Abstract
The hypothalamo-pituitary-adrenocortical (HPA) axis is responsible for initiation of glucocorticoid stress responses in all vertebrate animals. Activation of the axis is regulated by diverse afferent input to the hypothalamic paraventricular nucleus (PVN). This review discusses brain mechanisms subserving generation and inhibition of stress responses focusing on the contribution of the limbic system and highlighting recent conceptual advances regarding organization of stress response pathways in the brain. First, control of HPA axis responses to psychogenic stimuli is exerted by a complex neurocircuitry that involves oligosynaptic networks between limbic forebrain structures and the PVN. Second, individual stress-modulatory structures can have a heterogeneous impact on HPA axis responses, based on anatomical microorganization and/or stimulus properties. Finally, HPA axis hyperactivity pursuant to chronic stress involves a substantial functional and perhaps anatomical reorganization of central stress-integrative circuits. Overall, the data suggest that individual brain regions do not merely function as monolithic activators or inhibitors of the HPA axis and that network approaches need be taken to fully understand the nature of the neuroendocrine stress response.
Keywords: HPA axis, hippocampus, amygdala, medial prefrontal cortex, glucocorticoid
The hypothalamo-pituitary-adrenocortical (HPA) axis is a critical adaptive system that maximizes survival potential in the face of physical or psychological challenge. The principal end-products of the HPA axis, glucocorticoid hormones, act on multiple organ systems, including the brain, to maintain homeostatic balance. While glucocorticoids are beneficial for short-term survival, prolonged exposure can lead to serious metabolic, immune and psychological dysfunction,1 requiring that glucocorticoid secretion be a tightly regulated process. Therefore, termination of the glucocorticoid response is well-controlled by efficient feedback inhibition mechanisms.
Activation of the HPA axis can occur reflexively in response to physical challenge. These ‘reactive’ responses are driven by ascending brain systems or circumventricular organs, which send direct projections to the hypothalamic paraventricular nucleus (PVN) (FIG. 1).2 Neurons of the PVN produce corticotrophin releasing hormone (CRH), the primary ACTH secretagogue, and thereby control ACTH release and subsequent glucocorticoid secretion. Reactive responses are initiated by stimuli that signal a direct threat to homeostasis or survival (so-called ‘systemic stressors’). Activation of the HPA axis and glucocorticoid secretion may also occur in the absence of frank threat, perhaps serving to prepare the organism for potential homeostatic challenge. Such ‘anticipatory’ responses are initiated through comparison of environmental stimuli to either innate programs (e.g., instinctual fear of predators) or memories (e.g., prior experience with a painful stimulus) (so-called ‘psychogenic stressors’). As would be expected, anticipatory responses rely heavily on limbic and associational systems capable of integrating the significance of observed external events and rely on indirect connections with the PVN (FIG. 1).2
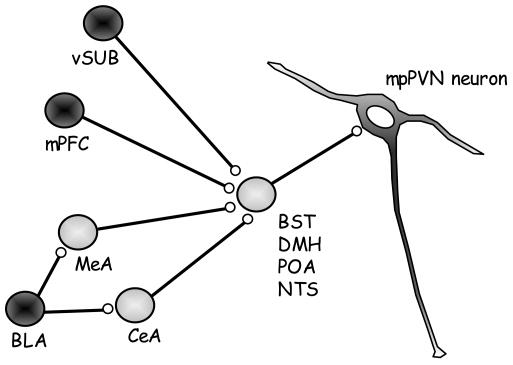
FIGURE 1. Major indirect projections of the limbic system to the medial parvocellular PVN.
Some of the direct projections innervated by the limbic system include the bed nucleus of the stria terminalis (BST), dorsomedial hypothalamus (DMH), preoptic area (POA) and the nucleus of the solitary tract (NTS). Innervation of direct projections by predominately glutamatergic inputs (dark circles) from the ventral subiculum (vSUB) and medial prefrontal cortex (mPFC) and predominately GABAergic inputs (light circles) from the medial amygdala (MeA) and central amygdala (CeA). Although the basolateral amygdala (BLA) innervates some PVN projecting neurons its primary outflow is glutamatergic inputs to the MeA and CeA.
Temporal prolongation of stress exposure (‘chronic stress’) causes marked enhancement in basal HPA tone as well as stress reactivity. These changes occur despite high resting or cumulative glucocorticoid secretion, suggesting that mechanisms are in place to bypass negative feedback inhibition of the HPA system.3 The net result is baseline glucocorticoid hypersecretion, adrenal hypertrophy and/or thymic atrophy;4, 5 an increase in central tone of the HPA axis (4, 6–9; e.g., enhanced CRH and vasopressin synthesis); down regulation of glucocorticoid receptors (GR) in key feedback regions;4, 8 facilitation of corticosteroid responses to novel stressors;3 reduction in glucocorticoid negative feedback efficacy;10 and depression-like behavioral changes.11 All of these sequelae can be associated with stress-induced changes in central pathways responsible for HPA axis regulation, prominently including pathways that mediate anticipatory stress responses. Neurocircuitry responsible for the transition to the chronic stress state have yet to be clearly established.
The goal of the current review is to summarize work studying limbic mechanisms responsible for regulation of the HPA axis during acute and chronic stress. This paper focuses on the hippocampus, amygdala and prefrontal cortex, key central circuits that are 1) involved in regulation of anticipatory stress responses and 2) implicated in pathologies associated with chronic stress.
ROLE OF THE HIPPOCAMPUS
The hippocampus exerts a trans-synaptic influence on the PVN that is primarily inhibitory in nature. Initial observations using loss-of-function approaches demonstrate that hippocampal lesions elevate basal glucocorticoid levels.12, 13 Ablation of the dorsal hippocampus or lateral fornix (which carries information from the hippocampus to the hypothalamus) disrupt the diurnal corticosteroid rhythm and elevates resting corticosteroids.14, 15 Lesions of the hippocampus proper prolong corticosteroid response to some stressors, like restraint16 and novelty (open field17), while responses to other stressors, such as ether inhalation,18 are unaffected. These studies provide evidence that the hippocampus is involved in control of both the circadian glucocorticoid rhythm and inhibition of HPA axis responses to stress, the latter of which is specific for stimulus modality.
Both GR and mineralocorticoid receptors are expressed in high abundance in the hippocampus.19–21 This allows the hippocampus to detect a wide range of circulating glucocorticoid concentrations and places it in position to modulate negative feedback inhibition of the axis by stress levels of glucocorticoids. In agreement with this assessment, cortisol implants in the hippocampus flatten the corticosteroid circadian rhythm.22 In addition, blocking GR diminishes the inhibitory influence of the hippocampus on the HPA response to stress.23 Finally, deletion of the hippocampal and cortical GR in mouse increases AM corticosteroid secretion and causes resistance to dexamethasone negative feedback inhibition of the HPA axis,24 futher consistent with a role of the hippocampal GR, alone or in combination with cortical and lateral amygdala GR, in negative feedback regulation of the axis.
Regulation of HPA axis stress responses are controlled in large part by the ventral hippocampus/ventral subiculum (vSUB). Stimulation of the ventral hippocampus produces inhibition of HPA activity.25 Our group has demonstrated that vSUB lesions enhance PVN CRH peptide and mRNA expression and enhance HPA axis and PVN fos responses to restraint stress, but do not affect basal morning or evening corticosterone secretion. Lesions of the vSUB also prolong HPA axis responses to novelty, but do not affect responses to ether inhalation and result in a somewhat paradoxical reduction in responsiveness to hypoxia (see Table 1 for summary of data).17, 26, 27 Overall, the data are consistent with an inhibitory role of the vSUB on anticipatory stress responses.
TABLE 1.
Effect of ventral subiculum lesion on acute stress regulation
| Measure | Change | Comments |
|---|---|---|
| Basal AM corticosterone | nc | |
| Basal PM corticosterone | nc | |
| Corticosterone: Restraint | ↑ | 60 min |
| Corticosteone: Ether | nc | |
| Corticosterone: Novelty | ↑ | 120 min |
| Corticosterone: Hypoxia | ↓ | 30, 60 min |
| PVN CRH mRNA | ↑ | Basal: no increase following acute stress |
| PVN CRH peptide | ↑ | Basal: no increase following acute stress |
| PVN AVP mRNA | nc | |
| PVN Fos mRNA | ↑ | 60 min |
The vSUB is a point of outflow for hippocampal information destined for the hypothalamus. Efferents from the vSUB contact PVN projecting neurons in the bed nucleus of the stria terminalis (BST), the medial preoptic area, dorsomedial hypothalamus and other hypothalamic nuclei.2 These relay areas are densely populated with GABAergic neurons, many of which project directly to the PVN,28 allowing for a putative two-neuron relay between glutamatergic hippocampal outflow and GABA neurons controlling the HPA response to stressors (FIG.1).
The prominent role of the vSUB in acute responses to psychogenic stress led us to test its role in controlling physiological responses to chronic variable stress (CVS), a model of unpredictable stress used extensively in our laboratory.4 We hypothesized that loss of vSUB inhibition would exacerbate chronic stress-induced physiological changes. As can be observed in Table 2, chronic variable stress induced adrenal hypertrophy, thymic atrophy and reduced weight gain, as previously observed in our laboratory.4 Lesions of the vSUB did not alter the impact of CVS on any of these measures. Lesions of the vSUB increased basal CRH mRNA expression in unstressed animals, consistent with previous studies.17, 27 However, CVS exposure did not result in further increases in CRH mRNA levels. Finally, vSUB lesions did not affect resting corticosterone levels in CVS-exposed animals. Overall, the data suggest that the integrity of the vSUB is not required for regulation of responses to chronic stress.
TABLE 2.
Effect of ventral subiculum lesion on physiological responses to chronic variable stress
| Handled | Stressed | |||
|---|---|---|---|---|
| Saline | Ibotenate | Saline | Ibotenate | |
| Body Weight Change (gm) | 63.8 ± 10.9 | 62.5 ± 4.1 | 16.3 ± 4.4* | 19.8 ± 5.5* |
| Thymus Weight (mg/gm BW × 100) | 93.3 ± 5.5 | 84.1 ± 4.0 | 66.5 ± 6.6* | 62.6 ± 5.0* |
| Adrenal Weight (mg/gm BW × 100) | 11.1 ± 0.4 | 10.2 ± 0.6 | 15.4 ± 0.7* | 14.8 ± 0.6* |
| PVN CRH mRNA** (corrected gray level) | 73.8 ± 1.4 | 86.5 ± 3.0 | 87.6 ± 4.4 | 78.6 ± 2.6 |
Chronic variable stress significantly decreased body weight gain and thymus size and increased adrenal size. Ibotenate lesions of the vSUB did not alter any of the physiological variables in the handled animals nor did ibotenate lesions affect the response to chronic variable stress.
There was a significant interaction between ibotenate lesion and chronic variable stress on CRH mRNA levels in the PVN. BW, body weight.
P < 0,05 versus corresponding handled control.
ROLE OF THE AMYGDALA
Electrical stimulation of the amygdala increases corticosteroid secretion in rats,29 monkeys30 and humans.31 When the amygdala is destroyed there is a reduction in the stress response to olfactory stimuli but not to ether.13, 32 However, there is a marked subregional localization of stress-integrative functions within the amygdala. The central amygdaloid nucleus (CeA) is highly responsive to systemic stressors such as inflammatory challenge and hemorrhage,33, 34 and lesions specific to this region attenuate HPA axis responses to these types of stimuli, but not to restraint.35–37 In contrast, the medial amygdaloid nucleus (MeA) is differentially Fos responsive to stimuli such as noise, restraint, and forced swim,33, 34, 38–40 and lesions of this region reduce responses to restraint but not systemic interleukin-1β injection.35 Lesions of the MeA blocked the stress-induced decrease in median eminence stores of CRH and blocked the increase in ACTH and corticosterone to photic and acoustic stimuli but not to ether inhalation,41 again consistent with a stressor-specific role of this region.
The CeA and MeA have limited projections to the PVN (mainly to pre-autonomic neurons).42, 43 Therefore, like the vSUB, the CeA and MeA must affect PVN neurons via indirect pathways. Both nuclei are composed of primarily GABAergic projection neurons and thus are likely to function by removing inhibitory inputs to the PVN.2 Like the vSUB, the CeA and MeA have heavy projections to the BST, albeit to different subdivisions.44 The CeA and MeA projections to the BST are GABAergic45 and a sizable component of projections from the BST to the PVN are also GABAergic, suggesting that activation of the HPA axis may result from a disinhibitory process (see FIG.1). In the case of the MeA, there is a clear functional connection with the HPA mediated by the BST, as the corticosterone response to stimulation of the MeA can be inhibited by lesions of the BST.46 Therefore, the increase in HPA activity seen in response to activation of the CeA and MeA may be at least partially the result of the removal of inhibitory inputs from the BST to the PVN.43
The CeA projects prominently to anterior and lateral subdivisions of the BST, whereas MeA efferents preferentially innervate posterior and medial divisions.44 This differentiation may have functional consquences. Lesions of the anterolateral BST decrease basal CRH expresision and reduce HPA axis responses, whereas posteromedial lesions exacerbate CRH expression and stress response magnitude.47
In addition to the BST, the MeA and CeA also project to other PVN-projecting, stress regulatory nuclei. The MeA heavily innervates the preoptic area, another highly GABAergic region implicated in HPA axis inhibition. The CeA projects strongly to the nucleus of the solitary tract (NTS),2 a brainstem structure involved in activation of reactive stress responses.
The basolateral amygdala (BLA) shows pronounced Fos activation to psychogenic stressors, such as restraint, swim and footshock, but not following cytokine stimulation,33 suggesting a role in regulation of anticipatory stress. However, lesions of the BLA do not affect basal HPA activity or HPA axis responses to social interaction, novelty, restraint, ether, or cold,41 indicating that the BLA is not necessary for normal elaboration of the acute stress response.
Projections from the BLA primarily, but not exclusively, target other amygdaloid regions. Thus, a sizable proportion of BLA output is channeled through the CeA and the MeA.2 However, it is important to note that the BLA also projects to the anterodorsal BST and other PVN projecting nuclei,44 suggesting that this region is positioned to interact with the PVN independent of other amygdaloid nuclei.
The amygdala has received some attention as a possible mediator of chronic-stress induced drive of the HPA axis. Numerous studies report enhanced expression of CRH mRNA in the CeA following high dose steroid treatment48 or exposure to chronic stressors such as immobilization or neuropathic pain,49 but not CVS.50 These data indicate that CeA CRH induction is associated with chronic stress in some, but not all models. However, direct tests of CeA involvement in chronic stress have proven equivocal. For example, damage to the CeA is not able to inhibit development of physiological symptoms of chronic stress or block stress-induced increases in CRH mRNA expression in the CVS model.36 Notably, work from our group has also shown that MeA lesions are not effective in modulating central or peripheral HPA responses to chronic stress (unpublished observations), casting further doubt on the role of the primary amygdalar outflow in chronic stress integration.
On the other hand, the BLA, which is not involved in acute stress regulation, appears to have a role in chronic stress integration. In chronic stress animals, inactivation of the BLA with muscimol enhanced HPA activation following a novel (but not familiar) stressor,51 suggesting that the BLA serves to dampen responses within the context of chronic stress. In addition, the BLA receives input from the posterior paraventricular thalamus, which promotes facilitation of HPA response in animals under chronic stress.52
ROLE OF THE MEDIAL PREFRONTAL CORTEX
The medial prefrontal cortex is clearly involved in processing of stressful information. In rodent models, all divisions of the medial prefrontal cortex manifest robust c-fos induction and enhanced glucose utilization following acute exposure to numerous stressors (c.f.,38, 53–55). Lesion studies indicate that bilateral lesions of the prelimbic (PL) component of the medial prefrontal cortex enhance ACTH and corticosterone responses increase PVN Fos activation following stress.56–58 Like the hippocampus, the role of the PL is stimulus-specific: the HPA axis response to restraint is enhanced by lesions of this area, whereas the response to ether inhalation (a systemic stressor) is not affected by lesions.57, 58
The role of the infralimbic (IL) cortex is more complex. Electrolytic lesions of the IL cortex enhance ACTH secretion and PVN Fos activation following interleukin-1β administration, but not following restraint, suggesting involvement in inhibition of responses to systemic stressors.59 However, a recent study by Radley et al60 demonstrates that ibotenate lesions of the IL region reduce restraint-induced Fos activation in PVN CRH neurons, suggesting that this region of the medial prefrontal coretex may in fact enhance HPA responses to psychogenic stressors.
The influence of medial prefrontal cortex on stress responsiveness may be lateralized. Bilateral or right IL cortex lesions suppress corticosterone secretion, whereas unilateral, left-sided lesions do not.61 Importantly, neither dorsal nor ventral medial prefrontal cortex lesions affect basal AM or PM ACTH and corticosterone levels,57, 58 indicating that the medial prefrontal cortex selectively modulates stress-induced HPA axis activity. Thus, the data suggest a differential involvement of the PL and IL in regulating HPA axis responses to stressors that follow psychogenic vs. systemic pathways, respectively.
The PL and IL interact with numerous sites that may be responsible for communicating inhibition of HPA axis activity to psychogenic vs. systemic stressors, respectively. The PL connects to PVN-projecting regions of preoptic area, which is known to modulate HPA axis activation, and to the dorsal raphe, which contains serotonin neurons that are implicated in direct or indirect activation of the HPA axis.2, 62, 63 In addition, the PL has possible oligosynaptic connections with PVN via the BLA and paraventricular thalamus, and indeed appears to interconnect with the vSUB.62, 63 The IL has direct projections to the anteroventral BST, CeA, and NTS, all of which are implicated in HPA axis activation, as well as the dorsomedial and lateral hypothalamus, areas thought to be involved in HPA axis inhibition.2, 63, 64
There are numerous instances in which putative PL-PVN or IL-PVN relay sites are differentially activated during anticipatory vs. systemic stress responses. In general, PVN-interacting targets of the PL, such as the BLA and posterior BST, show greater Fos induction during anticipatory responses (e.g., respond to restraint, footshock or predator exposure but not ether inhalation, hypoxia or interleukin-1β injection).33, 38, 40, 65 Many targets of the IL are also differentially Fos activated by psychogenic stressors, including the anteroventral BST, dorsomedial hypothalamus and lateral hypothalamus.33, 38, 40, 65, 66 In the NTS, Fos is expressed during both anticipatory and reflexive responses.33 However, there is evidence to suggest that different NTS cell populations are activated by psychogenic vs. systemic stressors,39 raising the possibility that IL inputs to this region may mediate NTS responses in a modality specific manner, perhaps accounting for inhibitory actions of the IL on HPA responses to systemic stressors.
The medial prefrontal cortex plays a role in modulation of responses to chronic stress. In female rats, lesions of the medial prefrontal cortex enhance stress-induced Fos activation of the PVN and plasma catecholamine release (males not tested), suggesting that the medial prefrontal cortex affords some degree of protection from the effects of chronic stress.67 In addition, chronic stress causes retraction of prefrontal cortical dendrites,68 reduces prefrontal dopamine content69 and sensitizes norepinephrine release,70 indicating that alterations in medial prefrontal cortex function occur as a consequence of prolonged stress exposure.
LIMBIC INTEGRATION OF STRESS RESPONSES: SHIFTING PRIORITIES?
The data summarized above indicate that subregions of the amygdala, hippocampus and medial prefrontal cortex regulate HPA axis responses to acute stress. The regulatory role of these structures in stress integration is highly differentiated, dependent on both stressor type and functional variation among the anatomically defined subdivisions of these structures. In the case of the hippocampus, it is possible that basal feedback inhibition and psychogenic stress inhibition are processed by different topographical divisions of the structure (dorsal vs. ventral hippocampus). In the amygdala, stimulation of responses to psychogenic vs. systemic stimuli are conferred by different output regions (medial vs. central nuclei). Finally, in the medial prefrontal cortex, excitatory and inhibitory functions may be controlled by neighboring cortical subdivisions (infralimbic vs. prelimbic). Thus, regulation of HPA axis (or other) responses to stressors is a distributed process that needs to be understood from the perspective of the very specialized roles of individual components of a limbic regulatory network.
The involvement of a given limbic region in acute stress integration does not necessarily extend into the realm of chronic stress. There are numerous regions (vSUB, CeA, MeA) that are necessary for normal responsivity to acute stress but do not affect primary indices of the chronic stress syndrome, either positively or negatively. Conversely, regions that are not required for acute stress regulation, such as the BLA, may be essential for processing of chronic stimulus exposure. Thus, the rules appear to change when stress becomes chronic. The mechanisms that underlie the ‘chronicity switch’ from acute to chronic time domains lies at the crux of understanding stress-related pathologies.
The complexities of limbic stress integration have implications for understanding stress related disease states (e.g., depression and PTSD). Given the data from experimental animals, it is likely that stress processing deficits in human will involve disruptions in integrated circuit regulation, rather than a disturbance of function that is localized to a given region or center. Thus, the challenge for the future will be design of therapies or pharmaceuticals that can specifically target neural networks responsible for inappropriate processing or interpretation of stressful information.
Acknowledgments
This work was supported by NIH grants MH049698, MH069725, MH069680, AG12962 and DK059803.
References
- 1.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 2.Herman JP et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Akana SF et al. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131:57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 4.Herman JP, Adams D, Prewitt CM. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 5.Ulrich-Lai YM et al. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–73. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 6.DeGoeij DC, Binnekade R, Tilders FJ. Chronic stress enhances vasopressin but not corticotropin-releasing factor secretion during hypoglycemia. Am J Physiol. 1992;263:E394–399. doi: 10.1152/ajpendo.1992.263.2.E394. [DOI] [PubMed] [Google Scholar]
- 7.Chappell PB et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez, et al. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–37. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 9.Albeck DS et al. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17:4895–903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi K et al. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–97. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 11.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 12.Fendler K, Karmos G, Telegdy G. The effect of hippocampal lesion on pituitary-adrenal function. Acta Physiol Acad Sci Hung. 1961;20:293–7. [PubMed] [Google Scholar]
- 13.Knigge KM. Adrenocortical response to stress in rats with lesions in hippocampus and amygdala. Proc Soc Exp Biol Med. 1961;108:18–21. doi: 10.3181/00379727-108-26832. [DOI] [PubMed] [Google Scholar]
- 14.Fischette CT et al. Differential fornix ablations and the circadian rhythmicity of adrenal corticosteroid secretion. Brain Res. 1980;195:373–87. doi: 10.1016/0006-8993(80)90073-6. [DOI] [PubMed] [Google Scholar]
- 15.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Sapolsky RM et al. Elevation of hypophysial portal concentrations of adrenocorticotropin secretagogues after fornix transection. Endocrinology. 1989;125:2881–7. doi: 10.1210/endo-125-6-2881. [DOI] [PubMed] [Google Scholar]
- 17.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–59. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 18.Magarinos AM, Somoza G, De Nicola AF. Glucocorticoid negative feedback and glucocorticoid receptors after hippocampectomy in rats. Horm Metab Res. 1987;19:105–9. doi: 10.1055/s-2007-1011753. [DOI] [PubMed] [Google Scholar]
- 19.Herman JP et al. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–94. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 20.Reul JM, deKloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 21.Reul JM, deKloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986;24:269–72. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- 22.Slusher MA. Effects of cortisol implants in the brainstem and ventral hippocampus on diurnal corticosteroid levels. Exp Brain Res. 1966;1:184–94. doi: 10.1007/BF00236870. [DOI] [PubMed] [Google Scholar]
- 23.Feldman S, Weidenfeld J. Glucocorticoid receptor antagonists in the hippocampus modify the negative feedback following neural stimuli. Brain Res. 1999;821:33–7. doi: 10.1016/s0006-8993(99)01054-9. [DOI] [PubMed] [Google Scholar]
- 24.Boyle MP et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–8. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casady RL, Taylor AN. Effect of electrical stimulation of the hippocampus upon corticosteroid levels in the freely-behaving, non-stressed rat. Neuroendocrinology. 1976;20:68–78. doi: 10.1159/000122470. [DOI] [PubMed] [Google Scholar]
- 26.Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145:3763–8. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- 27.Herman JP et al. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 28.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: Evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 29.Redgate ES, Fahringer EE. A comparison of the pituitary adrenal activity elicited by electrical stimulation of preoptic, amygdaloid and hypothalamic sites in the rat brain. Neuroendocrinology. 1973;12:334–43. doi: 10.1159/000122182. [DOI] [PubMed] [Google Scholar]
- 30.Mason JW. Plasma 17-hydroxycorticosteroid levels during electrical stimulation of the amygdaloid complex in conscious monkeys. Am J Physiol. 1959;196:44–8. doi: 10.1152/ajplegacy.1958.196.1.44. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher BB et al. The effect of electrical stimulation of medial temporal lobe structures in epileptic patients upon ACTH, prolactin, and growth hormone. Neurology. 1987;37:299–303. doi: 10.1212/wnl.37.2.299. [DOI] [PubMed] [Google Scholar]
- 32.Feldman S, Conforti N. Amygdalectomy Inhibits adrenocortical responses to somatosensory and olfactory stimulation. Neuroendocrinology. 1981;32:330–4. doi: 10.1159/000123182. [DOI] [PubMed] [Google Scholar]
- 33.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 34.Thrivikraman KV, Su Y, Plotsky PM. Patterns of Fos-Immunoreactivity in the CNS induced by repeated hemorrhage in conscious rats: Correlations with pituitary-adrenal axis activity. Stress. 1997;2:145–158. doi: 10.3109/10253899709014745. [DOI] [PubMed] [Google Scholar]
- 35.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–22. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 36.Prewitt CM, Herman JP. Hypothalamo-Pituitary-Adrenocortical regulation following lesions of the central nucleus of the amygdala. Stress. 1997;1:263–280. doi: 10.3109/10253899709013746. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Day TA, Buller KM. The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1beta administration. Neuroscience. 1999;94:175–83. doi: 10.1016/s0306-4522(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 38.Cullinan WE et al. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 39.Dayas CV et al. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 40.Figueiredo HF et al. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–58. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 41.Feldman S et al. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658:21–6. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- 42.Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: Possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 43.Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15:173–85. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 44.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 45.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–31. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 46.Feldman S, Conforti N, Saphier D. The preoptic area and bed nucleus of the stria terminalis are involved in the effects of the amygdala on adrenocortical secretion. Neuroscience. 1990;37:775–9. doi: 10.1016/0306-4522(90)90107-f. [DOI] [PubMed] [Google Scholar]
- 47.Choi DC et al. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 49.Ulrich-Lai YM et al. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav. 2006;88:67–76. doi: 10.1016/j.physbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Ostrander MM et al. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–17. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–9. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- 52.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 53.Duncan GE, Johnson KB, Breese GR. Topographic patterns of brain activity in response to swim stress: Assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J Neurosci. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kollack-Walker S et al. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–59. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- 55.Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- 56.Brake WG et al. Enhanced nucleus accumbens dopamine and plasma corticosterone stress responses in adult rats with neonatal excitotoxic lesions to the medial prefrontal cortex. Neuroscience. 2000;96:687–95. doi: 10.1016/s0306-4522(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 57.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamo-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Figueiredo HF et al. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–64. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 59.Crane JW, Ebner K, Day TA. Medial prefrontal cortex suppression of the hypothalamic-pituitary-adrenal axis response to a physical stressor, systemic delivery of interleukin-1beta. Eur J Neurosci. 2003;17:1473–81. doi: 10.1046/j.1460-9568.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- 60.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–76. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–40. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sesack SR et al. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 63.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 64.Hurley KM et al. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–76. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 65.Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–7. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- 66.Thrivikraman KV, Nemeroff CB, Plotsky PM. Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic-pituitary-adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res. 2000;870:87–101. doi: 10.1016/s0006-8993(00)02405-7. [DOI] [PubMed] [Google Scholar]
- 67.Gerrits M et al. Increased stress vulnerability after a prefrontal cortex lesion in female rats. Brain Res Bull. 2003;61:627–35. doi: 10.1016/j.brainresbull.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 69.Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- 70.Nisenbaum LK, Abercrombie ED. Presynaptic alterations associated with enhancement of evoked release and synthesis of norepinephrine in hippocampus of chronically cold-stressed rats. Brain Res. 1993;608:280–7. doi: 10.1016/0006-8993(93)91469-9. [DOI] [PubMed] [Google Scholar]