Abstract
We attempted to determine whether the immune reactions elicited by aberrantly expressed testis antigens contribute to the beneficial responses to interferon (IFN)-α therapy and other therapies in patients with polycythemia vera (PV). We screened a human testis cDNA library using SEREX (serological analysis of tumor antigens by screening an expression cDNA library with sera from three patients with PV who had undergone IFN-α-induced or other therapeutics-induced remission). We identified two novel PV associated tumor antigens, PV65 (eIF-2α) and PV13 (protamine 2). These 2 antigens elicited IgG antibody reactions in a subset of PV patients but not in healthy donors, suggesting that they are authentic tumor antigens. Increased phosphorylation of PV65 in response to stimulation of IFN-α, and upregulation of PV13 in tumor cells might enhance their abilities in elicitation of immune reactions in patients. These findings provide new insights into the mechanism underlying the regulation of the self-antigen repertoire in eliciting anti-tumor immune reactions in patients with polycythemia vera, and suggest their potential as the targets of novel immunotherapy.
Keywords: Tumor antigens, IgG antibody reactions, SEREX, Polycythemia vera, Myeloproliferative diseases
Introduction
Myeloproliferative diseases (MPD) arise from the clonal expansion of a pluripotent hematopoietic progenitor, and include polycythemia vera (PV), essential thrombocythemia, agnogenic myeloid metaplasia, and chronic myeloid leukemia (CML) [1]. The current therapy for PV is non-specific and controversial [2]. Interferon-α (IFN-α) therapy induces a clinical remission with documented reversal to polyclonal hematopoiesis [2] in PV patients [3], thus making PV a model to study the antigenic mechanism of the immune reactions that may contribute to the beneficial responses to IFN-α therapy and other therapies. Cytogenetic response to IFN-α therapy in CML [4], is often associated with therapy-related autoimmunity [5], suggesting that anti-self antigen immune reactions may play an important role in controlling tumors. However, the potential self-antigens eliciting anti-PV immune reactions remained largely unknown until our recent report in characterization of a new unconventional tumor antigen MPD6 [6].
Because of the paucity of tumor cell lines in MPD, serological analysis of tumor antigens by screening expression cDNA libraries with sera from patients (SEREX) becomes a suitable method to identify self-tumor antigen targets [7].By applying SEREX to identify antigens that elicit immune reactions associated with CML remission, we identified two broadly immunogenic tumor antigens, CML66 [8] and CML28 [9]. These two SEREX antigens, similar to a class of testicular-cancer like antigens, have restricted expression limited to the testis, and are expressed in a variety of tumor cells [8,9] and myeloid progenitor cells [10]. Recently, we demonstrated that overexpression via alternative splicing mechanisms was the dominant mechanism for increased immunogenicity of these unmutated tumor antigens [11]. In addition, we proposed a novel “stimulation-responsive splicing” model in which non-canonical alternative splicing of the RNA transcripts of tumor antigens and autoantigens provides a structural basis for generation of untolerized self-antigen epitopes [12-14].
Identification of testicular-cancer antigens that induce high titers of IgG antibody reactions suggests that these antigens could also elicit T cell immune reactions, since the elicitation of IgG reactions requires T helper cell function. Our results in characterization of HLA-A2.1-restricted T cell antigen epitopes of tumor antigen CML66L have verified this conclusion [15]. Moreover, specific antibody reactions to CML66 and CML28 can also be detected in patients who had undergone PV remission responding to IFN-α therapy, suggesting that testicular-cancer antigens-elicited immune reactions may mediate PV remission [16]. Integrated humoral and cellular immune reactions to SEREX antigens are truly tumor specific, as demonstrated for the SEREX antigen NY-ESO-1 [17] and other tumor antigens [18].
The search for tumor antigens by screening testis expression cDNA library [7] (SEREX) is a useful approach to identify aberrantly expressed antigens, as reported [19]. For our purposes, the rationale underlying this approach is that testis, an immune-privileged site, does not normally present its self-antigens to the host immune system [20]. Notwithstanding, the aberrant expression of any given cancer-testis antigen could be found in polycythemia vera, if that antigen has specifically reacted to the sera derived from PV patients [21]. In this report, we attempted to identify SEREX antigens with sera from three PV patients who had undergone IFN-α-induced or other therapeutics-induced remission. These findings provide new insights into the mechanism underlying the regulation of the self-antigen repertoire in eliciting antitumor immune reactions in patients with myeloproliferative diseases, and suggest their potential as the targets of novel immunotherapy [22].
Materials and methods
Serum Samples
In accordance with a protocol approved by the Institutional Review Board at Temple University and established guidelines, serum samples were obtained from patients with PV and CML receiving IFN-α therapy enrolled into Baylor College of Medicine (eight patients with PV receiving IFN-α treatment, 8 PV patients receiving other treatments), M.D. Anderson Cancer Center (10 patients with CML receiving IFN-α treatment), New York Presbyterian Hospital-Weill Cornell Medical Center (13 PV patients receiving IFN-α treatment, 26 PV patients receiving other treatments, six PV patients having not yet received any treatments) Institutional Review Board-approved trials. The therapeutic regimens other than IFN-α for the 34 patients with PV included imatinib mesylate (Gleevec, 16 patients), hydroxyurea (12 patients), hydroxyurea plus agrelin (three patients), agrelin plus phlebotomy (one patient), phlebotomy (one patient), and thalidomide (one patient). The patients in the other treatment group have not been treated with IFN-α.
Human testis CDNA library screening by SEREX
The library screening was performed as described previously [23]. Briefly, XL-1 Blue MRF’ Escherichia coli (Stratagene, La Jolla, CA) were infected with the recombinant phages of a human testis expression cDNA library (BD Clontech, Palo Alto, CA), and expression of recombinant proteins was induced by incubation with isopropyl β-d-thiogalactoside (IPTG) (Fisher, Pittsburgh, PA) treated nitrocellulose membranes (Schleicher and Schuell Bioscience, Keene, NH). The filters were then incubated with sera from the PV patients, diluted at 1:500 in TBST at 4°C. The serum was pre-absorbed against lysate of the phage and the E. coli strain to minimize nonspecific antibody binding (Stratagene). Visualization of the antigen-antibody complex was accomplished by staining with the Sigma Fast™ BCIP/NBT substrate (Sigma, St. Louis, MO). DNA sequencing was performed by SeqWright (Houston, TX).
In vitro transcription and translation (TNT)
The TNT with plasmid pTriplEx (BD Clontech, Palo Alto, CA) containing cDNA encoding PV13 and plasmid pTriplEx without cDNA insert (as a negative control) were performed according to the manufacturer’s protocol (Promega, Madison, WI) [24].
Bioinformatic analyses
To determine whether cloned sequences were related or identical to genes, proteins, or protein domains in the databases, sequence analyses were performed using the NCBI-GenBank databases (http://www.ncbi.nlm.nih.gov/), NCBI-conserved domain databases, and the PROSITE analysis (http://us.expasy.org/cgi-bin/scanprosite) [25]. The gene organizations such as intron, exon, chromosome location, were analyzed through searches in the NCBI-LocusLink website, and the NCBI-AceView website [25]. In addition to the Northern blot analyses, the gene expression data for genes were analyzed by searching the NCBI-UniGene website, and the NCBI-SAGEmap database (SAGE, Serial Analysis of Gene Expression). The cis-acting transcription factor binding sites in promoters of antigens were searched in the TRANSFAC database (http://www.cbil.upenn.edu/cgi-bin/tess/tess?RQ=SEA-FR-QueryS).
Northern blot and Southern blot
Multiple tissue Northern blots were prepared with purified polyadenylated RNAs (2 μg/lane) (Ambion, Austin, TX). Hybridizations were conducted with the probes of PV65 and PV13, as reported previously [8].
Quantitative RT-PCR
Granulocytes were separated by differential centrifugation and isopyknic density gradient separation using standard protocols. RNA was prepared from granulocytes using TRI reagent (Molecular Research Center, Cincinnati, OH) in combination with RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturers’ protocols to avoid DNA contamination. Quantitative real-time RT-PCR was used to quantify PV65 mRNA in total RNAs isolated from peripheral blood granulocytes using the TaqMan One-Step RT-PCR master mix reagents (Applied Biosystems, Foster City, CA) for Real time PCR, which were performed on an ABI Prism 7000 sequence detection system (Applied Biosystems). The primers and TaqMan probe for PV65 with targeted center of sequence at 1402 bp were purchased from Applied Biosystems (Hs00230684_m1, https://myscience.appliedbiosystems.com/ge/servlet/com.celera.web.cdsentry.servlets.GetCdsEntryListServlet?cmd=cmdGeReport&assayType=geneExpression&assayAcc=Hs00230684_m1). The 18S ribosomal RNA used as a reference was labeled with the VIC fluorophore, targeted to center of the sequence at 606 bp, and also was purchased from Applied Biosystems (Hs99999901_s1, https://myscience.appliedbiosystems.com/ge/servlet/com.celera.web.cdsentry.servlets.GetCdsEntryListServlet?cmd=cmdGeReport&assayType=geneExpression&assayAcc=Hs99999901_s1). Expression of PV65 and 18S was analyzed in separate reactions. An amount of RNA was used which gave a linear range of response for PV65 and 18S (typically 100 ng-0.01 ng RNA/reaction). We used a universal RT-PCR protocol recommended by the manufacturer, that is, reverse transcription at 48°C for 30 min, denaturation and polymerase activation at 95°C for 10 min, followed by 50 cycles of denaturation at 92°C for 15 s and annealing/extension/plate reading at 60°C for 1 min. RT-PCRs for each sample and each gene were done in duplicate. PCR without reverse transcriptase was performed for each sample to control for the possible interference from genomic DNA contamination. The threshold amplification cycles (CT) at the reporter signal was normalized with the reference gene human 18S (h18S), ΔCT [ΔCT=CTX-CTR, the difference in threshold cycles for the target gene X and the reference (R)] was calculated [2,26].Mean ΔCT was compared among each sample group with the ANOVA test [27].
Phage plaque assay
The phage plaque assay was performed as described previously [23]. Phages from positive clones of interest were mixed with the cDNA insert-free phage of the cDNA library (generously provided by Dr. C.J. Wu at Harvard Medical School, MA) as internal negative controls at a ratio of 1:5.
Western blot
Western blot procedures were performed as described [8]. PV65 (eIF-2α) proteins on the blots were visualized, respectively, with 1:1000 diluted eIF-2α antibody (Cell Signaling Technology, Beverly, MA), and Phospho-eIF-2α (Ser51) Antibody (Cell Signaling).
Transcriptional clonality assay [2]
The genotypes for exonic polymorphisms of 5 X-chromosome genes (MPP1, IDS, G6PD, BTK and FHL1) of three patients with PV were determined [2]. To examine the clonality of hematopoietic cells in these patients before and after IFN-α therapy, the peripheral blood specimens were drawn every 3 months and RNA was extracted from platelets and granulocytes. The mRNA expressions of the informative polymorphic genes were then assayed using single-stranded conformation polymorphism (SSCP) analysis as previously described [2].
Results
Identification of two novel PV-associated SEREX antigens
We performed SEREX screening of a human allogeneic testis expression cDNA library using diluted sera collected from the patients with PV who responded to IFN-α therapy. We chose sera from three patients with PV for cDNA library screening based on two criteria: (1) the patients’ diseases had undergone remission, as judged by conversion from monoclonal to polyclonal hematopoiesis as determined by analyses of the patients platelets and granulocytes by transcriptional based X-chromosome inactivation assay [2] (not shown); and (2) improved blood counts [2,3]. Initial screening of 1 × 106 λ recombinant phage clones led to identification of 15 positive clones (not shown). Subsequently, after several rounds of the purification of positive phage plaques and further confirmation on their antigen specificities, two independent cDNA clones were identified.
A novel PV-associated SEREX tumor antigen, PV65
The DNA analysis of the first clone with a 619 bp insert showed that it was identical to eukaryotic translation initiation factor 2α (eIF-2α) (GenBank accession: NM_032025) [28]. The eIF-2α is a 585 aa protein with a molecular size of 65 kDa (Fig. 1A), which was thus referred to as PV65 [28].
Figure 1.
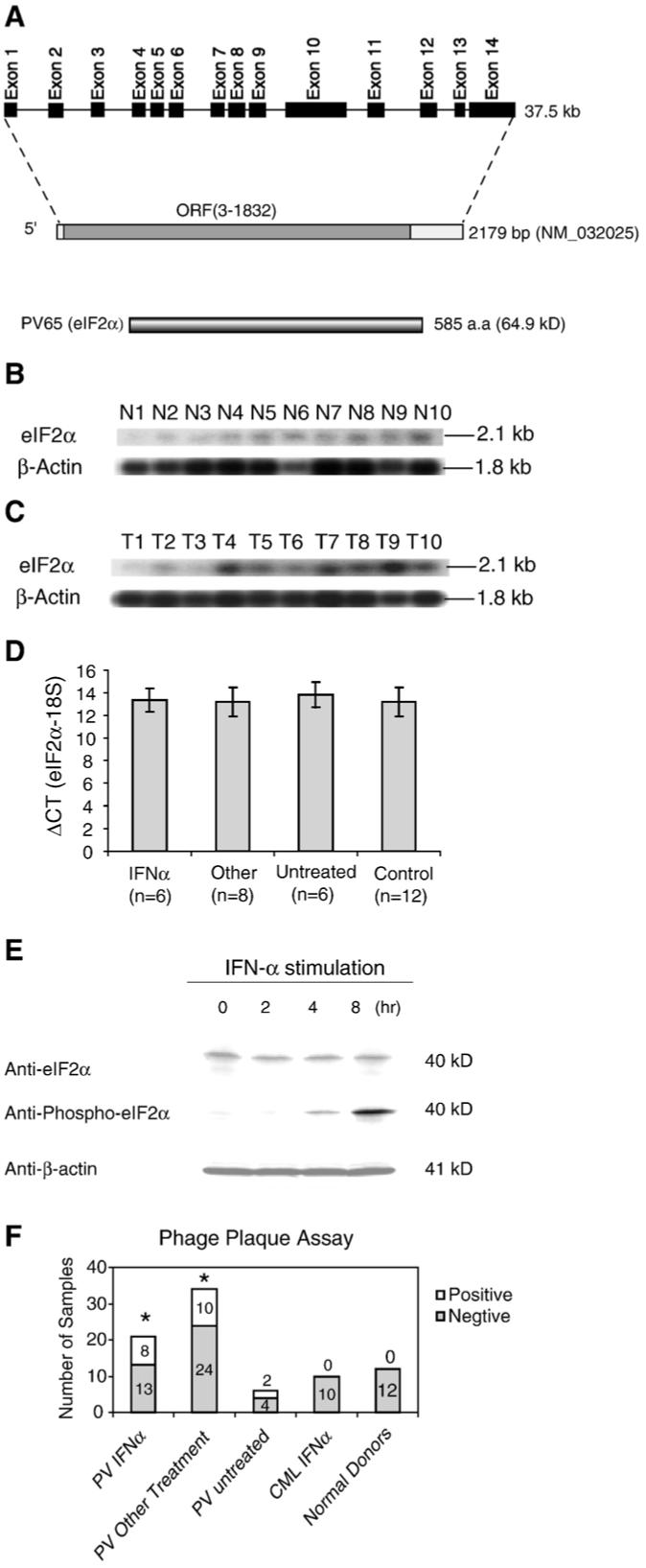
The novel tumor antigen PV65 (eIF-2α). (A) Schematic representation of the genomic structure, mRNA, and protein structure of tumor antigen PV65 (eIF-2α, GenBank accession number: NM_032025). (B) The expression of PV65 transcripts in normal tissues detected by Northern blot. The lanes N1 to N10 indicate various normal tissues in the order of brain (N1), liver (N2), placenta (N3), small intestine (N4), colon (N5), thymus (N6), spleen (N7), prostate (N8), testis (N9), and ovary (N10), respectively. The hybridization analyses of the normal tissue and tumor cell expression (BD Clontech) with 32P-labelled specific probes, as indicated, were performed, respectively. The transcript sizes are indicated with kilobases (kb). (C) The expression of PV65 transcripts in tumor cells detected by Northern blot. The lanes T1 to T10 indicate various tumor cells in the order of acute T cell leukemia (Jurkat cells) (T1), Burkitt’s lymphoma (CA46) (T2), breast cancer (MDA-MD-453) (T3), Burkitt’s lymphoma (Namalwa) (T4), epidermal carcinoma (A-431) (T5), uterine carcinoma (MES-SA) (T6), Burkitt’s lymphoma (Raji) (T7), osteosarcoma (MG-63) (T8), histiocytic lymphoma (U-937) (T9), and cervical adenocarcinoma (Hela S3) (T10), respectively. (D) The expression of PV65 transcripts in the granulocytes from PV patients and healthy donors detected by quantitative RT-PCR. The expression levels of PV65 transcripts are expressed as the ΔCT (PV65-18S). Low ΔCT values indicate higher expression of the specific gene. The experiments were repeated for three times. The mean and standard deviation for each group were calculated. (E) Western blot analyses of PV65 protein and phosphorylated PV65 protein in K562 myeloid leukemia cells. The expression levels of PV65 protein and phosphorylated PV65 protein in K562 cells at 0, 2, 4, and 8 h after stimulation by IFN-α were assayed with Western blots using anti-PV65 and anti-phosphorylated PV65, respectively. The Western blot analysis for the house keeping protein control β-actin was also performed using anti-β-actin as protein loading control. (F) The IgG antibody reactions to the tumor antigen PV65 detected by phage plaque assay. The detection rates in each group are presented with the empty column as the positive (on the top) and the solid column as the negative (on the bottom). The experiments were repeated for three times. The representative results are shown. The groups, whose detection rates of the IgG antibody reactions to PV65 are statistically higher than that of healthy donors (the Chi-Square Goodness-of-Fit Test; p < 0.05), are marked with *.
Furthermore, the Northern blot showed the expression of PV65 was low in normal tissues (Fig. 1B), but was significantly upregulated in some tumor cells, i.e. Burkitt’s lymphoma (T4, and T7), osterosarcoma (T8), and histocytic lymphoma (T9) (Fig. 2C) in comparison to that of β-actin housekeeping gene control. Previous reports and the data deposited in the NCBI-Unigene website in NCBI-GenBank showed that the PV65 expression was increased in some solid tumors including mammary tumors [29], melanomas, and colon cancers [30], compared to normal tissues, suggesting that PV65 expression might be modulated in patients with PV. To test this possibility, we performed quantitative PCR assay in measuring PV65 expression in granulocytes from patients with PV. As shown in Fig. 1D, PV65 expression [the ΔCT (PV65-18S)] in patients with PV was not significantly higher than that in healthy donor controls (p > 0.05), suggesting that the increased immunogenicity of PV65 in patients with PV might not be due to the higher expression of PV65 in granulocytes in patients with PV. Of note, this expression pattern of PV65 is not unique since a previous study also reported that a solid tumor associated gene 1 (STAG1/PMEPA1) is upregulated in some solid tumors but not in leukemia samples [31]. It is also noteworthy that the discrepancy between the numbers of patients and healthy controls in Fig. 1D and that in Fig. 1F resulted from the limited volumes of some blood samples, in which high quality RNAs could not be prepared but the sera could be prepared for performing the experiments presented in Fig. 1F.
Figure 2.
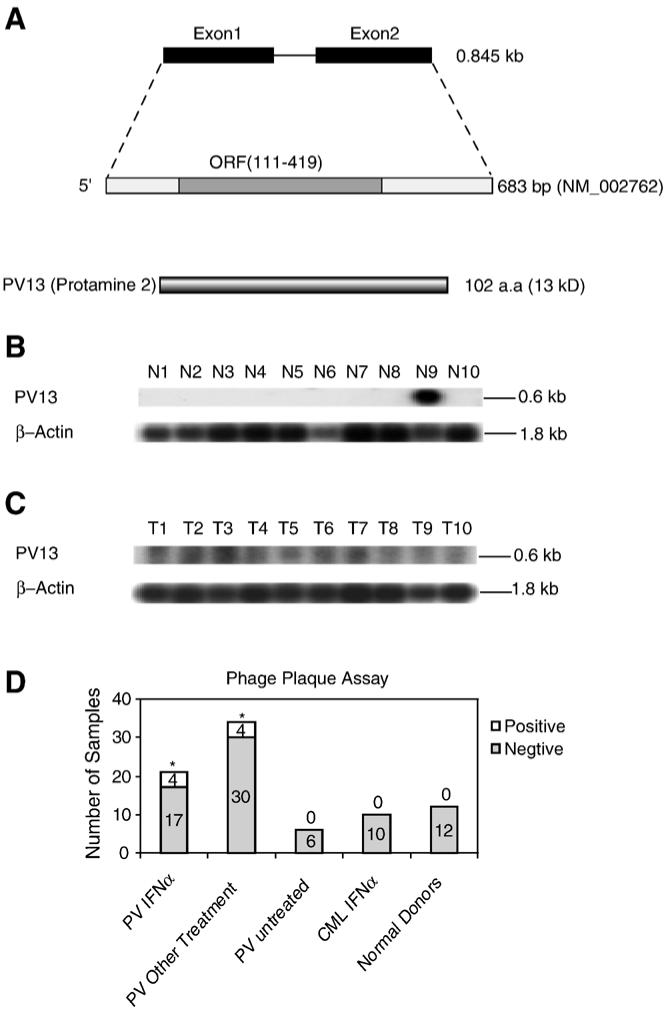
The novel tumor antigen PV13 (protamine 2). (A) Schematic representation of the genomic structure, mRNA, and protein structure of tumor antigen PV13 (protamine 2, GenBank accession number: NM_002762). (B) The expression of PV13 transcripts in normal tissues detected by Northern blot. The lanes N1 to N10 indicate various normal tissues in the order of brain (N1), liver (N2), placenta (N3), small intestine (N4), colon (N5), thymus (N6), spleen (N7), prostate (N8), testis (N9), and ovary (N10), respectively. The hybridization analyses of the normal tissue and tumor cell expression (BD Clontech) with 32P-labelled specific probes, as indicated, were performed, respectively. The transcript sizes are indicated with kilobases (kb). (C) The expression of PV13 transcripts in tumor cells detected by Northern blot. The lanes T1 to T10 indicate various tumor cells in the order of acute T cell leukemia (Jurkat cells) (T1), Burkitt’s lymphoma (CA46) (T2), breast cancer (MDA-MD-453) (T3), Burkitt’s lymphoma (Namalwa) (T4), epidermal carcinoma (A-431) (T5), uterine carcinoma (MES-SA) (T6), Burkitt’s lymphoma (Raji) (T7), osteosarcoma (MG-63) (T8), histiocytic lymphoma (U-937) (T9), and cervical adenocarcinoma (Hela S3) (T10), respectively. (D) The IgG antibody reactions to the tumor antigen PV13 detected by phage plaque assay. The detection rates in each group are presented with the empty column as the positive (on the top) and the solid column as the negative (on the bottom). The experiments were repeated for three times. The representative results are shown. The groups whose detection rates of the IgG antibody reactions to PV13 are statistically higher than that of healthy donors (the Chi-Square Goodness-of-Fit Test; p < 0.05) are marked with *.
Furthermore, since phosphorylation may increase the immunogenicity of the proteins [32], we tested the possibility whether PV65 can be phosphorylated by RNA-dependent protein kinase PKR [33] activated via IFN-α stimulation in cultured K562 myeloid leukemia cells. Since there are no cultured PV cell lines available, using K562 myeloid leukemia cells due to the similarity among myeloproliferative diseases in our study was well justified. By using anti-PV65 (eIF-2α) antibody and anti-phosphorylated PV65 (eIF-2α) antibody to perform Western blots, as shown in Fig. 1E, the results showed that IFN-α stimulation did not significantly upregulate the expression of PV65 protein in myeloid leukemia cells, which corresponded well to that of PV65 expression in granulocytes in patients with PV detected by quantitative PCR (Fig. 1D). Interestingly, IFN-α stimulation significantly promoted its phosphorylation of PV65 (Fig. 1E), presumably via PKR [33], which was also demonstrated in hematopoietic cells [34]. Thus, this result shown in Fig. 1E required no confirmation in PV cells. These results also suggested that the immunogenicity of the antigen PV65 might be associated with its critical role in IFN-α-induced inhibition of tumor growth and the phosphorylation of PV65 [32].
The results from phage plaque assay with the sera of three PV patients used in SEREX screening, showed that the serum sample from one patient recognized both of the two PV associated antigens, sera from two patients recognized one antigen, respectively, either PV65 or PV13 (data not shown). In addition, as shown in Fig. 1F, anti-PV65 IgG antibodies were not detected in any of the serum samples from 12 healthy donors, suggesting that PV65 was an authentic tumorassociated antigen. In contrast, anti-PV65 IgG antibodies were detected in 8 out of 21 serum samples (38.1%) from PV patients receiving IFN-α therapy and also detected in 10 out of 34 serum samples (29.4%) from PV patients receiving other treatments. The detection rates of anti-PV65 IgG antibodies in both groups of PV patients were significantly higher than that of healthy donors (p < 0.05). Of note, anti-PV65 IgG antibodies were detected in two out of six patients with PV who have not received any treatment. Because of the limited number in this group, it was difficult to have a statistical comparison between the untreated group of PV patients and the two groups of PV patients receiving IFN-α and other therapies in detection of anti-PV65 IgG antibodies. Our previous reports on the identification of the novel tumor antigens CML66L [8] and CML28 [9] suggested that the immunogenicity of the antigens CML66L and CML28 was enhanced in IFN-α responders. We also noticed that the detection rates of anti-PV65 antibody responses (Fig. 1F) in PV patients receiving IFN-α were higher than that in PV patients receiving other treatment. However, there were no statistical differences between these two groups of PV patients in their reactions to these novel antigens (p > 0.05). Furthermore, in comparison to patients with PV, IgG antibody reactions to PV65 were not detected in 10 patients with CML who received IFN-α treatment (p > 0.05), suggesting that humoral reactions to PV65 might play a specific role in patients with PV. However, future extensive studies may be needed to verify this finding. Finally, there were no significant differences in the detection of IgG antibody reactions to PV65 among the PV patients receiving the therapeutics other than IFN-α (not shown). Taken together, the anti-PV65 IgG antibody reactions were detected in a fraction of patients with PV who received IFN-α and other therapies. Of note, the phage plaque assay used in this study does not detect non-phosphorylated proteins. Thus, some humoral immune reactions directed against phosphorylated PV65 might be missed out.
Another PV-associated SEREX tumor antigen, PV13 (protamine 2)
The second identified clone had a 737 bp insert. This clone was identical to protamine 2 (Prm2) (GenBank accession: NM_002762)(Fig. 2A). The Protamine 2 gene spans 0.85 kb with 2 exons. The protamine 2 is a spermatid-specific basic protein with 102 aa [35] with a molecular size of 13 kDa, which was thus referred to as PV13. PV13 was previously found to be one of the major DNA-binding proteins in sperm, and functional in packaging of DNA in a small volume [35].
In addition, the Northern blot analyses showed that expression of protamine 2 in 10 normal tissues was restricted to testis (N1-N10, Fig. 2B). In contrast, high expression was found in tumor cells (Fig. 2C), especially in acute T cell leukemia (T1), Burkitt’s lymphoma (T2, T4, and T7), breast carcinoma (T3), and uterine carcinoma (T6). These results suggested that protamine 2 upregulation in tumor cells was not as pronounced as that of self-tumor antigens CML66 [8,36] and CML28 [9]. As reported previously [37], the technical problem in differentiating PV13 genomic DNA and PV13 cDNA prevented us from performing quantitative PCR on RNA samples from patients with PV, however, aberrant upregulation of testis antigen protamine 2 in the PV malignant clones might result in the increased immunogenicity of this antigen in patients with PV enhanced by IFN-α therapy.
The results from phage plaque assay showed that PV13 was not recognized by sera from normal healthy donors, suggesting that they were authentic tumor-associated antigens (Fig. 2D). In contrast, IgG antibody reactions to PV13 was detected in 19.1% of patients with PV who were treated by IFN-α, and in 11.8% of patients with PV who received other treatments, which was significantly higher than the reaction rates in healthy donors (p<0.05) (Fig. 2D). Of note, anti-PV13 IgG antibodies were not detected in the samples from six patients with PV who have not received any treatment (Fig. 2D). Because of the limited number in this group, it was difficult to make a statistical comparison between the untreated group of PV patients and the two groups of PV patients receiving IFN-α and other therapies in detection of anti-PV13 IgG antibodies. Furthermore, similar to antigen PV65, in comparison to patients with PV, IgG antibody reactions to PV13 were not detected in 10 patients with CML who received IFN-α treatment (p<0.05), suggesting that humoral reactions to PV13 might play a specific role in patients with PV. Finally, similar to PV65, there were no significant differences in the detection of IgG antibody reactions to PV13 among the PV patients receiving the therapeutics other than IFN-α (not shown). These results suggested that the immunogenicity of PV13 was significantly enhanced in patients with PV who received IFN-α and other therapies.
Discussion
Despite significant progress, the self-antigen targets that may mediate immune reactions contributing to beneficial responses to therapies in patients with myeloproliferative diseases (MPD) remained poorly defined before our recent report in characterization of a new unconventional tumor antigen MPD6 [6]. Recent reports showed that mRNA of polycythemia rubra vera-1 (PRV-1) is overexpressed in mature peripheral blood granulocytes from patients with PV [38,39]. The issue of whether PRV-1 can serve as a differentiation antigen eliciting specific anti-PV immune responses is under investigation. Prchal and co-workers have reported reversion of clonal to polyclonal hematopoiesis in PV patients who responded to therapies [2]. These studies have laid a foundation for definition of therapeutic immune reactions in patients with PV. Further, Prchal’s laboratory also suggested that multiple genetic defects are involved in the early pathogenesis of PV [40], reflecting tumor heterogeneity, presumably antigen heterogeneity, and immune reaction heterogeneity as well [41]. Therefore, it would be beneficial that the future antigen-specific vaccine, based on the screening from this report, encompasses a broader array of tumor antigens to encompass various subpopulations of tumor cells expressing different tumor antigens [41].
By applying SEREX, we have identified two novel antigens that are capable of eliciting potent humoral immune responses associated with PV remission. To our knowledge, this is a part of the first such a study in BCR-ABL negative MPD[1,6]. The analyses showed that these novel tumor antigens are not identical to any hematologic malignancy antigens identified previously with SEREX [17] or other approaches[22,42]. These antigens were identified using sera from female patients, however, the chromosomal locations of these gene loci (PV65 at 3q25, and PV13 at 16p13.2) showed that they were encoded by genes located in autosomal chromosomes, but not in the male-specific Y chromosome [43], suggesting that anti-tumor immune reactions in PV patients might be partially mediated by the novel self-tumor antigens identified in this study.
IFN-α-induced, double-stranded (ds) RNA-activated protein kinase (PKR) is a key mediator of the antiviral and antiproliferative effects of IFN [44]. A known physiological substrate of PKR activity is the α subunit of the eukaryotic translation initiation factor eIF-2α [45]. As shown for other IFN-α inducible genes [46], we found that increased phosphorylation of eIF-2α, via IFN-α stimulation or activation of IRF-1 [47,48] and PKR [44] potentially associated with other therapies, might enhance their abilities to elicit immune responses in patients. Posttanslational modification, including protein phosphorylation has been shown to be an important pathway for self-proteins to gain the immunogenicity [32].
A recent report from our laboratory showed that upregulation via alternative splicing is a novel mechanism for the generation of the immunogenicity of self-tumor antigen CML66-L [11]. Our results further demonstrated here that PV13 is upregulated in tumor cells, suggesting that overexpression of PV13 antigen in tumor cells is one of the mechanisms for eliciting immune reactions. A recent study reported that PV13 (protamine 2) is a tumor antigen (MAD-CT-1) associated with a subgroup of patients with prostate cancer (4.6%) [49]. Cumulatively, our results and others’ suggest that PV13 (protamine 2) may be a broadly immunogenic tumor antigen, which corresponds well with our results on PV13 expression in various tumor cells. As suggested previously, lower rates of antibody reactions to PV13 in patients with PV and prostate cancer are similar to the expression rates of cancer testis antigens (2-31%) [50], which may result in the status of methylation-demethylation of tumor antigen promoters in tumor cells [17].
Correlation of antigen-specific IgG immune reactions with remission in PV patients suggests that immune reactions mediated by these novel antigens may contribute to the MPD remission. This may be further verified in the future studies. Most recently, a gain-of-function acquired somatic mutation (V617F) of the tyrosine kinase JAK2 has been identified in most patients with PV and other MPDs [51-55]. The important issue of how the activating mutation of JAK2 may modulate the expression of tumor antigens and anti-tumor immune reactions is under investigation [56]. Taken together, our results suggest that novel PV associated tumor antigens may elicit anti-tumor humoral immune reactions in patients with PV. This provides new insights into the mechanism underlying the regulation of the self-antigen repertoire in eliciting anti-tumor immune reactions in patients with myeloproliferative diseases and suggests their potential as the targets of novel immunotherapy.
Acknowledgments
We are grateful to Drs. B. Ashby at Temple University, C.J. Wu, at Harvard Medical School, S.A. Krawetz at Wayne State University, Candice Price and Alec Merber at New York Presbyterian Hospital-Weill Cornell Medical Center, Ms. K. Franks at Baylor College of Medicine, and Ms. Diane Durr at Temple University for their assistance. This work was partially supported by NIH grant AI054514 (XFY); funds from Leukemia and Lymphoma Society and the Myeloproliferative Disorders Foundation (XFY), and funds from the Cancer Research and Treatment Fund (RTS).
References
- [1].Gilbert HS. Current management in polycythemia vera. Semin. Hematol. 2001;38:25–28. doi: 10.1016/s0037-1963(01)90137-4. [DOI] [PubMed] [Google Scholar]
- [2].Liu E, Jelinek J, Pastore YD, Guan Y, Prchal JF, Prchal JT. Discrimination of polycythemias and thrombocytoses by novel, simple, accurate clonality assays and comparison with PRV-1 expression and BFU-E response to erythropoietin. Blood. 2003;101:3294–3301. doi: 10.1182/blood-2002-07-2287. [DOI] [PubMed] [Google Scholar]
- [3].Lengfelder E, Berger U, Hehlmann R. Interferon alpha in the treatment of polycythemia vera. Ann. Hematol. 2000;79:103–109. doi: 10.1007/s002770050563. [DOI] [PubMed] [Google Scholar]
- [4].Fujii S. Role of interferon-alpha and clonally expanded T cells in the immunotherapy of chronic myelogenous leukemia. Leuk. Lymphoma. 2000;38:21–38. doi: 10.3109/10428190009060316. [DOI] [PubMed] [Google Scholar]
- [5].Sacchi S, Kantarjian H, O’Brien S, Cohen PR, Pierce S, Talpaz M. Immune-mediated and unusual complications during interferon alfa therapy in chronic myelogenous leukemia. J. Clin. Oncol. 1995;13:2401–2407. doi: 10.1200/JCO.1995.13.9.2401. [DOI] [PubMed] [Google Scholar]
- [6].Xiong Z, Liu E, Yan Y, Silver RT, Yang F, Chen IH, Chen Y, Verstovsek S, Wang H, Prchal J, Yang XF. An unconventional antigen translated by a novel internal ribosome entry site elicits antitumor humoral immune reactions. J. Immunol. 2006;177:4907–4916. doi: 10.4049/jimmunol.177.7.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad Sci. U. S. A. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang XF, Wu CJ, McLaughlin S, Chillemi A, Wang KS, Canning C, Alyea EP, Kantoff P, Soiffer RJ, Dranoff G, Ritz J. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc. Natl. Acad Sci. U. S. A. 2001;98:7492–7497. doi: 10.1073/pnas.131590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang XF, Wu CJ, Chen L, Alyea EP, Canning C, Kantoff P, Soiffer RJ, Dranoff G, Ritz J. CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res. 2002;62:5517–5522. [PMC free article] [PubMed] [Google Scholar]
- [10].Wu CJ, Biernacki M, Kutok JL, Rogers S, Chen L, Yang XF, Soiffer RJ, Ritz J. Graft-versus-leukemia target antigens in chronic myelogenous leukemia are expressed on myeloid progenitor cells. Clin. Cancer Res. 2005;11:4504–4511. doi: 10.1158/1078-0432.CCR-05-0036. [DOI] [PubMed] [Google Scholar]
- [11].Yan Y, Phan L, Yang F, Talpaz M, Yang Y, Xiong Z, Ng B, Timchenko NA, Wu CJ, Ritz J, Wang H, Yang XF. A novel mechanism of alternative promoter and splicing regulates the epitope generation of tumor antigen CML66-L. J. Immunol. 2004;172:651–660. doi: 10.4049/jimmunol.172.1.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ng B, Yang F, Huston DP, Yan Y, Yang Y, Xiong Z, Peterson LE, Wang H, Yang XF. Increased noncanonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J. Allergy Clin. Immunol. 2004;114:1463–1470. doi: 10.1016/j.jaci.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang F, Chen IH, Xiong Z, Yan Y, Wang H, Yang X-F. Model of stimulation-responsive splicing and strategies in identification of immunogenic isoforms of tumor antigens and autoantigens. Clinical Immunology. 2006;121:121–133. doi: 10.1016/j.clim.2006.06.007. [DOI] [PubMed] [Google Scholar]
- [14].Xiong Z, Shaibani A, Li YP, Yan Y, Zhang S, Yang Y, Yang F, Wang H, Yang XF. Alternative splicing factor ASF/SF2 is down regulated in inflamed muscle. J. Clin. Pathol. 2006;59:855–861. doi: 10.1136/jcp.2005.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yan Y, Chen Y, Yang F, Chen IH, Xiong Z, Wang J, Lachman LB, Wang H, Yang X-F. HLA-A2.1-restricted Tcells are reacted to SEREX-defined tumor antigen CML66L and suppressed by CD4 +CD25+ regulatory T cells. Intl J Immunopathol and Pharmacol. Sept 29; doi: 10.1177/039463200702000109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang X-F, Yan Y, Phan L, Xiong Z, Jelinek J. Upregulation of cancer-testis antigens in polycythemia vera cells suggests their potential role in immune responses against myeloproliferation. Blood. 2002;100:348b. [Google Scholar]
- [17].Chen Y. SEREX review, Cancer Immunity. http://www. cancerimmunity.org/SEREX/(2004)
- [18].Yang X, Mirkovic D, Zhang S, Zhang QE, Yan Y, Xiong Z, Yang F, Chen IH, Li L, Wang H. Processing sites are different in the generation of HLA-A2.1-restricted T cell reactive tumor antigen epitopes and viral epitopes. International Journal of Immunopathology and Pharmacology. 19(4) doi: 10.1177/039463200601900415. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gure AO, Tureci O, Sahin U, Tsang S, Scanlan MJ, Jager E, Knuth A, Pfreundschuh M, Old LJ, Chen YT. SSX: a multigene family with several members transcribed in normal testis and human cancer. Int. J. Cancer. 1997;72:965–971. doi: 10.1002/(sici)1097-0215(19970917)72:6<965::aid-ijc8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [20].Streilein JW. Unraveling immune privilege. Science. 1995;270:1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- [21].Chen YT, Old LJ. Cancer-testis antigens: targets for cancer immunotherapy. Cancer J. Sci. Am. 1999;5:16–17. [PubMed] [Google Scholar]
- [22].Yang F, Yang XF. New concepts in tumor antigens: their significance in future immunotherapies for tumors. Cell Mol. Immunol. 2005;2:331–341. [PubMed] [Google Scholar]
- [23].Wu CJ, Yang XF, McLaughlin S, Neuberg D, Canning C, Stein B, Alyea EP, Soiffer RJ, Dranoff G, Ritz J. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J. Clin. Invest. 2000;106:705–714. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang XF, Weber GF, Cantor H. A novel Bcl-x isoform connected to the T cell receptor regulates apoptosis in T cells. Immunity. 1997;7:629–639. doi: 10.1016/s1074-7613(00)80384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ng B, Yang Fan, Huston David P., Yan Yan, Yang Yu, Xiong Zeyu, Peterson Leif E., Wang Hong, Yang Xiao-Feng. Increased non-canonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J. Allergy Clin. Immunol. 2004;114:1463–1470. doi: 10.1016/j.jaci.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang J, Shridhar R, Dai Q, Song J, Barlow SC, Yin L, Sloane BF, Miller FR, Meschonat C, Li BD, Abreo F, Keppler D. Cystatin m: a novel candidate tumor suppressor gene for breast cancer. Cancer Res. 2004;64:6957–6964. doi: 10.1158/0008-5472.CAN-04-0819. [DOI] [PubMed] [Google Scholar]
- [27].Rosner B. Multisample Inference. Fifth edn. Duxbury; Australia, Canada, Mexico, Singapore, Spain, United Kingdom, United States: 2000. [Google Scholar]
- [28].Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol. Cell. Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM. Expression of a translationally regulated, dominantnegative CCAAT/enhancer-binding protein beta isoform and up-regulation of the eukaryotic translation initiation factor 2alpha are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–4386. [PubMed] [Google Scholar]
- [30].Rosenwald IB, Wang S, Savas L, Woda B, Pullman J. Expression of translation initiation factor eIF-2alpha is increased in benign and malignant melanocytic and colonic epithelial neoplasms. Cancer. 2003;98:1080–1088. doi: 10.1002/cncr.11619. [DOI] [PubMed] [Google Scholar]
- [31].Rae FK, Hooper JD, Nicol DL, Clements JA. Characterization of a novel gene, STAG1/PMEPA1, upregulated in renal cell carcinoma and other solid tumors. Mol. Carcinog. 2001;32:44–53. doi: 10.1002/mc.1063. [DOI] [PubMed] [Google Scholar]
- [32].Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000;2:101–114. doi: 10.1186/ar75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- [34].Dimitrova DI, Yang X, Reichenbach NL, Karakasidis S, Sutton RE, Henderson EE, Rogers TJ, Suhadolnik RJ. Lentivirus-mediated transduction of PKR into CD34(+) hematopoietic stem cells inhibits HIV-1 replication in differentiated T cell progeny. J. Interferon Cytokine Res. 2005;25:345–360. doi: 10.1089/jir.2005.25.345. [DOI] [PubMed] [Google Scholar]
- [35].Balhorn R, Cosman M, Thornton K, Krishnan VV, Corzett M, Bench G, Kramer C, Lee J, IV, Hud NV, Allen M, Prieto M, Meyer-Ilse W, Brown JT, Kirz J, Zhang X, Bradbury EM, Maki G, Braun RE, Breed W. Protamine mediated condensation of DNA in mammalian sperm. In: Gagnon C, editor. The Male Gamete: From Basic Knowledge to Clinical Applications. Cache River Press; Vienna, IL: 1999. pp. 55–70. [Google Scholar]
- [36].Yan Y, Xiong Z, Yang F, Yang Y, Ng B, Lachman L, Wang H, Yang X-F. Broadly immunogenic SEREX antigen CML66-L elicits humoral and cellular immune responses. FASEB J. 2004;18(4):A58. [Google Scholar]
- [37].Steger K, Fink L, Klonisch T, Bohle RM, Bergmann M. Protamine-1 and -2 mRNA in round spermatids is associated with RNA-binding proteins. Histochem. Cell Biol. 2002;117:227–234. doi: 10.1007/s00418-002-0385-3. [DOI] [PubMed] [Google Scholar]
- [38].Tefferi A, Lasho TL, Wolanskyj AP, Mesa RA. Neutrophil PRV-1 expression across the chronic myeloproliferative disorders and in secondary or spurious polycythemia. Blood. 2004;103:3547–3548. doi: 10.1182/blood-2003-10-3505. [DOI] [PubMed] [Google Scholar]
- [39].Pahl HL. PRV-1 mRNA expression and other molecular markers in polycythemia rubra vera. Curr. Hematol. Rep. 2003;2:231–236. [PubMed] [Google Scholar]
- [40].Kralovics R, Stockton DW, Prchal JT. Clonal hematopoiesis in familial polycythemia vera suggests the involvement of multiple mutational events in the early pathogenesis of the disease. Blood. 2003;102:3793–3796. doi: 10.1182/blood-2003-03-0885. [DOI] [PubMed] [Google Scholar]
- [41].Bhattachary-Chatterjee M, Nath Baral R, Chatterjee SK, Das R, Zeytin H, Chakraborty M, Foon KA. Counterpoint. Cancer vaccines: single-epitope anti-idiotype vaccine versus multiple-epitope antigen vaccine. Cancer Immunol. Immunother. 2000;49:133–141. doi: 10.1007/s002620050612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2004 doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu. Rev. Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- [44].Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- [45].Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase. PKR, EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad Sci. U. S. A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Matikainen S, Lehtonen A, Sareneva T, Julkunen I. Regulation of IRF and STAT gene expression by retinoic acid. Leuk. Lymphoma. 1998;30:63–71. doi: 10.3109/10428199809050930. [DOI] [PubMed] [Google Scholar]
- [48].Chelbi-alix MK, Bobe P, Benoit G, Canova A, Pine R. Arsenic enhances the activation of Stat1 by interferon gamma leading to synergistic expression of IRF-1. Oncogene. 2003;22:9121–9130. doi: 10.1038/sj.onc.1207090. [DOI] [PubMed] [Google Scholar]
- [49].Hoeppner LH, Dubovsky JA, Dunphy EJ, McNeel DG. Humoral immune responses to testis antigens in sera from patients with prostate cancer. Cancer Immun. 2006;6:1. [PubMed] [Google Scholar]
- [50].Scanlan MJ, Jager D. Challenges to the development of antigen-specific breast cancer vaccines. Breast Cancer Res. 2001;3:95–98. doi: 10.1186/bcr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee C, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- [52].Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- [53].Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, Zhao ZJ. Identification of an acquired JAK2 mutation in polycythemia vera. J. Biol. Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- [55].Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- [56].Verma A, Kambhampati S, Parmar S, Platanias LC. Jak family of kinases in cancer. Cancer Metastasis Rev. 2003;22:423–434. doi: 10.1023/a:1023805715476. [DOI] [PubMed] [Google Scholar]


