 0.1× to 10× concentrations (0.09 pmol/ul to 9.3 pmol/ul) and 95% of genes between 0.1× to 50× (0.09 pmol/ul to 47 pmol/ul). The linear relationship between RNA and fluorescence is quite strong for RNA concentrations of 0.1× to 10× having average R2 values of 0.97, and most genes (88%) have R2 values greater than 0.95 for these four concentrations. The genes most affected by biological saturation do not have a high fluorescence; if anything, they are less than the average (genes with R2<0.8 for 1× to 50× have a mean that is 60% of the mean for all other genes). The two possible explanations for biological saturation with low fluorescence are that the synthesis of amino allyl labeled RNA for these genes is strongly truncated or that there is less DNA printed on the array for these genes. Truncation of amino allyl labeling would produce many more short probes with few labels per probe. Thus, to produce a similar fluorescence many more molecules would be necessary and these would saturate the DNA on the array. These problems can be avoided by using moderate amounts of probe (<10 pmol/ul). We typically avoid this problem by using 0.7 to 2 pmol/ul. Using concentrations of RNA up to 50× (47 pmol/ul) is feasible, but our data suggest that at this high of a concentration some genes will biologically saturate the cDNA on the array and therefore should be avoided.
If RNA samples are amino allyl labeled using one round of T7-RNA synthesis [22] versus two rounds of T7-RNA synthesis, 11% of genes have significant differences in fluorescence at a p-value of 0.01. Although this difference in gene expression for single versus double labeling is not large, it may be unacceptably high. Thus, we would suggest that for any one experiment that a researcher uses only single or double labeling procedures but not both within an individual experiment. Approximately half (59%) of genes with a significant difference between single and double labeling were greater for single labeling. The greater fluorescence for single labeling than that for double labeling would occur if cDNA or RNA synthesis was truncated with each round of labeling. Truncation would occur if the synthesis of cDNA or RNA were incomplete forming shorter nucleotide sequences with less fluorescence per RNA.
We used blood to test the effect of different RNA isolations, amino allyl labeling and hybridizations. The first experiment used a single blood isolation that was divided into four equal samples. There are few differences in expression, 2.4% at a p-value of 0.01 (i.e., six versus the expected three false positives). If a Bonferroni correction was applied none of these genes would be significant. Therefore, technical errors do not necessarily contribute significant amounts of variation. Similar conclusions were made about microarray results among laboratories: many different laboratories yielded similar results using different varieties of platforms [13]–[15], [33]–[36]. However, a few laboratories yielded different results. Together these data suggest that good experimental practice can minimize the effect of technical variation.
In a separate experiment, five individuals were bled once every two weeks during a six-week period, resulting in four serial blood samples from each individual. Any differences in expression among sampling times could be due to technical variation, of which there is very little as shown by the previous experiment, or biological variation. That is, although fish appeared healthy, had normal blood glucose, and the stress hormone, cortisol, did not vary significantly (p>0.1), gene expression could vary significantly for unknown biological reasons. Yet, for the five individuals there are few, if any, meaningful differences in gene expression (only one individual had more than the expected number of false positives, Fig. 3). These data confirm the observation that technical errors do not necessarily affect microarray measures. Importantly, these data also suggest that for a tissue or blood sample there is little random stochastic variation in gene expression. These data are in contrast to other publications suggesting that mRNA expression is noisy and has large stochastic variation [37], [38]. The important distinction is that for a single cell, transcription is pulsatile, occurring in bursts [37], [38], and for an individual cell this creates large stochastic variation in mRNA expression. However, our results demonstrate that for millions of cells, this variation is not apparent across a 6-week time course. We suggest that if there is a large stochastic variation in each cell, sampling of millions of cells masks this variation such that the amount of expression from any one gene is stable over time.
The microarrays used here have array elements for essential metabolic genes (Table 1) and are similar to the array elements used in previous work demonstrating larger inter-individual variation in gene expression [1], [9], [16], [17]. While the data presented here addresses the sources of variation in many microarray experiments, the lack of temporal variation in gene expression in our study may only reflect the expression of the metabolic genes. However, these results are similar to studies of gene expression in humans where the same individuals were sampled over a time period of 24 hours to four weeks [39]–[41]. These studies also found relative stable expression of a more diverse set of genes when the same individuals were sampled over time. Thus, although there are good biochemical or molecular reasons to expect stochastic variation in gene expression, this variation is not necessarily observed using routine sampling methods.
Microarrays are a useful technology for observing differences in gene expression and data extracted from microarrays can be reliably reproduced. With reasonable care, any experiment involving microarrays is capable of obtaining biological data that is not masked by technical variation thereby providing a true representation of the transcriptome under a particular set of conditions. However, caution is required before making conclusions about the biological nature of the data until the sources of technical variation are understood.
Acknowledgments
We thank Dr. M. F. Oleksiak for production of the Fundulus heteroclitus EST collection, statistical analysis, and creative thought.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by NSF/OCE 0221879; NIH/NHLBI HL65470-01A2, and NSF-NIEHS P50ES12736 and NSF OCE-0432368. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Oleksiak MF, Churchill GA, Crawford DL. Variation in gene expression within and among natural populations. Nature Genetics. 2002;32:261–266. doi: 10.1038/ng983. [DOI] [PubMed] [Google Scholar]
- 2.de Koning DJ, Haley CS. Genetical genomics in humans and model organisms. Trends in Genetics. 2005;21:377–381. doi: 10.1016/j.tig.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Townsend JP, Cavalieri D, Hartl DL. Population genetic variation in genome-wide gene expression. Molecular Biology and Evolution. 2003;20:955–963. doi: 10.1093/molbev/msg106. [DOI] [PubMed] [Google Scholar]
- 4.Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, et al. Genetics of gene expression surveyed in maize, mouse and man. Nature. 2003;422:297–302. doi: 10.1038/nature01434. [DOI] [PubMed] [Google Scholar]
- 5.Gibson G, Riley-Berger R, Harshman L, Kopp A, Vacha S, et al. Extensive sex-specific nonadditivity of gene expression in Drosophila melanogaster. Genetics. 2004;167:1791–1799. doi: 10.1534/genetics.104.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brem RB, Yvert G, Clinton R, Kruglyak L. Genetic dissection of transcriptional regulation in budding yeast. Science. 2002;296:752–755. doi: 10.1126/science.1069516. [DOI] [PubMed] [Google Scholar]
- 7.Enard W, Khaitovich P, Klose J, Zollner S, Heissig F, et al. Intra- and interspecific variation in primate gene expression patterns. Science. 2002;296:340–343. doi: 10.1126/science.1068996. [DOI] [PubMed] [Google Scholar]
- 8.Monks SA, Leonardson A, Zhu H, Cundiff P, Pietrusiak P, et al. Genetic inheritance of gene expression in human cell lines. American Journal of Human Genetics. 2004;75:1094–1105. doi: 10.1086/426461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oleksiak MF, Roach JL, Crawford DL. Natural variation in cardiac metabolism and gene expression in Fundulus heteroclitus. Nature Genetics. 2005;37:67–72. doi: 10.1038/ng1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yvert G, Brem RB, Whittle J, Akey JM, Foss E, et al. Trans-acting regulatory variation in Saccharomyces cerevisiae and the role of transcription factors. Nature Genetics. 2003;35:57–64. doi: 10.1038/ng1222. [DOI] [PubMed] [Google Scholar]
- 11.Baugh LR, Hill AA, Brown EL, Hunter CP. Quantitative analysis of mRNA amplification by in vitro transcription. Nucleic Acids Research. 2001;29 doi: 10.1093/nar/29.5.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quackenbush J. Microarray data normalization and transformation. Nature Genetics. 2002;32:496–501. doi: 10.1038/ng1032. [DOI] [PubMed] [Google Scholar]
- 13.Irizarry RA, Warren D, Spencer F, Kim IF, Biswal S, et al. Multiple-laboratory comparison of microarray platforms. Nature Methods. 2005;2:345–349. doi: 10.1038/nmeth756. [DOI] [PubMed] [Google Scholar]
- 14.Larkin JE, Frank BC, Gavras H, Sultana R, Quackenbush J. Independence and reproducibility across microarray platforms. Nature Methods. 2005;2:337–343. doi: 10.1038/nmeth757. [DOI] [PubMed] [Google Scholar]
- 15.Bloom G, Yang IV, Boulware D, Kwong KY, Coppola D, et al. Multi-platform, multi-site, microarray-based human tumor classification. American Journal of Pathology. 2004;164:9–16. doi: 10.1016/S0002-9440(10)63090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehead A, Crawford DL. Variation within and among species in gene expression: raw material for evolution. Molecular Ecology. 2006;15:1197–1211. doi: 10.1111/j.1365-294X.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead A, Crawford DL. Neutral and adaptive variation in gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5425–5430. doi: 10.1073/pnas.0507648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YH, Buckley MJ, Dudoit S, Speed TP. Comparison of methods for image analysis on cDNA microarray data. Journal of Computational and Graphical Statistics. 2002;11:108–136. [Google Scholar]
- 19.Gold D, Coombes K, Medhane D, Ramaswamy A, Ju ZL, et al. A comparative analysis of data generated using two different target preparation methods for hybridization to high-density oligonucleotide microarrays. Bmc Genomics. 2004;5:-. doi: 10.1186/1471-2164-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Haaften RIM, Schroen B, Janssen BJA, van Erk A, Debets JJM, et al. Biologically relevant effects of mRNA amplification on gene expression profiles. Bmc Bioinformatics. 2006;7:-. doi: 10.1186/1471-2105-7-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vangelder RN, Vonzastrow ME, Yool A, Dement WC, Barchas JD, et al. Amplified Rna Synthesized from Limited Quantities of Heterogeneous Cdna. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eberwine J. Amplification of mRNA populations using aRNA generated from immobilized oligo(dT)-T7 primed cDNA. Biotechniques. 1996;20:584–&. doi: 10.2144/19962004584. [DOI] [PubMed] [Google Scholar]
- 23.Paschall JE, Oleksiak MF, VanWye JD, Roach JL, Whitehead JA, et al. FunnyBase: a Systems Level Functional Annotation of Fundulus ESTs for the Analysis of Gene Expression. BMC Genomics. 2004;5:96. doi: 10.1186/1471-2164-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr MK, Churchill GA. Experimental design for gene expression microarrays. Biostatistics. 2001;2:183–201. doi: 10.1093/biostatistics/2.2.183. [DOI] [PubMed] [Google Scholar]
- 25.Kerr MK, Churchill GA. Statistical design and the analysis of gene expression microarray data. Genetical Research. 2001;77:123–128. doi: 10.1017/s0016672301005055. [DOI] [PubMed] [Google Scholar]
- 26.Oleksiak MF, Churchill GA, Crawford DL. Variation in gene expression within and among natural populations. Nature Genetics. 2002;32:261–266. doi: 10.1038/ng983. [DOI] [PubMed] [Google Scholar]
- 27.Dudoit S, Gendeman RC, Quackenbush J. Open source software for the analysis of microarray data. Biotechniques. 2003:45–51. [PubMed] [Google Scholar]
- 28.Whitehead A, Crawford D. Variation in tissue-specific gene expression among natural populations. Genome Biology. 2005;6:R13.11–13.14. doi: 10.1186/gb-2005-6-2-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitehead A, Crawford DL. Neutral and adaptive variation in gene expression. Proc Natl Acad Sci U S A. 2006;103:5425–5430. doi: 10.1073/pnas.0507648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford DL, Oleksiak MF. The biological importance of measuring individual variation. J Exp Biol. 2007;210:1613–1621. doi: 10.1242/jeb.005454. [DOI] [PubMed] [Google Scholar]
- 31.Currie S, Tufts BL. Synthesis of stress protein 70 (Hsp70) in rainbow trout (Oncorhynchus mykiss) red blood cells. Journal of Experimental Biology. 1997;200:607–614. doi: 10.1242/jeb.200.3.607. [DOI] [PubMed] [Google Scholar]
- 32.Koldkjaer P, Pottinger TG, Perry SF, Cossins AR. Seasonality of the red blood cell stress response in rainbow trout (Oncorhynchus mykiss). Journal of Experimental Biology. 2004;207:357–367. doi: 10.1242/jeb.00747. [DOI] [PubMed] [Google Scholar]
- 33.Bammler T, Beyer RP, Bhattacharya S, Boorman GA, Boyles A, et al. Standardizing global gene expression analysis between laboratories and across platforms. Nature Methods. 2005;2:351–356. doi: 10.1038/nmeth754. [DOI] [PubMed] [Google Scholar]
- 34.Tan PK, Downey TJ, Spitznagel EL, Xu P, Fu D, et al. Evaluation of gene expression measurements from commercial microarray platforms. Nucleic Acids Research. 2003;31:5676–5684. doi: 10.1093/nar/gkg763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yauk CL, Berndt ML, Williams A, Douglas GR. Comprehensive comparison of six microarray technologies. Nucleic Acids Research. 2004;32:-. doi: 10.1093/nar/gnh123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beissbarth T, Fellenberg K, Brors B, Arribas-Prat R, Boer JM, et al. Processing and quality control of DNA array hybridization data. Bioinformatics. 2000;16:1014–1022. doi: 10.1093/bioinformatics/16.11.1014. [DOI] [PubMed] [Google Scholar]
- 37.Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. Plos Biology. 2006;4:1707–1719. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blake WJ, Kaern M, Cantor CR, Collins JJ. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- 39.Cobb JP, Mindrinos MN, Miller-Graziano C, Calvano SE, Baker HV, et al. Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci U S A. 2005;102:4801–4806. doi: 10.1073/pnas.0409768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eady JJ, Wortley GM, Wormstone YM, Hughes JC, Astley SB, et al. Variation in gene expression profiles of peripheral blood mononuclear cells from healthy volunteers. Physiol Genomics. 2005;22:402–411. doi: 10.1152/physiolgenomics.00080.2005. [DOI] [PubMed] [Google Scholar]
- 41.Whitney AR, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, et al. Individuality and variation in gene expression patterns in human blood. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1896–1901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]