Summary
Recently there has been renewed interest in poxvirus pathogenesis, especially with regard to infection via the respiratory route. Members of this family are known to produce a number of proteins that have the potential to negatively regulate the immune response. Vaccinia virus (VACV) has been used for a number of years as a model for the study of poxvirus infection. We have previously reported a dose dependent decrease in virus-specific CD8+ T cells following respiratory infection with VACV. In this study we have evaluated whether more generalized immunosuppressive effects are also observed following infection with a high dose of VACV. We have found that mice infected intranasally with a high, but non-lethal, dose of VACV exhibited significant weight loss as well as decreased thymocyte number. Although these mice mounted an immune response, there was a significant increase observed in bystander T and B cell apoptosis. While increased death was apparent in both naïve and activated/memory T cells populations, naive T cells appeared more sensitive to this effect. These findings are important for our understanding of poxvirus regulation of the immune response and extends our previous understanding of VACV mediated immunosuppression to include generalized apoptosis in the naïve and activated/memory repertoires.
Keywords: Vaccinia virus, respiratory infection, immunosuppression, apoptosis
1. Introduction
While it has been decades since smallpox has been eradicated, there is still a justifiable concern about its reemergence, either intentional or unintentional. Because the vaccination program ended worldwide in 1980, many are left unprotected against this deadly virus, which has a 30% mortality rate (Wollenberg and Engler,2004). Variola virus, which is normally transmitted via the respiratory route (Baron,2003), is quite stable in aerosol form and one can envision that during intentional release of aerosolized virus, individuals closest to the point of release would be exposed to high infectious doses via the respiratory route (Harper,1961). For this reason, it is important that we have a better understanding of the immune responses generated to high and lethal dose virus infections of the respiratory tract.
Vaccinia virus is the prototypic member of the poxvirus family and serves as the highly effective vaccine against variola virus infection. VACV has been used as a model dissect the cellular immune response to poxvirus infection, (e.g. (Belyakov et al.,2003; Harrington et al.,2002; Reading and Smith,2003; Wyatt et al.,2004; Xu et al.,2004)). Our previous studies using a recombinant vaccinia virus expressing the model antigen ovalbumin (VACV-Ova) revealed a dose-dependent decrease in the number of virus-specific CD8+ T cells present on day 12 following i.n. infection of C57BL/6 mice (Parks and Alexander-Miller,2002). Such a decrease was reported in another study following i.p. infection of BALB/c mice (Ramirez et al.,2000). The purpose of the studies described herein was to determine whether the negative regulatory effects observed for virus-specific CD8+ T cells extended to non-virus specific immune cells, i.e. bystander cells. For example, generalized apoptosis in peripheral lymphocytes has been reported following infection with a number of pathogens, including respiratory syncytial virus (RSV), lymphocytic choriomeningitis virus (LCMV), Ebola, SARS, measles virus, and influenza virus (Baize et al.,1999; Carrero et al.,2004; Hassan and Curtiss, III,1994; He et al.,2005; McNally et al.,2001; Okada et al.,2000; Roe et al.,2004; Tumpey et al.,2000).
C57BL/6 mice were intranasally infected with a low, high, or lethal dose of VACV-Ova, the virus construct used in our previous studies (Parks, G. D. and Alexander-Miller, M. A.,2002). High and lethal doses of VACV-OVA were found to result in increased apoptosis of all lymphocyte populations examined. These included CD4+ and CD8+ T lymphocytes as well as B cells. Apoptosis was observed in both CD44high and CD44low populations and thus did not correlate with the differentiation state of the cell. The loss observed with a high, but sublethal dose, was a systemic effect and did not appear to be due to the presence of infectious virus and was not a result of immunosuppressive glucocorticoids, fas/fasL interactions, type I IFN, IL-6, or IFNγ. These findings suggest that high dose poxvirus infection of the respiratory tract has the potential to result in a generalized loss of lymphocytes. Such a loss could potentially affect not only the generation of the immune response to the poxvirus, but to other pathogens with which the host may come into contact.
2. Materials and Methods
2.1 Mice
Female C57BL/6 mice (age 6–8 weeks) were purchased from Charles River Laboratories. All mice were housed in a specific pathogen free facility. All research performed on mice in this study complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Use Committee.
2.2 Virus and Infections
VACV-Ova (WR strain) was a kind gift from J. Bennink (NIH) (Restifo et al.,1995). VACV-Ova was generated by insertion of the ovalbumin gene under the control of the VACV P7.5 promoter into the VACV thymidine kinase gene by homologous recombination, resulting in the generation of thymidine kinase-negative progeny as described previously (Chakrabarti et al.,1985). Virus was expanded by infection of HeLa cell cultures, which underwent 3 freeze-thaw cycles to release infectious virus. Virus was then pelleted and titered by plaque formation on BSC-1 monolayers. For infections, mice were anesthetized intraperitoneally with avertin (2,2,2-tribromoethanol). Mice were then infected intranasally with low (5×102 PFU), high (5×104 PFU), or lethal (5×105 PFU) doses of VACV-Ova in 50µl PBS or 50µl PBS alone for mock-infected mice.
2.3 Preparation of lymphocytes
Mice were weighed and then euthanized at the indicated time points postinfection by cardiac puncture (to obtain plasma for inflammatory cytokine detection) followed by cervical dislocation. Thymus and spleen were harvested and processed in PBS. Following lysis of RBC, cells were washed in PBS and counted.
2.4 Adoptive Transfers
Splenocytes from wild-type WT C57BL/6 or C57BL/6 IFNγR−/− mice, both positive for the Ly5.2 antigen, were processed in PBS followed by RBC lysis. A total of 4.2×107 splenocytes from WT or IFNγR−/− (Ly5.2+) mice were transferred i.v. into congenic C57BL/6 mice expressing the Ly5.1 antigen.
2.5 Antibody Staining and Flow Cytometry
Cells were stained with anti-CD8 (clone 53-6.7), anti-CD4 (clone RM4-5), anti-CD19 (clone 1D3), and anti-CD44 (clone IM7) (all from BD Pharmingen, San Diego, CA except CD44 Pacific Blue which was purchased from BioLegend, San Diego, CA). For detection of apoptosis, fixed and permeabilized cells were stained with anti-active caspase 3 (clone C92-605) (BD Pharmingen, San Diego, CA). Donor cells in adoptive transfer experiments were detected with an antibody to Ly5.2 (CD45.2) (eBioscience, San Diego, CA). After staining, cells were fixed in 2% paraformaldehyde and events were acquired on a FACSCalibur flow cytometer or a FACSAria cell sorter (BD, San Diego, CA). Debris was excluded on the basis of forward and side scatter and data were analyzed using Cell Quest software (BD, San Diego, CA).
2.6 Plasma Corticosterone Detection
Blood was drawn from mice by cardiac puncture between the hours of 7 and 9AM less than 5 minutes after perturbation of the cage. Plasma was obtained by centrifugation at 3000 rpm in the presence of heparin and stored at −80°C until use. Plasma corticosterone was detected by 3H radioimmunoassay per manufacturer’s instructions (MP Biomedicals, Solon, OH). Corticosterone concentrations were determined by extrapolation from a standard curve.
2.7 Inflammatory Cytokine Detection
IL-12p70, tumor necrosis factor alpha (TNF-α), IL-10, IL-6, IFN-γ, and MCP-1 were detected using the cytometric bead array (CBA) kit as per the manufacturer's instructions (BD, San Diego, CA).
2.8 Virus Titers
Lung, spleen, thymus, MLN, and ovary homogenates were titrated for the presence of VACV-Ova by plaque formation on BSC-1 monolayers as previously described (Buller et al.,1985). The number of plaques was multiplied by the dilution factor to obtain the number of PFU/tissue.
2.9 Statistics
Student’s t test was used to compare the means of responses.
3. Results
3.1 Infection of C57BL/6 mice with increasing doses of VACV resulted in significant weight loss and thymocyte loss
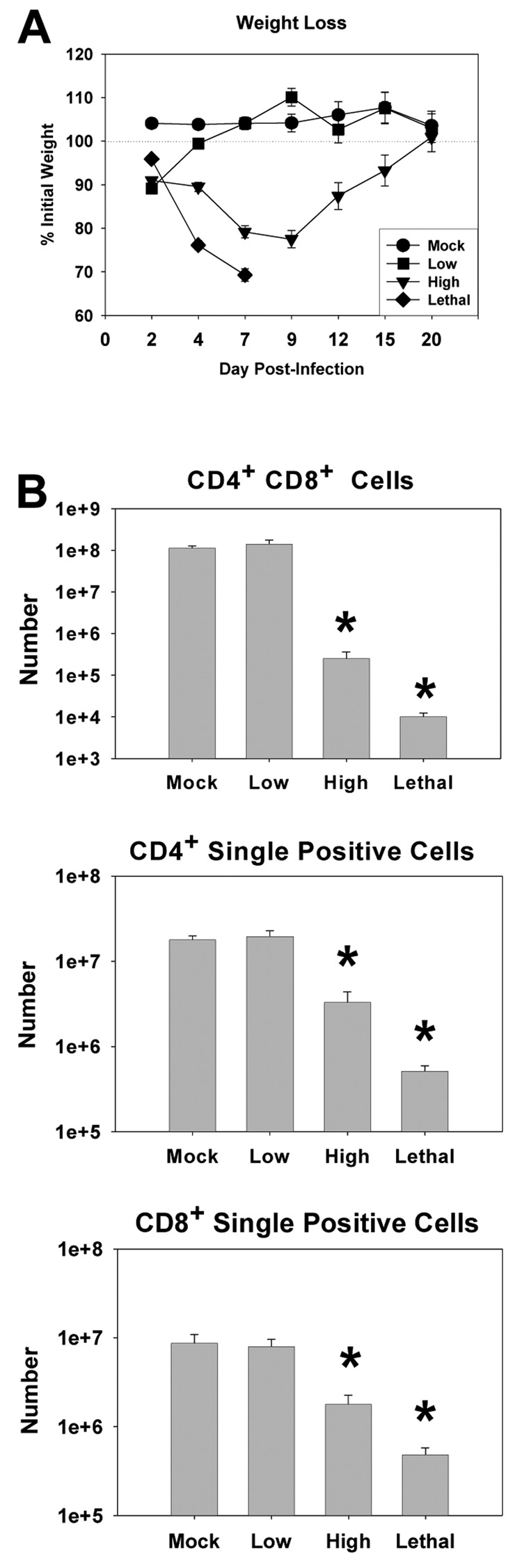
C57BL/6 mice were infected i.n. with a low (5×102 PFU), high (5×104 PFU), or lethal (5×105 PFU) dose of VACV-Ova and weight changes were monitored at various times postinfection (Fig. 1A). Mice infected with a low dose of VACV experienced some weight loss (10.9 ± 1.5%) but quickly recovered and went on to gain weight and maintain normal weight after day 2. Throughout the course of the infection, these mice had no apparent signs of illness. High dose-infected mice however, had lost 20.8 ± 0.9% of their initial weight by day 7 postinfection and eventually returned to their original weight by day 20 as they recovered from the infection. These mice also had labored breathing and ruffled fur during the peak of weight loss. Lethal dose-infected mice had lost 30.7 ± 0.3% of their initial weight by day 7 postinfection, when they were euthanized for humane reasons. These mice were hunched, had significantly labored breathing, ruffled fur, and were minimally responsive to manipulation.
Figure 1. Dose dependent weight loss and decreased thymocyte number in C57BL/6 mice following infection with VACV-Ova.
C57BL/6 mice were infected i.n. with low, high, or lethal doses of VACV-Ova or were mock-infected (PBS). A) Changes in body weight for mock-infected mice (circles) or mice infected with low (squares), high (triangles), lethal (diamonds) doses were calculated as a percentage of initial body weight. B) Thymocyte numbers were calculated by determining percentage of the population that was double positive (CD8+CD4+) and single positive (CD8+ or CD4+) at day 7 p.i. and multiplying by the total number of recovered cells. Percentages were obtained following gating on the live lymphocyte population. Each data point or bar represents the mean of 5 mice ± SEM. * P < 0.05 compared to low dose-infected mice.
Thymocyte loss has been noted in other models of virus infection (Adair,2000; Orange et al.,1995; Rojko et al.,1996; Sanchez-Cordon et al.,2002). Thus we determined whether such an effect occurred following vaccinia infection. The data presented in figure 1B show a dramatic loss of thymocytes as a result of both high and lethal dose VACV-Ova infection. Loss of cells was most apparent in the double positive (CD8+CD4+) population, but was also present in single positive (CD4+ or CD8+) cells (Figure 1B). Loss of thymocytes was accompanied by decreased tissue size and weight (data not shown). Of note, no loss of thymocytes was apparent in low dose infected mice. Infection of these mice, however, had occurred as measured by the generation of an adaptive immune response following infection with this dose ((Parks, G. D. and Alexander-Miller, M. A.,2002) and data not shown). This dose-dependent loss of thymocytes correlated with an increase in the percent and total number of thymocytes staining positive for active caspase 3, suggesting that infection with high or lethal dose induced apoptosis of these cells (data not shown). Thymocyte levels eventually recovered by day 40 postinfection in high dose-infected mice (data not shown). Together, these results indicated that intranasal infection with a high or lethal dose of VACV-Ova, but not a low dose, resulted in significant weight loss, thymocyte loss, and obvious signs of illness.
3.2 High and lethal dose VACV infection resulted in apoptosis of T cells in the spleen
We previously showed that intranasal infection with increasing doses of VACV-Ova resulted in a reduction in the IFNγ-secreting antigen-specific CD8+ T cell response in the spleen (Parks, G. D. and Alexander-Miller, M. A.,2002). This finding suggested the potential for negative regulation of the immune response as a result of high doses of VACV-Ova. We hypothesized that the immunoregulatory effects of vaccinia virus infection may extend to bystander lymphocytes, i.e. those not specific for VACV antigens.
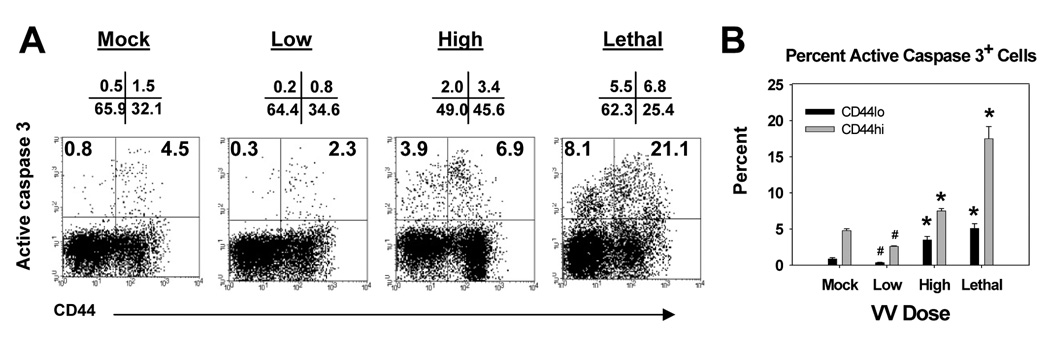
We initially tested this hypothesis by determining whether the level of apoptosis in T cells increased in mice following intranasal infection with a low versus high or lethal dose of VACV-Ova. Cells undergoing apoptotic death were identified by staining for the presence of active caspase 3. Days 4, 7, 9, 12 and 15 postinfection were examined. No differences in the percentage of cells that stained positive for active caspase 3 were found in the T cells present at d4, 9, 12 or 15 (data not shown). However at d7 we observed an increase in the percent CD8+ and CD4+ T cells undergoing apoptosis (Fig. 2 and 3). In CD8+ T cells, there was an increase in the percentage of active caspase 3+ cells in both CD44lo and CD44hi population in high dose and lethal dose infected mice compared to mock infected mice (Fig. 2). Dot plots from a representative animal are shown in figure 2A and averaged data from multiple animals in figure 2B. Interestingly the percentage of CD44hi cells that were positive for active caspase 3 was significantly reduced in mice infected with the low dose of virus compared to the baseline of mock infected mice (Fig. 2). Given that CD44hi cells in infected mice were likely activated effector cells as opposed to CD44hi cells in mock infected mice, which were presumably resting memory cells, this finding may suggest that effector cells have a reduced rate of apoptosis compared to memory cells. Whether this was the case is not known, as direct determination of apoptosis in virus-specific cells in the spleen at this time was hampered by the low number of these cells.
Figure 2. Infection with a high or lethal dose of VACV-Ova resulted in increased apoptosis in naïve and activated CD8+ T cells in the spleen.
C57BL/6 mice were infected i.n. with low (5×102), high (5×104), or lethal (5×105) dose of VACV-Ova or were mock-infected (PBS) and spleens harvested at day 7. A) Representative flow cytometric data showing active caspase 3 staining of CD44lo and CD44hi cells following gating on CD8+ T cells. Numbers above the dot plot are the percentage of cells in each quadrant. The numbers in the quadrants are the percentage of CD44lo (upper left) or active CD44hi (upper right) cells that are active caspase 3+ positive. B) Averaged data showing the percent of CD44lo or CD44hi CD8+ T cells staining positive for active caspase 3. * = significant increase, p<0.05; # = significant decrease, p<0.05.
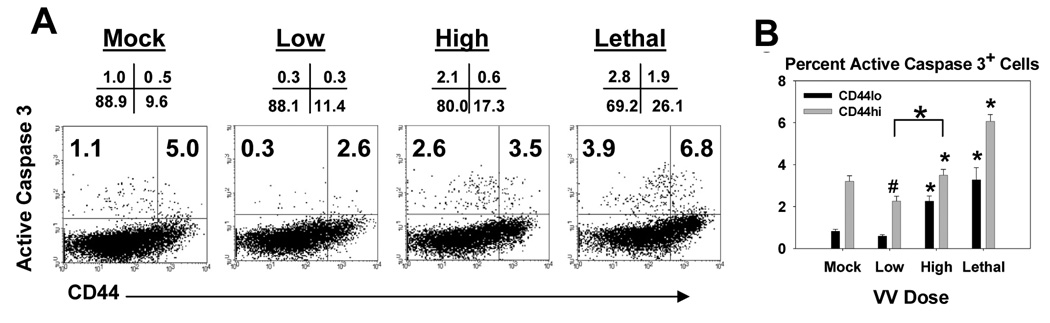
Figure 3. Infection with a high and lethal dose of VACV-Ova resulted in increased apoptosis in naïve and activated CD4+ cells in the spleen.
C57BL/6 mice were infected i.n. with low (5×102), high (5×104), or lethal (5×105) dose of VACV-Ova or were mock-infected (PBS) and spleens harvested at day 7. A) Representative flow cytometric data showing active caspase 3 staining of CD44lo and CD44hi cells following gating on CD4+ T cells. Numbers above the dot plot are the percentage of cells in each quadrant. The numbers in the quadrants are the percentage of CD44lo (upper left) or active CD44hi (upper right) cells that are active caspase 3+ positive. B) Averaged data showing the percent of CD44lo or CD44hi CD4+ T cells staining positive for active caspase 3. * = significant increase, p<0.05; # = significant decrease, p<0.05.
CD4+ T cells also showed evidence of increased apoptosis in high and lethal dose infected mice. As shown in figure 3, CD44lo CD4+ T cells from high dose infected mice exhibited a significant increase in the percentage of cells that were positive for active caspase 3 compared to those from mock infected mice (Fig. 3). Comparison of CD44hi T cell populations under these two conditions did not reveal a significant increase in apoptosis. However, apoptosis in CD44hi cells from high dose infected mice was significantly increased compared to low dose infected mice. Thus there appeared to be increased apoptosis in effector cells in the context of high compared to low dose infection. Increases in the percentage of both CD44lo and CD44hi cells that were positive for active caspase 3 were observed following infection with the lethal dose.
3.3 High and lethal dose VACV infection resulted in apoptosis of B cells in the spleen
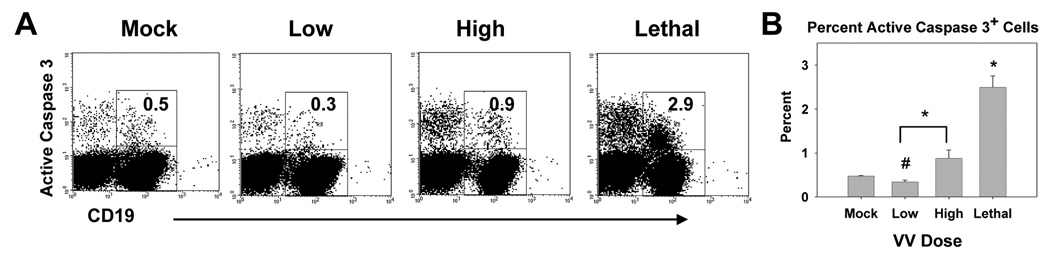
Finally we determined whether increased apoptosis following infection with high or lethal doses of VACV-Ova also occurred in B cells. CD19+ cells present at d7 postinfection had a significant reduction in active caspase 3+ cells following infection with the low dose of virus compared to mock infected animals (Fig. 4), suggesting a protective effect in cells during the course of an optimal immune response as is the case with the low dose infection. When compared to low dose infected mice, there was a statistically significant increase in the percentage of active caspase 3+ B cells in the high dose infected animals. As with T cells, infection with the lethal dose resulted in a significant increase in active caspase 3+ B cells (Fig. 4). Together these results, along with those above, suggest a generalized increase in death of lymphocyte populations following intranasal infection with high and lethal doses of VACV-Ova. The increased apoptosis in T and B cells in high and lethal dose compared to low dose infected mice correlated with a decrease in the total number of lymphocytes recovered (data not shown).
Figure 4. Infection with a high and lethal dose of VACV-Ova resulted in increased apoptosis in B cells in the spleen.
C57BL/6 mice were infected i.n. with low (5×102), high (5×104), or lethal (5×105) dose of VACV-Ova or were mock-infected (PBS) and spleens harvested at day 7. A) Representative flow cytometric data showing active caspase 3 staining of CD19+ cells. The number in the dot plot is the percentage of CD19+ cells that stained positive for active caspase 3+. B) Averaged data showing the percent of CD19+ cells staining positive for active caspase 3. * = significant increase, p<0.05; # = significant decrease, p<0.05.
3.4 Virus spread following infection with VACV
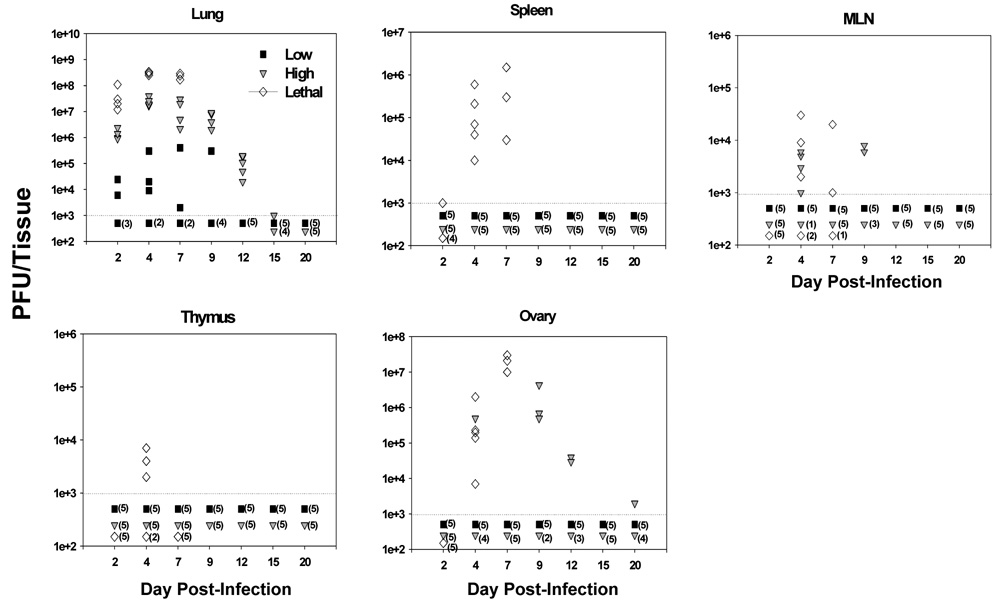
One potential explanation for the generalized loss of lymphocytes was direct cytopathic effects as a result of high level virus replication in the spleen following high or lethal dose infection. To test for this possibility, C57BL/6 mice were infected intranasally with the various doses and lungs, MLN, spleen, thymus and ovary were harvested and titered for the presence of VACV-Ova. In mice infected with a low dose of VACV-Ova, the only tissue with detectable virus was the lung, and even then only about half of the mice had detectable virus in that tissue (Fig. 5). However, the failure to detect virus in these mice did not mean that they had not been infected, since mice developed a vaccinia-specific immune response despite not having detectable virus (data not shown). In contrast, high dose infection resulted in high levels of VACV-Ova in the lungs, with virus levels peaking at day 4 and eventual clearance by day 15 in all mice. Some high dose infected mice (4/5 at day 4 and 2/5 at day 9) also had detectable virus in the MLN, though at levels barely above the limit of detection. Also, in some high dose-infected animals, virus was also detected in the ovaries, a tissue that is highly permissive for replication of the WR strain of VACV. Importantly, however, titratable VACV was not found in the spleen or thymus of high dose infected mice.
Figure 5. VACV-Ova was not detectable in the spleen following high dose infection.
C57BL/6 mice were infected i.n. with the low (black squares), high (gray triangles), or lethal (open diamonds) dose of VACV-Ova. Lung, spleen, MLN, thymus, and ovary were harvested at the indicated timepoints and VACV-Ova titers from each whole tissue determined. Each symbol represents one mouse, with numbers in parentheses indicating the number of mice with organ titers below the limit of detection (103 PFU).
In contrast, virus was detected in all of the tested tissues of mice infected with the lethal dose, with the highest levels present in the lung. These results show that, while in some cases high dose i.n. infection with VACV-Ova resulted in virus spread to the ovary and MLN, significant levels of virus were never found in the thymus or spleen, sites where a high level of cell death was detected. This, along with unpublished data from our lab showing that VACV only minimally infects thymocytes and splenic T cells in vitro, suggested that lymphocyte and thymocyte apoptosis after high dose infection was not a result of direct cytopathic effects.
3.5 Infection with lethal, but not high doses of VACV resulted in increased plasma corticosterone levels during infection
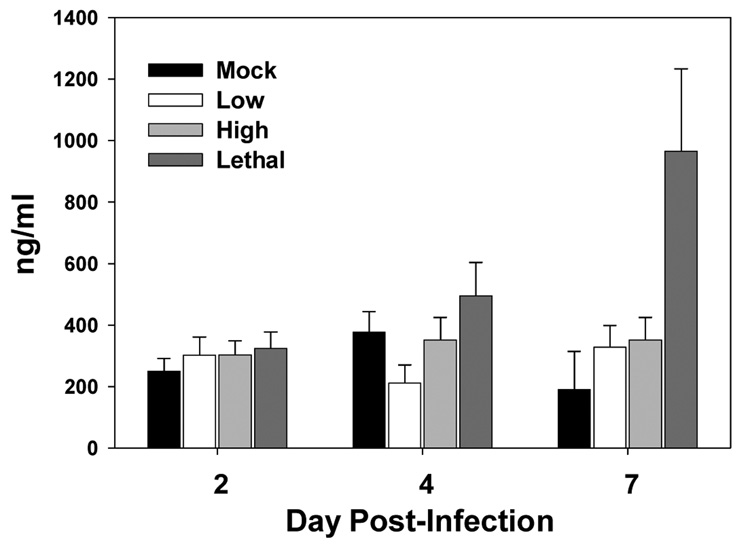
Others have shown that the A44L gene of VACV encodes for a 3-β-hydroxysteroid dehydrogenase, which is an enzyme necessary for the production of glucocorticoids (GC) (Moore and Smith,1992; Reading et al.,2003; Sroller et al.,1998). GC are steroids normally produced in response to stress and have broadly immunosuppressive effects, including cell death (Blotta et al.,1997; Kim et al.,2001; Piemonti et al.,1999; Zacharchuk et al.,1990). Thus infection with VACV virus has the potential to significantly increase the amount of immunosuppressive GC that are produced. To determine if the systemic lymphocyte apoptosis that occurred in the spleen was the result of high levels of virus induced GC, corticosterone, the main GC in rodents, was measured following infection with the low, high, or lethal dose of virus. The only condition that resulted in a significant increase in plasma corticosterone was lethal dose infection (Fig. 6). Systemic corticosterone levels in low and high dose infected mice remained at or near baseline levels and were not significantly different from one another. These results suggested that GC do not play a role in lymphocyte apoptosis in the spleen during high dose infection with VACV. However we cannot rule out the possibility that high GC levels contributed to the loss of cells in mice infected with the lethal dose of virus.
Figure 6. Lethal, but not high, dose VACV-Ova infection resulted in elevated plasma corticosterone levels.
C57BL/6 mice were infected i.n. with the low, high, or lethal dose of VACV-Ova or were mock-infected. Plasma corticosterone levels were determined at the indicated times postinfection by radioimmunoassay. Each bar represents 5 mice ± SEM. * =p <0.05 compared to low dose-infected mice.
3.6 Infection with the high and lethal doses of VACV resulted in increased systemic levels of inflammatory cytokines
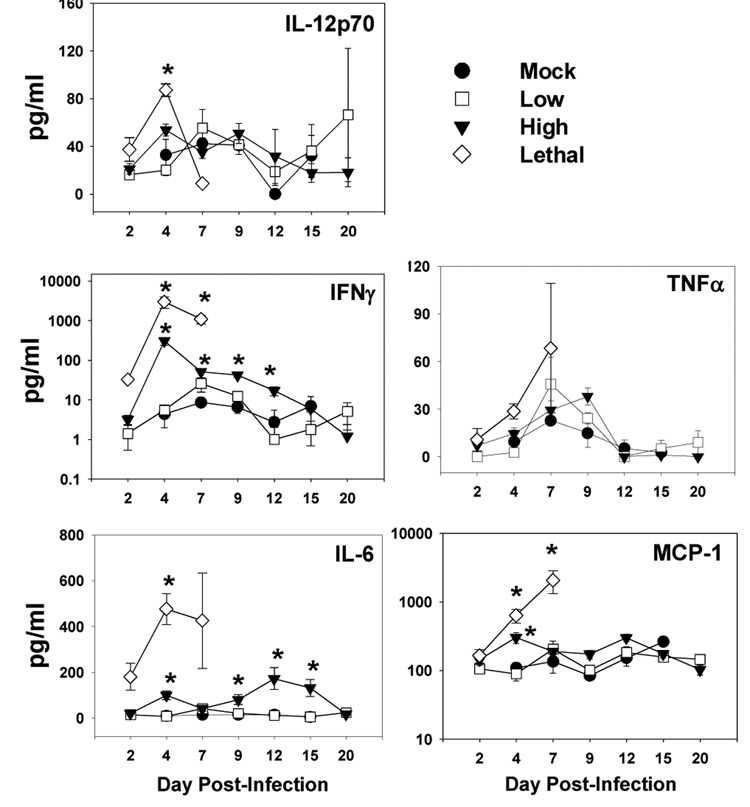
Cytokines such as TNFα and IFNγ have been shown to induce lymphocyte apoptosis (Alexander-Miller et al.,1998; Badovinac et al.,2000; Chan et al.,2003; Dalton et al.,2000; Florido et al.,2005; Martins et al.,1999; Speiser et al.,1996). Thus we determined plasma cytokine levels throughout the course of infection with low, high, and lethal doses of VACV-Ova (Fig. 7). In low dose infected mice there was a slight increase in circulating IFNγ at day 7, but all other cytokines tested were not at levels significantly above mock-infected mice. In contrast, high dose infection resulted in a greatly elevated amount of IFNγ which peaked at d4 p.i. at a level 50-fold higher than mock-infected mice. Significant increases in MCP-1 and IL-6 were also detected. A similar set of cytokines were increased in mice infected with the lethal dose. Here, a large increase in IFNγ was present as early as d2 p.i., peaking at d4 with a level 500-fold above mock infected mice. IL-12p70, MCP-1, and IL-6 also increased in lethal dose infected mice. Thus high (and lethal) dose infected mice exhibit a significantly altered cytokine profile compared to low dose infected mice.
Figure 7. High and lethal dose VACV-Ova infection resulted in elevated levels of inflammatory cytokines.
C57BL/6 mice were infected i.n. with the low (squares), high (triangles), or lethal (diamonds) dose of VACV-Ova or were mock-infected (circles). The level of inflammatory cytokines (IL-12p70, TNFα, IFNγ, MCP-1, and IL-16) in the plasma at the indicated timepoints was determined by CBA (see Materials and Methods). Each data point represents 5 mice ± SEM. * = p < 0.05 compared to low dose-infected mice.
4. Discussion
Poxviruses have acquired an impressive arsenal of weapons with which to combat the immune response (Dunlop et al.,2003; Haga and Bowie,2005). In support of this we previously observed a dose-dependent decrease in the virus-specific CD8+ T cell response generated following intranasal infection (Parks, G. D. and Alexander-Miller, M. A.,2002). In this report we tested the hypothesis that the negative regulation of the immune system extended to other lymphocyte populations. These studies were focused on the possibility that there was a systemic effect of infection that extended to lymphocyte populations other than those directly responding to infection. We found that a high, but non-lethal, virus dose resulted in a significant increase in the percent of lymphocytes staining positive for active caspase 3 in the spleen. While caspase 3 activation has been shown to occur during T cell activation in the absence of apoptosis (Alam et al.,1999; Miossec et al.,1997; Sabbagh et al.,2004; Wilhelm et al.,1998), our findings argue against this interpretation as 1) there was a considerable decrease in CD4+, CD8+ and B cell numbers in the spleen in high versus low dose infected mice and 2) there was an increase in active caspase 3+ cells within the naïve T cell populations. Thus our data support the interpretation that the active caspase 3 detected following infection with high dose virus is a marker for cells undergoing apoptosis. In support of this we see increased staining for 7AAD in mice receiving the high dose of virus (data not shown). The increased apoptosis in lymphocytes following high dose i.n. vaccinia virus infection was not restricted to the spleen as a similar effect was observed in the mediastinal lymph node (data not shown). We note that we have not ruled out a contribution of differential trafficking to the reduced number of lymphocytes found in the mice infected with a high dose. However, we do not favor this hypothesis given the detection of death by two approaches in lymphocyte populations in these mice.
One possibility to explain cell death was a direct cytopathic effect of infection. However, our data suggest that this is not the case as increased death in the spleen occurred in the absence of detectable virus in this tissue. Further following intranasal infection with a high but non-lethal dose of a recombinant vaccinia virus expressing eGFP, which resulted in increased apoptosis and reduced cell recovery from the spleen, we found that cells undergoing apoptosis did not express eGFP (data not shown). Together these results suggest that direct cytopathic effects as a result of infection were not responsible for death in these cells.
Peripheral lymphocyte apoptosis is a common sign of disease progression resulting from infection not only with certain viruses (among them is respiratory syncytial virus (RSV), lymphocytic choriomeningitis virus (LCMV), Ebola, SARS, measles, influenza) but also a number of bacteria (Salmonella typhimurium, Listeria monocytogenes), e.g. (Baize, S. et al. 1999; Carrero, J. A. et al. 2004; Hassan, J. O. and Curtiss, R., III,1994; He, Z. et al. 2005; McNally, J. M. et al. 2001; Okada, H. et al. 2000; Roe, M. F. et al. 2004; Tumpey, T. M. et al. 2000). In some cases death was found to be mediated by IFNγ (Dalton, D. K. et al. 2000; Florido, M. et al. 2005; Schwacha and Eisenstein,1997). Death in these models is often observed even at very early times postinfection, i.e. d3 p.i. However we saw no evidence of increased death at this time. The death in our model was evident at d7, a time that correlates with peak virus titers, just prior to the initiation of clearance as measured by reduced virus titers in the lung. This may suggest the requirement for high levels of a viral protein or a soluble mediator produced as a result of high virus titers. Our cytokine analyses revealed increased levels of IFNγ and IL-6 in mice infected with the high dose of virus. We tested a potential role for these cytokines in the apoptosis observed using mice deficient in IL-6 or IFNγ receptor (data not shown). We found that lymphocyte apoptosis was unchanged, suggesting that neither was involved in apoptosis. Further we have also evaluated the contribution of type I IFN. However, apoptosis remained unchanged in the absence of type I IFN signaling (data not shown).
Given that none of the cytokines tested were responsible for lymphocyte death, we tested the possibility that fas/fasL interactions or high level systemic glucocorticoid production contributed to the death of lymphocytes in our system. The latter seemed an attractive candidate given a previous report where deletion of the A44L gene, which produces a 3β-HSD homologue, resulted in decreased levels of plasma GC, reduced signs of illness and an increased number of T cells in the lung (Moore, J. B. and Smith, G. L.,1992; Reading, P. C. et al. 2003; Sroller, V. et al. 1998). However, the lack of increased GC levels in mice infected with the high (but non-lethal) dose of vaccinia virus did not support this hypothesis. It remains possible that high levels of GC play a role during lethal infection, but this has not been tested. The fas/fasL pathway also did not appear to be involved (data not shown). Thus the mediator responsible for the increased apoptosis remains elusive.
Interestingly there was evidence for increased apoptosis in both naïve and memory/activated T cell populations. However, naïve cells seemed to have an increased susceptibility to this effect, as CD4+ and CD8+ naïve cells from high dose infected mice had a 3.8-fold and 9.2-fold increase in active caspase 3+ cells compared to low dose infected mice. While also increased in the CD44hi population, the extent of the increase was not as great, 1.5-fold for CD4+ cell and 2.9-fold for CD8+ cells. This could reflect increased resistance to this effect in activated cells. A difference in the susceptibility to death between naïve and activated/memory cells has been observed in other infections, i.e. LCMV (Bahl et al.,2006; McNally, J. M. et al. 2001), however, the pattern differs from our results with vaccinia virus infection. In contrast to our findings, while naïve cells showed some increased death following infection with LCMV, memory (CD44hi) CD8+ T cells were the most affected (McNally, J. M. et al. 2001). These data suggest that the mechanism responsible for the apoptosis in these two viral models may be distinct.
It is clear that from our studies that once the viral burden reaches a significant level (as was the case for high dose infected animals), a significant amount of apoptosis occurred in the systemic lymphocyte population, which appeared not to be due to direct infection with virus. Whether this loss of lymphocytes is impacting the rate of vaccinia virus clearance in these animals is unknown, although it is certainly possible that this is the case. In addition it has often been observed that one infection predisposes the infected individual to a secondary infection. In the case of vaccinia virus infection of the respiratory tract, this may occur by a number of mechanisms. For example, the damage that occurs during infection is likely to increase the ability of a second pathogen to infect via the epithelial surface. Compounding the issue, the increased apoptosis observed in naïve lymphocyte populations when high vaccinia viral burdens are reached could decrease the individual’s ability to mount an immune response to the second pathogen.
In summary the studies presented here show that a high, but non-lethal dose of VACV administered via the respiratory route resulted in apoptosis of naïve and activated T cells and B cells, as well as single positive and double positive thymocytes. Our findings suggest that direct cytopathic effects, GC, fas/fasL, IFNγ, IL-6, or type I IFN were not responsible for triggering death in these populations. These findings have important implications for our understanding of poxvirus pathogenesis and provide insights into how the immune response is negatively regulated as virus burden increases.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants R21 AI56096 (to M.A. A-M.) and P01 AI 060642 (to S.B.M.). We thank Dr. Griff Parks and Dr. Beth Hiltbold for helpful suggestions with regard to this manuscript and Mark Landrum and Ellen Young for technical assistance. We are also grateful to Dr Jack Bennink for provision of the recombinant vaccinia viruses expressing ovalbumin and eGFP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Adair BM. Immunopathogenesis of chicken anemia virus infection. Dev.Comp Immunol. 2000;24:247–255. doi: 10.1016/s0145-305x(99)00076-2. [DOI] [PubMed] [Google Scholar]
- Alam A, Cohen LY, Aouad S, Sekaly RP. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J.Exp.Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Miller MA, Derby MA, Sarin A, Henkart PA, Berzofsky JA. Supraoptimal peptide-major histocompatibility complex causes a decrease in bc1-2 levels and allows tumor necrosis factor alpha receptor II- mediated apoptosis of cytotoxic T lymphocytes. J.Exp.Med. 1998;188:1391–1399. doi: 10.1084/jem.188.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J.Immunol. 2006;176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat.Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- Baron S. Smallpox: The main site of transmission is the oropharynx. Journal of Dental Research. 2003;82:252. doi: 10.1177/154405910308200401. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc.Natl.Acad.Sci.USA. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J.Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- Buller RML, Smith GL, Cremer K, Notkins AL, Moss B. Decreased Virulence of Recombinant Vaccinia Virus Expression Vectors Is Associated with A Thymidine Kinase-Negative Phenotype. Nature. 1985;317:813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J.Exp.Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Brechling K, Moss B. Vaccinia Virus Expression Vector - Coexpression of Beta-Galactosidase Provides Visual Screening of Recombinant Virus Plaques. Molecular and Cellular Biology. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J.Biol.Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J.Exp.Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop LR, Oehlberg KA, Reid JJ, Avci D, Rosengard AM. Variola virus immune evasion proteins. Microbes and Infection. 2003;5:1049–1056. doi: 10.1016/s1286-4579(03)00194-1. [DOI] [PubMed] [Google Scholar]
- Florido M, Pearl JE, Solache A, Borges M, Haynes L, Cooper AM, Appelberg R. Gamma interferon-induced T-cell loss in virulent Mycobacterium avium infection. Infect.Immun. 2005;73:3577–3586. doi: 10.1128/IAI.73.6.3577-3586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga IR, Bowie AG. Evasion of innate immunity by vaccinia virus. Parasitology. 2005;130 Suppl:S11–S25. doi: 10.1017/S0031182005008127. [DOI] [PubMed] [Google Scholar]
- Harper GJ. Airborne micro-organisms: survival tests with four viruses. J.Hyg.(Lond) 1961;59:479–486. doi: 10.1017/s0022172400039176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Most RR, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J.Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan JO, Curtiss R., III Virulent Salmonella typhimurium-induced lymphocyte depletion and immunosuppression in chickens. Infect.Immun. 1994;62:2027–2036. doi: 10.1128/iai.62.5.2027-2036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhao C, Dong Q, Zhuang H, Song S, Peng G, Dwyer DE. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int.J.Infect.Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KD, Choe YK, Choe IS, Lim JS. Inhibition of glucocorticoid-mediated, caspase-independent dendritic cell death by CD40 activation. J.Leukoc.Biol. 2001;69:426–434. [PubMed] [Google Scholar]
- Martins GA, Vieira LQ, Cunha FQ, Silva JS. Gamma interferon modulates CD95 (Fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypanosoma cruzi infection: a possible role in immune response control. Infect.Immun. 1999;67:3864–3871. doi: 10.1128/iai.67.8.3864-3871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J.Virol. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec C, Dutilleul V, Fassy F, Diu-Hercend A. Evidence for CPP32 activation in the absence of apoptosis during T lymphocyte stimulation. J.Biol.Chem. 1997;272:13459–13462. doi: 10.1074/jbc.272.21.13459. [DOI] [PubMed] [Google Scholar]
- Moore JB, Smith GL. Steroid hormone synthesis by a vaccinia enzyme: a new type of virus virulence factor. EMBO J. 1992;11:1973–1980. doi: 10.1002/j.1460-2075.1992.tb05251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Kobune F, Sato TA, Kohama T, Takeuchi Y, Abe T, Takayama N, Tsuchiya T, Tashiro M. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch.Virol. 2000;145:905–920. doi: 10.1007/s007050050683. [DOI] [PubMed] [Google Scholar]
- Orange JS, Salazarmather TP, Opal SM, Spencer RL, Miller AH, Mcewen BS, Biron CA. Mechanism of interleukin 12-mediated toxicities during experimental viral-infections - role of tumor-necrosis-factor and glucocorticoids. J.Exp.Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks GD, Alexander-Miller MA. High avidity cytotoxic T lymphocytes to a foreign antigen are efficiently activated following immunization with a recombinant paramyxovirus, simian virus 5. J.Gen.Virol. 2002;83:1167–1172. doi: 10.1099/0022-1317-83-5-1167. [DOI] [PubMed] [Google Scholar]
- Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, Socci C, Di CV. Glucocorticoids affect human dendritic cell differentiation and maturation. J.Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- Ramirez JC, Gherardi MM, Esteban M. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J.Virol. 2000;74:923–933. doi: 10.1128/jvi.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Moore JB, Smith GL. Steroid hormone synthesis by vaccinia virus suppresses the inflammatory response to infection. J.Exp.Med. 2003;197:1269–1278. doi: 10.1084/jem.20022201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Smith GL. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J.Gen.Virol. 2003;84:1973–1983. doi: 10.1099/vir.0.19285-0. [DOI] [PubMed] [Google Scholar]
- Restifo NP, Bacik I, Irvine KR, Yewdell JW, McCabe BJ, Anderson RW, Eisenlohr LC, Rosenberg SA, Bennink JR. Antigen processing in vivo and the elicitation of primary CTL responses. J.Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- Roe MF, Bloxham DM, White DK, Ross-Russell RI, Tasker RT, O'Donnell DR. Lymphocyte apoptosis in acute respiratory syncytial virus bronchiolitis. Clin.Exp.Immunol. 2004;137:139–145. doi: 10.1111/j.1365-2249.2004.02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko JL, Hartke JR, Cheney CM, Phipps AJ, Neil JC. Cytopathic feline leukemia viruses cause apoptosis in hemolymphatic cells. Prog.Mol.Subcell.Biol. 1996;16:13–43. doi: 10.1007/978-3-642-79850-4_2. [DOI] [PubMed] [Google Scholar]
- Sabbagh L, Kaech SM, Bourbonniere M, Woo M, Cohen LY, Haddad EK, Labrecque N, Ahmed R, Sekaly RP. The selective increase in caspase-3 expression in effector but not memory T cells allows susceptibility to apoptosis. J.Immunol. 2004;173:5425–5433. doi: 10.4049/jimmunol.173.9.5425. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cordon PJ, Romanini S, Salguero FJ, Nunez A, Bautista MJ, Jover A, Gomez-Villamos JC. Apoptosis of thymocytes related to cytokine expression in experimental classical swine fever. Journal of Comparative Pathology. 2002;127:239–248. doi: 10.1053/jcpa.2002.0587. [DOI] [PubMed] [Google Scholar]
- Schwacha MG, Eisenstein TK. Interleukin-12 is critical for induction of nitric oxide-mediated immunosuppression following vaccination of mice with attenuated Salmonella typhimurium. Infect.Immun. 1997;65:4897–4903. doi: 10.1128/iai.65.12.4897-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser DE, Sebzda E, Ohteki T, Bachmann MF, Pfeffer K, Mak TW, Ohashi PS. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur.J.Immunol. 1996;26:3055–3060. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- Sroller V, Kutinova L, Nemeckova S, Simonova V, Vonka V. Effect of 3-beta-hydroxysteroid dehydrogenase gene deletion on virulence and immunogenicity of different vaccinia viruses and their recombinants. Arch.Virol. 1998;143:1311–1320. doi: 10.1007/s007050050377. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J.Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Wagner H, Hacker G. Activation of caspase-3-like enzymes in nonapoptotic T cells. Eur.J.Immunol. 1998;28:891–900. doi: 10.1002/(SICI)1521-4141(199803)28:03<891::AID-IMMU891>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Engler R. Smallpox, vaccination and adverse reactions to smallpox vaccine. Curr.Opin.Allergy Clin.Immunol. 2004;4:271–275. doi: 10.1097/01.all.0000136758.66442.28. [DOI] [PubMed] [Google Scholar]
- Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc.Natl.Acad.Sci.USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J.Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- Zacharchuk CM, Mercep M, Chakraborti PK, Simons SS, Jr, Ashwell JD. Programmed T lymphocyte death. Cell activation- and steroid-induced pathways are mutually antagonistic. J.Immunol. 1990;145:4037–4045. [PubMed] [Google Scholar]