Abstract
One difference between the excitator model and other theoretical models of coordination is the mechanism of discrete movement initiation. In addition to an imperative signal common to all discrete movement initiation, the excitator model proposes that movements are initiated when a threshold element in state space, the so-called separatrix, is crossed as a consequence of stimulation or random fluctuations. The existence of a separatrix predicts that false starts will be caused by mechanical perturbations and that they depend on the perturbation's direction. The authors tested this prediction in a reaction-time task to an auditory stimulus. Participants applied perturbations in the direction of motion (i.e., index finger flexion) or opposed to the motion prior to the stimulus on 1/4 of the trials. The authors found false starts in 34% and 9% of trials following flexion perturbations and extension perturbations, respectively, as compared with only 2% of trials without perturbations, confirming a unique prediction of the excitator model.
Keywords: coordination dynamics, excitator model, false starts, model testing
Inspired by the theories of nonlinear dynamic systems and self-organization, researchers in the field of coordination dynamics seek to identify the laws and mechanisms underlying motor pattern generation, persistence, and change (for reviews, see Amazeen, Amazeen, & Turvey, 1998; Kelso, 1995). Laws of coordination are manifested functionally and emerge as a result of the interaction of the end effectors within constraints imposed by anatomy, environmental context, and task. One instantiation of task differences often discussed in the field of motor control is the division into rhythmic or discrete movement tasks (e.g., Hogan & Sternad, 2007; Huys, Jirsa, Studenka, Rheaume, & Zelaznik, 2008; Huys, Studenka, Rheaume, & Zelaznik, Jirsa, 2008). The way in which this distinction has been drawn provides a means for differentiating approaches to motor control and coordination.
Current theoretical models of coordination can be broadly sorted into three categories, each of which places a different emphasis on the distinction between discrete and rhythmic movements. One approach, used extensively to describe discrete movements, is based on the idea of optimization. This approach suggests that movements are organized to minimize some objective function (e.g., for jerk, see Flash & Hogan, 1985; for torque change, see Uno, Kawato, & Suzuki, 1989; for energy minimization, see Chapman, 1968; Hatze, 1977; for variance in the end position, see Harris & Wolpert, 1998; for overviews, see Nelson, 1983; Todorov, 2004). These models reproduce features of the movement that play a role in the coordination of the end effector, such as the shape of the trajectories (e.g., Morasso, 1981) or the speed–accuracy trade-off (Fitts, 1954; Plamondon & Alimi, 1997). Unfortunately, optimization approaches do not provide a mechanism for the initiation of movement and have not been extended to accommodate pattern formation between multiple effectors (e.g., between-finger coordination) or perception–action interactions with the environment. Another approach uses equilibrium-seeking models (Bizzi, Polit, & Morasso, 1976; Feldman, 1966; Kelso, 1977) including the α (Polit & Bizzi, 1978; Polit & Bizzi, 1979) and λ models (Balasubramaniam & Feldman, 2004; Feldman, 1966; Latash, 1993). These biomechanically and neurophysiologically motivated models provide a plausible mechanism by which the end position of a movement is reached, and they are well suited for describing discrete movements (but for expansions to rhythmic movements, see Adamovich, Levin, & Feldman, 1994; Feldman & Levin, 1995; Günther & Ruder, 2003; Kelso, Holt, Kugler, & Turvey, 1980; Latash, 1992). To describe rhythmic movements, researchers usually invoke an external periodic driving of the system parameters and explain the nature of that driving through a different mechanism. Last, researchers have proposed several functionally motivated mathematical models making reference to the dynamic nature of movement. These include Schöner's (1990) model, based on a set of Gonzalez–Piro oscillators, which assumes that discrete movements are captured by half cycles of a rhythmic movement, and Sternad, Dean, and Schaal's (2000) model of mutual inhibition of rhythmic and discrete movements. These dynamical approaches suffer from either not having been rigorously tested (Schöner) or being limited to specific movement paradigms (Sternad et al., 2000; see also Schaal, Sternad, Osu, & Kawato, 2004).
Jirsa and Kelso (2005) offered a unifying perspective through their excitator model. The excitator model proposes that movements, either discrete or rhythmic, can be described by a flow field in the state space or, equivalently, phase space (i.e., the space spanned by the state variables). Generally, the state variables are the variables that unambiguously identify the dynamic state of a system; for most movements, position and velocity suffice. The flow in phase space prescribes the evolution of the time-dependent state variables and corresponds to the rate of change of the state variables. In other words, the phase flow is simply an alternative expression of the deterministic component of any dynamic system (e.g., Strogatz, 1994; see also Saltzman & Kelso, 1987). The essential contribution of the excitator is not necessarily to capture another quantitative aspect of movement trajectories, but to provide the perspective of phase flow topologies as invariants of dynamics and, hence, of movement classes: Two dynamic systems with the same flow topology mathematically belong to the same equivalence class and can be transformed into each other (at least locally; see Huys, Jirsa, et al., 2008; Huys, Studenka, et al., 2008). In this sense, the excitator model offers a taxonomy of models of movement generation (of the same dimension) at a high level of abstraction and includes previous models of constant phase flow (see Huys, Jirsa, et al., 2008; Huys, Studenka, et al., 2008). The description of movement in terms of force fields (e.g., Balasubramaniam & Feldman, 2004; Bizzi, Chapple, & Hogan, 1982; Feldman, 1980a; Feldman, 1980b; Latash, 1993) is related but not equivalent, as force fields do not provide a unique description of the system dynamics. It is generally possible, however, to rewrite the force fields in terms of phase flow fields.
So what is gained by the excitator model's abstract approach? Systems with constant phase flows can be contrasted to systems with time-dependent phase flows. The latter include the equilibrium-point models and others (e.g., Sternad et al., 2000) in which the system parameters are changed on a temporal scale comparable with the movement and in which some of the complexity of the movement is captured by the time course of the system parameter changes. On the other hand, systems with a constant phase flow (excitators) by definition have constant system parameters and are only subject to unspecific stimuli.1 In other words, the phase flow is the same before and after the short-term stimulation. For models of this type, the majority of the complexity of the movement process is captured by the flow itself. The benefit of the excitator approach lies in the implementation of the phase flow pattern: Once the phase flow pattern is established, it does not need to be controlled any longer, unlike otherwise. In robotics, a similar approach toward the coding of different modes of operation is known as motion description language (Brockett, 1988; Manikonda, Krishnaprasad, & Hendler, 1998), in which phase flows of movement control are turned on and off for varying behavioral situations.
If constant phase flows exist, the question naturally arises about the nature of the neural substrate of the phase flow codes. The equilibrium-point (EP) approach identifies a physiologically plausible basis by which movements are controlled: A descending neural signal sets a value of λ, and the invariant characteristics (and hence force fields) emerge as a result of the intrinsic properties of the system (e.g., the stretch reflex). The neural substrate of the phase flow codes is not known, but it is open to investigation using modern imaging methods and neurophysiologically relevant quantities. To make a historic comparison from neuroscience, the FitzHugh–Nagumo system (FitzHugh, 1961; Nagumo, Arimoto, & Yoshizawa, 1962) offers a functional description of the single neuron dynamics without reference to neurobiology, which is captured by the Hodgkin–Huxley equations (Hodgkin & Huxley, 1952). Depending on the question asked by the neuroscience researcher, either the functional model or the biophysical model is used. Still, there are hints about the neural basis of phase flow codes in the literature. For example, Rabinovich, Huerta, and Varona (2006) showed that a small network of spiking neurons is capable of generating phase flows of various (though not arbitrary) topologies. On the network level, Graziano, Taylor, Moore, and Cooke (2002) have shown that long-lasting stimulations of primary motor and premotor networks in the monkey brain result in robust and reproducible trajectories in the physical space. These results clearly demonstrate that these brain areas are implicated in the coding of the procedural aspects of movement (in contrast to just coding for the endpoint), though the precise details of the code remain unclear at this point. It seems fair to say that the equilibrium approaches offer a reasonable explanation for the generation of simple movements ranging from the level of neurophysiology to the level of dynamic implementation. When the movements considered become more complex (in a functional sense), the phase flow codes offer a dynamic means to capture this complexity. So far, the latter approach is impoverished by its absence of neurophysiological insight—akin to the early behavioral evidence for the EP hypothesis (e.g., Asatryan & Feldman, 1965; Feldman, 1966; Kelso, 1977; Polit & Bizzi, 1978)—though it is attractive because of deep roots in the theory of dynamic systems. There is a clear need for further testing of the functional implications of phase flow codes. This is what we sought to do in the present study.
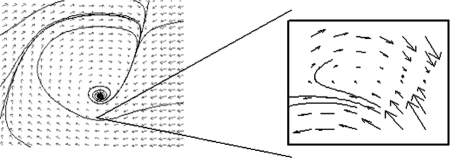
More specifically, in the present article, we focus on a particular type of movement, the discrete flexion–extension movement of a single end effector. Jirsa and Kelso (2005) argued that there are some archetypical phase flow patterns in movement control. They suggested that the particular dynamics of a discrete flexion–extension movement is determined by a monostable phase flow pattern with the characteristic topology shown in Figure 1 (the equations for the monostable phase flow can be found in the Appendix). A separatrix, shown in the insert of Figure 1, is a subset of points in the state space, that segregates the flow field into two regions.
FIGURE 1.
Computational simulations of the excitator's monostable phase flow (for details, see V. K. Jirsa & J. A. S. Kelso, 2005). On the left, the phase space is spanned by the state variables position (horizontal) and velocity (vertical). The flow field is indicated through vectors illustrating the direction and magnitude of the rate of change. Multiple example trajectories are plotted as they evolve in time toward a fixed point. The insert represents a magnification of the flow field in the neighborhood of the separatrix, and the diverging flows are visible.
For movements to be initiated in the monostable phase flow pattern, the effector must move into the region where the flows are directed away from the fixed point in Figure 1. Typically, this is accomplished through an informational term, such as an imperative stimulus in a reaction-time task, which pushes the system across the separatrix (for an example of an informational push, see Shaw & Kinsella-Shaw, 2007). In speeded reaction-time tasks, however, movements occurring before or in the absence of a stimulus, referred to as false starts, are sometimes observed (Collet, 1999; Tresilian & Plooy, 2006). Monostable phase flows propose two mechanisms for false starts. First, noise inherent in the system or fluctuations can cause crossing of the separatrix even in the absence of the imperative stimulus. With this mechanism, the frequency of false starts is a function of the strength of the noise and of the distance from the fixed point to the separatrix. Second, false starts may also be elicited by mechanical perturbations of the effector used in the task. In the present article, we exploit this second possibility. If a perturbation is applied in the direction of motion (e.g., to the left in Figure 1), the perturbation can bring the finger across the separatrix and initiate a movement, which implies that false starts should be increased following flexion perturbations. If perturbations are applied away from the intended direction of motion (e.g., to the right in Figure 1), the effector is moved away from the separatrix, decreasing the probability of a false start. These predictions are in contrast to what would be expected on the basis of equilibrium–point approaches. Movement initiation in equilibrium–point approaches occurs when the equilibrium point, and hence the flow field, shift in response to the imperative stimulus: Presumably false starts are a result of anticipating the stimulus. Perturbations in equilibrium-point models are used to demonstrate the return to the initial position (e.g., Feldman, 1966; Kelso & Holt, 1980) and are not predicted to cause false starts. Indeed, the return to the initial position may suggest that perturbations away from the intended direction of motion are more likely to cause false starts: The effector, by returning to the initial position, would be moving in the direction of motion and may presumably assist in movement initiation. Thus, response to perturbations during the initiation process provides a way to test a basic and unique prediction of the Jirsa and Kelso (2005) excitator model.
To examine this issue, we used a variation of a reaction-time task with perturbations (see also Valls-Sole, Rothwell, Goulart, Cossu, & Munoz, 1999). Participants performed a simple reaction-time task, flexing the right index finger in response to an auditory stimulus. A visual warning signal was given, followed by a foreperiod of either 1.5, 2.0, or 2.5 s. On one quarter of the trials, a mechanical perturbation was applied to the index finger either in the direction of the instructed movement (i.e., flexion) or in the direction away from the instructed movement (i.e., extension). The perturbation, if applied, was 250, 200, 150, 100, or 50 ms before the auditory stimulus. Variations in the foreperiod and time of perturbation provided the necessary window to examine whether the separatrix was stationary or it moved as a function of anticipation. The frequency of false starts was used as the primary measure for the existence and movement of the separatrix: If the separatrix exists, false starts should be more frequent following perturbations, and if the separatrix moves, the frequency of false starts should vary with both the foreperiod and time of perturbation. In addition, the model predicts a directional effect of the perturbation, with false starts occurring more often in flexion perturbation trials than in extension perturbation trials or trials without perturbations. On the other hand, if perturbations do not cause false starts to become more common, a key feature of the excitator's monostable phase flow, the separatrix, comes into serious question.
Method
Participants
In all, 8 participants (3 men, 5 women), all right handed by self-report, took part in the experiment. The procedures were authorized by the university's institutional review board, and informed consent was obtained prior to the experiment.
Apparatus
Participants placed their right index finger in a manipulandum that restricted finger movement to the metacar-pophalangeal joint. The manipulandum was attached to a potentiometer, which allowed measurement of the finger position. The manipulandum was connected to a Honeywell 22VM51-020-5 torque motor (Honeywell, Morristown, NJ) by a gear system, and we used this to provide perturbations (Kelso & Holt, 1980). The torque motors were controlled by a PC with a National Instruments PCI 6733 16-bit 8-channel analog output card (National Instruments, Austin, TX) using a MATLAB program (MathWorks, Natick, MA), and they were set so that the resulting perturbation shifted the unloaded manipulandum 5° in 25 ms. The National Instruments card was also used to create the auditory stimulus (50-ms duration, 440-Hz frequency, volume adjusted to the loudest setting at which the participants felt comfortable), which was sent to a set of headphones worn by the participant, and the visual warning signal (50-ms duration), which was given to the participants using a red LED placed 40 cm from the manipulandum. The finger position and envelope of the auditory stimulus and torque motor signal were collected on a second computer using a National Instruments PCI 6024 16-bit 8-channel analog input card with a MATLAB interface. All data were sampled at 1,000 Hz.
Task
The task was a simple reaction-time task in which participants were asked to respond to an auditory stimulus as quickly as possible by flexing their right index finger. Participants were instructed to begin each trial with the index finger in an extended position. A visual warning signal was provided, followed by a foreperiod of 1.5, 2.0, or 2.5 s.2 The foreperiod was followed by the auditory stimulus. To keep the participants from anticipating a perturbation, we applied mechanical perturbations to the finger in either the flexion or extension direction in one quarter of the trials. The perturbation was applied 250, 200, 150, 100, or 50 ms before the auditory stimulus (see Figure 2 for an experiment schematic). At each combination of foreperiod and perturbation time, we collected 12 trials for both flexion and extension perturbations and 360 trials at each of the three foreperiods. The trials were presented in random order and were collected in blocks of 12 trials, with each trial in a block separated by a variable 2–4-s time interval. One practice block was given prior to the beginning of the experiment. Each block of 12 trials lasted approximately 75 s, and the experiment took approximately 1.5 hr.
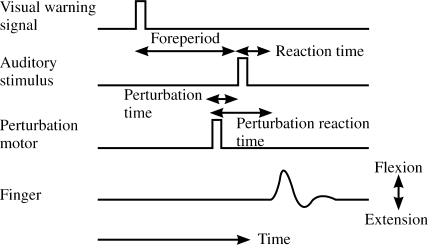
FIGURE 2.
False start paradigm showing events of a single trial. A visual warning signal is given, followed by a fore period of 1.5, 2.0, or 2.5 s, and then an imperative auditory stimulus is provided. On one quarter of the trials, a mechanical perturbation is given prior to the auditory stimulus at one of five times (250, 200, 150, 100, or 50 ms before the auditory stimulus).
Participants were instructed to respond as quickly as possible to the auditory stimulus. No target amplitude was specified, but participants were instructed to move the finger at least 20°, an amplitude that participants exceeded with a normal flexion movement. To maintain motivation throughout the experiment, we paid participants on the basis of their responses. Prior to the beginning of the experiment, participants performed 10 discrete finger movements as fast as possible, and we recorded the movement time for the first 20° of the flexion movements. Participants were given $0.05 for each response during the experiment when their movement times for the first 20° of the response were less than 75% of the established movement time.3 Feedback about the number of trials in which the movement time was below the standard was given at the end of each block of 12 trials. Financial reward for the participants varied from $5.25 to $9.30, suggesting that this procedure helped maintain participants' engagement in the task.
Analysis
It is typical that movement onsets are identified by finding when the velocity or acceleration of an effector exceeded a threshold (e.g., Teasdale, Bard, Fleury, Young, & Proteau, 1993). When applied to the current experiment, this method by itself often gave incorrect movement onsets when perturbations are applied: The velocity or acceleration thresholds were often exceeded either by the perturbation itself or by the finger returning to the original position (e.g., Feldman, 1966), even though a movement did not necessarily ensue. For this reason, an additional position threshold had to be applied to identify false starts so that a movement onset would be identified only if the subsequent movement was of greater amplitude than that seen in a return to the original position. Movement onsets were defined as the first time point when the finger velocity exceeded 60°/s and when the finger moved 5° in the flexion direction from a baseline position. To reduce biases as a result of a perturbation or lack thereof, we changed the baseline position so that the same subsequent movement amplitude was required to exceed the position threshold.4 When no perturbation was applied, the baseline was the mean finger position for the interval between 400 and 300 ms preceding the auditory stimulus. For the perturbation trials, the baseline was set as the maximum (flexion perturbation trials) or minimum (extension perturbation trials) position of the finger in the 23 ms following the perturbation onset. The addition of the position threshold made the estimate of movement onset more conservative: Movement onsets were identified on average 26 ms ± 12 ms later in the no-perturbation conditions when the position and velocity threshold was used than they were with a simple velocity threshold. (The same test could not be applied to the conditions with perturbations because the velocity threshold alone yielded false movement onset.)
False starts were defined as responses in which the movement onset preceded the auditory stimulus. Again, this is a conservative definition of false starts, as false starts in track and field are any starts that begin before 100 ms after the gun (Collet, 1999). Thus, this definition provides a minimum for the frequency of false starts. In all trials in which no false start was identified, reaction time was calculated as the time difference between the onset of the auditory stimulus and the onset of the finger movement. In trials with false starts, a perturbation response time—defined as the time difference between the onset of the perturbation and the onset of the finger movement—was calculated.
Statistics were run on the percentage of false starts, reaction time, and perturbation response time using three-way repeated measures analyses of variance (ANOVAs): 3 (foreperiods) × 6 (perturbation times) × 2 (perturbation direction). To render the percentage of false starts usable in an ANOVA, we performed an arctangent transform on the percentage of false starts prior to the ANOVA. Post hoc tests on significant effects were performed using a Tukey test.
Results
False Starts
Even with a conservative estimate of movement onset and a conservative definition of false starts, false starts were identified for all participants following perturbations. The ANOVA on the arctangent transform of the percentage of trials with false starts showed an effect of direction, F(1, 7) = 5.72, p < .05, and a significant interaction of perturbation time and direction, F(4, 28) = 9.38, p < .0001. The percentage of trials with false starts, collapsed across all participants and all three fore-periods, is shown in Table 1. False starts were rare when a perturbation was not applied, occurring in only 2% of the trials. When perturbations were applied, however, a significant increase in the percentage of false starts was found for both flexion and extension perturbations. With the exception of the −50-ms perturbation time, the percentage of false starts was greater for flexion perturbations than for extension perturbations, and it was significantly greater for the −150-, −200-, and −250-ms perturbation times. Taken together, these results show that perturbations cause false starts and that flexion perturbations produced more false starts than did extension perturbations, particularly when given at least 150 ms before the imperative stimulus.
TABLE 1. Percentage of Trials With False Starts at Each Perturbation Time, Collapsed Across All Participants and Three Foreperiods.
| Direction | Perturbation time (ms) |
|||||
| No perturbation | −50 | −100 | −150 | −200 | −250 | |
| Flexion (%) | 2 | 8 | 22a | 41a,b | 42a,b | 49a,b |
| Extension (%) | 2 | 13a | 16a | 12a | 1 | 1 |
Note. The no-perturbation condition is shown as a baseline for other measurements and is listed under both the flexion and extension columns.
A significant difference from the no-perturbation condition (p < .05).
A significant difference between the flexion and extension perturbations (p < .05).
Reaction Time and Perturbation Reaction Time
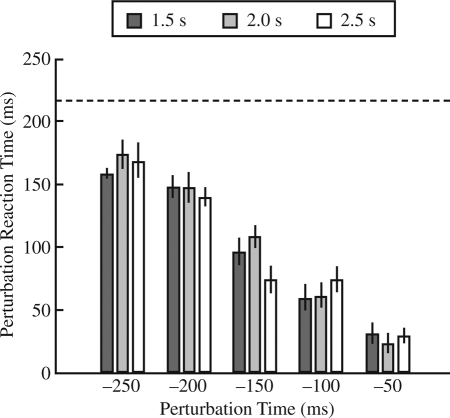
An ANOVA on the reaction time, using only those trials without false starts, showed no significant main effects or interactions (p > .05), with an overall mean of 217 ms ± 82 ms. On trials with false starts (and with perturbations), we performed a second ANOVA on the perturbation reaction time. A significant main effect of perturbation time, F(4, 28) = 16.33, p < .0001, and a significant interaction of perturbation time and foreperiod, F(8, 56) = 3.32, p < .01, were found. Figure 3 shows the perturbation reaction time as a function of perturbation time for each of the three foreperiods. A general pattern of a decrease in perturbation reaction time was found as the perturbation onset approached the auditory stimulus. This result is unsurprising as a long perturbation reaction time would result in a trial not being declared a false start for perturbations near the auditory stimulus. The significant interaction arose from the fact that the 2.5-s foreperiod in the −150-ms perturbation time condition resulted in a significantly lower perturbation reaction time than that found in the other two foreperiod conditions.
FIGURE 3.
Perturbation reaction time (in ms) for trials with false starts as a function of perturbation time (relative to the auditory stimulus) for all three foreperiod durations. The dashed line shows the mean reaction time across all conditions for the trials in which there were no false starts. Error bars indicate one standard error of the mean.
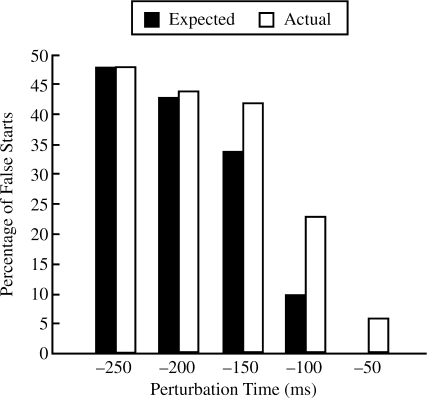
The analysis of the perturbation reaction time suffered from a potential confound; namely, that the perturbation reaction time had to be less than the time difference between the perturbation and the stimulus. Thus, the apparent decrease in the percentage of false starts as the perturbation onset approached the auditory stimulus may be an artifact caused by the conservative estimate of false starts: The movements could have been initiated in response to the perturbation, but the perturbation reaction time was too large for the movement to be classified as a false start. To address this problem, we examined the flexion perturbation conditions in more detail, using the distribution of perturbation reaction times at the −250-ms perturbation time condition as a baseline. At each perturbation time, the expected percentage of false starts was estimated by the percentage of trials in the −250-ms perturbation time condition in which the perturbation reaction time was less than the time difference between the perturbation and stimulus in the other perturbation time conditions (e.g., 200 ms in the −200-ms condition). The expected number of false starts and the actual percentage are shown in Figure 4. At each perturbation time, with the exception of the −250-ms condition, the expected number of false starts was less than the actual number of false starts. This observation suggests that movements initiated by the perturbation did not decrease as the perturbation onset neared the auditory stimulus and that the actual number of responses to the perturbation increased at small perturbation times.
FIGURE 4.
Percentage of false starts expected, given the distribution of perturbation response times in the −250-ms condition and the actual percentage of false starts as a function of perturbation time. At all perturbation times other than −250 ms, the actual percentage of false starts exceeded the expected percentage.
Discussion
A key prediction of the excitator model (Jirsa & Kelso, 2005) is that a mechanical perturbation of the effector used in a reaction-time task should result in false starts and that perturbations in the direction of the movement should result in more false starts than do perturbations away from the direction of movement. The experiment clearly showed that this prediction of the excitator model was confirmed: False starts were caused by mechanical perturbations of the effector used in the task, particularly when the perturbations were applied in the intended direction of motion. This finding is crucial for the existence of a separatrix because the separatrix is located at one side of the equilibrium point in phase space and hence requires the existence of nonuniformity in the likelihood of false starts. Actually, a separatrix at a uniform distance from the equilibrium point (like a circular structure in phase space) would require a third degree of freedom for the movement system to be able to return to the same point after a movement. The nonuniform distribution of false starts supports the viewpoint that the end effector acts as a two-dimensional dynamical system. Previous theories of discrete movement generation were not able to predict the increase in false starts following perturbations or their directional effect.
Alternative Explanations
To date, there have been few studies explicitly studying false starts as defined here: namely, responses prior to the imperative stimulus. The dearth of experimental evidence is not surprising. With false starts being present in only 2% of the unperturbed trials, it is difficult to see how to study them systematically without some means of increasing their number, as in the perturbations used here. As a result, only a few theories of false starts exist, and these theories cannot explain the results of the present article.
One possibility is that the method of identifying movement onsets introduces a bias that may explain the differences between flexion and extension perturbations. For example, flexion perturbations themselves exceed the velocity threshold in such a way that if the movement were to immediately continue with the same velocity, a movement onset (and therefore a false start) would be identified after the finger moved the five additional degrees required to exceed the position threshold, whereas the finger motion after an extension perturbation must be reversed before a movement onset can be identified. The large perturbation reaction times (see Figure 3) show that this did not occur: There was a delay between the offset of the perturbation and the onset of the movement of more than 100 ms at −250- and −200-ms perturbation times, which demonstrates that the movements were not merely continuations of the perturbations. In addition, the fact that false starts following extension perturbation actually decreased as the perturbation was further away from the imperative stimulus suggests that more false starts would not be found if there was more time in which to reverse the finger motion. Alternatively, a return to the initial position after a perturbation (e.g., Feldman, 1966) may assist a flexion movement following an extension perturbation and inhibit a flexion movement following a flexion perturbation. However, this explanation would suggest that extension perturbations, not flexion perturbations, would be more likely to elicit false starts—a prediction that is at odds with our predictions and results (see Table 1).
Anticipation may also explain false starts. One paradigm in the study of false starts is the use of a choice reaction-time task (Schmidt & Gordon, 1977). Schmidt and Gordon defined false starts as incorrect responses to a stimulus in a choice reaction-time task, which is different from the definition in the present article. In Schmidt and Gordon's paradigm, participants were asked to produce force in a direction specified by the stimulus. When the stimulus direction was varied in a predictable way, thereby creating expectancy or anticipation of the stimulus, participants sometimes produced forces in the incorrect direction on catch trials in which the stimulus direction was the opposite of that expected. These incorrect responses were labeled as false starts even though they occurred after the imperative stimulus and would not have been classified as false starts as defined in the present study. Nevertheless, anticipation could certainly play a role in false starts as observed in the present article. Evidence for anticipation in reaction-time tasks can be seen when foreperiod is kept constant (e.g., Quesada & Schmidt, 1970) or when only a small number of foreperiods are used (e.g., Aiken, 1964). When foreperiods were kept at a constant 1-s duration, reaction times were reduced to only 22 ms, suggesting that responses were made in anticipation of the stimulus rather than to the stimulus (Quesada & Schmidt; see also Engström, Kelso, & Holroyd, 1996). Even when a small number of foreperiods were used, reaction times varied systematically with foreperiod, decreasing as foreperiod lengthened (Aiken). These results highlight the importance of anticipation in reaction-time tasks, and they could provide an explanation for false starts on 2% of trials in the no-perturbation conditions. However, anticipation of the stimulus should be unaffected by perturbations, so other mechanisms must be invoked to explain the increase in false starts following perturbations.
Another possible explanation for false starts is that they arise from a startle response (e.g., Tresilian & Plooy, 2006; Valls-Sole et al., 1999; Valls-Sole et al., 1995). Acoustic startle stimuli applied after the imperative stimulus during a simple reaction-time task reduced the reaction time (Valls-Sole et al., 1995) while leaving the movement kinematics unaltered (Valls-Sole et al., 1999). This result was expanded by Tresilian and Plooy's study of the effects of acoustic startle signals applied before movement onset was expected. The acoustic signal led to false starts, prompting Tresilian and Plooy to posit that prepared motor commands were triggered by the auditory stimulus prior to the planned movement onset. Such an interpretation is not necessarily orthogonal to ours, if one is willing to adopt the identification of a phase flow with a prepared motor command. However, it must be pointed out that the phase flow codes for the motor commands in a dynamic manner and captures their characteristic dynamic properties (such as stability of trajectories, transients, and threshold behavior) in an unbiased fashion.
A further possible explanation for false starts is offered by a multisensory interference account (Calvert, 2001; Davis, 1959). Multisensory interference may posit that the mechanical perturbation is mistaken for the imperative stimulus and that movements are then initiated in response to the perturbation. Both startle and multisensory processes may play a role in the false starts observed in the current research. The monostable phase flow of the excitator model predicts that perturbations away from the direction of motion (i.e., extension perturbations) decrease the frequency of false starts more than they do in no-perturbation conditions. Instead, we observed an increase in the number of false starts with extension perturbations, although not to the same extent as with flexion perturbations. There are several possibilities for this increase in false starts. It may be that return to the initial posture demonstrated in equilibrium-point studies (e.g., Feldman, 1966) assisted in the subsequent flexion movement. However, this happened quite infrequently and does not explain the larger number of false starts following flexion perturbations. Alternatively, it may be that false starts in response to extension perturbations are because of, at least in part, a startle response or multisensory interference. Such accounts have a more difficult time explaining the directional effect of the perturbations. Whether the perturbation evokes a startle reflex (Tresilian & Plooy, 2006) or is mistaken for the auditory stimulus (Calvert), the direction of perturbation should not matter: False starts should be equally likely with both flexion and extension perturbations. Furthermore, if the perturbation is mistaken for the imperative stimulus, as the multisensory account suggests, the response to the perturbation should be equivalent to the response to the imperative stimulus. This implies, among other things, that the perturbation reaction time would be equal to the reaction time on trials without false starts. Because we observed neither equal percentages of false starts after flexion and extension perturbations (see Table 1) nor equal reaction and perturbation reaction times (see Figure 3), we infer that multisensory interference and startle response accounts, by themselves, are unable to explain our experimental findings.
Functional Interpretation of the Separatrix
If, as our experimental results suggest, the excitator model is favored, a question arises about the functional significance of the separatrix. The separatrix creates a local partitioning of the phase flow, resulting in the coexistence of the phase flow of the flexion or extension movement and the phase flow of the initial position. Hence, the phase flow of flexion or extension movement does not have to be created at initiation—it already exists. Huys, Jirsa, et al. (2008) suggested that the manipulation of phase flows is a more time-consuming, slower process than the actual movement itself. In contrast, the dynamical mechanism suggested by equilibrium–point approaches requires the annihilation of an existing equilibrium point and its phase flow and the creation of new ones at another location. In other situations, such as very slow movements or locomotion when no time-scale separation between phase flow creation and annihilation and movement is present, the existence of a separatrix may not have any advantage, and equilibrium–point control could be more useful (Huys, Jirsa, et al. 2008; Huys, Studenka, et al., 2008).
In the monostable phase flow pattern of the excitator, false starts can be triggered by both the noise inherent in the system (for a discussion of noise, see Harris & Wolpert, 1998; Kelso, Scholz, & Schöner, 1986) and perturbations, with the likelihood of these events causing false starts being a function of the location of the separatrix compared with the rest position. As the perturbations were of constant magnitude, and noise strength was not directly manipulated, the expectation is that the frequency of false starts provides a window into the relative change of the separatrix. Thus, if the separatrix changes, this should be reflected in variations in the frequency of false starts as the foreperiod and perturbation time are varied (e.g., Tresilian & Plooy, 2006). Conceptually, a changing separatrix could be a theoretical instantiation of anticipation (e.g., Aiken, 1964; Mowrer, 1940), with the separatrix moving toward the initial position for increasing levels of anticipation.
The results of the experiment were mixed in this regard. The percentage of false starts in the flexion perturbation conditions decreased when the perturbation occurred close to the auditory stimulus, a finding that suggests the separatrix either does not change or actually moves away from the fixed point. Nevertheless, the possibility of a separatrix that moves toward the initial finger position with time cannot be ruled out on the basis of the present experimental findings. As false starts were classified as such only when the response was initiated before the auditory stimulus, those trials with perturbation reaction times less than the perturbation time are identified as false starts. One hint that the separatrix may not be stationary is found in the analysis of the expected false starts given the distribution of perturbation reaction times (see Figure 4). If the separatrix were stationary, the percentage of false starts should have been identical to the expected false starts. Instead, the actual percentage of false starts exceeded the expected number of false starts, which suggests that the separatrix was moving closer to the initial finger position. On the other hand, the lack of an effect of foreperiod on the percentage of false starts is suggestive of a stationary separatrix. Further study is needed to satisfactorily resolve this question, perhaps by applying perturbations before the auditory stimulus even earlier than in the present study.
Nature of Phase Space
Regarding rhythmic bimanual coordination, researchers continue to debate the nature of the control laws involved in coordination; specifically, whether coordination is determined primarily (or even solely) by (a) informational, or perceptual, constraints (Mechsner, 2004; Mechsner, Kerzel, Knoblich, & Prinz, 2001); (b) neuromuscular-skeletal constraints (Carson & Riek, 1998; Carson, Riek, Smethurst, Parraga, & Byblow, 2000); or (c) both types of constraints with task context playing a role (Kelso, 1994; Kelso, Fink, DeLaplain, & Carson, 2001). Similar concerns may pertain to the excitator model. The excitator model, and the phase space in which it is embedded, could be informational in nature and not coupled to the effector used in the task. In this case, the excitator may be said to correspond to a model of neural activity, with flow fields and the separatrix being a general mechanism of movement initiation. If this is so, then any perturbation—regardless of whether mechanical–proprioceptive (as in the present article) or auditory (e.g., eliciting a startle reaction; see Valls-Sole et al., 1999)—should cause false starts, and false starts would be equally likely for flexion and extension perturbations. On the other hand, the excitator could be instantiated for a specific effector. In this case, only perturbations to the effector in the direction of the intended movement should cause false starts.
Our results indicate that the truth may lie between these two extremes. Although there were more false starts in the extension–perturbation conditions than in the no-perturbation conditions, there were more false starts when the perturbation was in the direction of the intended movement than when the perturbation was in the opposite direction. As previously discussed, it is possible that the increase in false starts with extension perturbations was the result of multisensory interference. Thus, it appears that multiple factors, both effector- and task-dependent, play a role in movement onset and in initiating a false start. The large directional effect of the perturbation, however, suggests that the phase space is primarily the phase space of the effector used. This is not meant to downplay neural contributions, but only to state that the phase flows of the excitator are likely to be instantiated at a neural level in an effector- and direction-specific fashion.
Conclusion
A key feature of the basic phase flow pattern of the excitator for discrete movement control is the existence of a separatrix. The separatrix divides the flow fields in phase space into distinct regions with coexisting flows toward and away from a fixed point. This feature generates a new hypothesis for false starts: False starts are caused by crossing the separatrix. Specifically, this hypothesis predicts that mechanical perturbations in the direction of an intended movement should elicit false starts. Our results are consistent with the existence of a separatrix with false starts occurring in a third of the trials in which a perturbation was applied in the direction of motion with perturbations away from the direction of motion resulting in fewer false starts. Furthermore, to our knowledge, these results are not expected on the basis of any other existing model of coordination, thereby enhancing the validity of the excitator model. We propose that human movement initiation and execution is a composite of the creation of a time-independent phase flow, including a separatrix, and an unspecific stimulus signal. This sort of movement preparation may have benefits regarding speed of movement and compactness of coding, and it provides a new way of thinking about movement.
ACKNOWLEDGMENTS
The authors thank Raoul Huys for preparing Figure 1. The research reported herein was supported by the U.S. Office of Naval Research Grant N000140510104 to Philip W. Fink and Viktor K. Jirsa; ATIP (Centre National de la Recherche Scientifique) and JS McDonnell Foundation grants to Viktor K. Jirsa; and U.S. Office of Naval Research Contract N00014-05-1-0117, National Institutes of Mental Health Grant MH42900, and National Institute of Neurological Disorders and Strokes Grant NS48229 to J. A. Scott Kelso.
APPENDIX
Monostable Phase Flow Equations
The excitator model (V. K. Jirsa & J. A. S. Kelso, 2005) proposes that movements arise from flow fields, and that the type of movement (i.e., discrete or rhythmic) is characterized by the topological properties of the flow field. The excitator model is described by the following equations:
| (1) |
where x is the position of the effector, y is the velocity of the effector, a is a constant parameter, I is an unspecific input, and τ is a time constant. The constant a and function g2(x,y) define the topology of the flow fields; therefore, the task constraints (e.g., perform a rhythmic or discrete movement) can be estimated from experimental data (R. Huys, V. K. Jirsa, B. Studenka, N. Rheaume, & H. N. Zelaznik, 2008; A. M. van Mourik, A. Daffertshofer, & P. J. Beek, 2006). To accommodate realistic movements, these functions also must satisfy a set of constraints (for details, see V. K. Jirsa & J. A. S. Kelso, 2005). For example, for discrete movements that begin and end at the same point, such as those studied in the present article, the functions and parameters in Equation 1 were determined by polynomial fit of experimental data as follows:
| (2) |
These parameters describe a flow field such as the one shown in Figure 1, with a single fixed point (hence the description mono-stable), a global attractor at x = 1.2, y = −0.1263, and a separatrix dividing the flow field into regions where the flow moves toward the fixed point and away from the fixed point.
In the context of rapid movements, such as those studied in the present article, the flow fields are assumed to change on a time scale much slower than the movement. Hence, the flow fields are assumed to be constant. The input, or informational, term I is not included in the flow fields, but rather represents a rapidly changing external influence such as the imperative stimulus used in reaction time tasks. Rather than changing the flow fields, I can, if large enough, move the effector across the separatrix, causing a movement to be initiated in response to this nonspecific stimulus. A second I can also be included to represent the mechanical perturbation, and in this context, the I is direction-specific and can be either negative, moving the effector away from the separatrix, or positive, moving the effector toward the separatrix. From this point of view, movement initiation and execution is understood as the interaction between an internally generated, constant flow field and a set of externally generated inputs.
NOTES
Technically, a stimulus may be viewed as a system parameter change acting on a time scale much faster than that of the process under consideration.
We used three distinct foreperiods rather than the more common variable foreperiod, because the primary measure of interest was the frequency of false starts, which requires multiple measurements at each time point tested. None of the participants reported noticing that only a small number of foreperiods were used.
Although participants were instructed to respond as quickly as possible, implying a minimized reaction time, the financial reward was tied to movement time, because the predicted presence of false starts would result in either rewarding false starts or discouraging them. Thus, we used movement time under the hypothesis that a participant motivated to respond as quickly as possible (i.e., a short reaction time) would also have a short movement time.
Some additional biases may remain, but these do not explain our results. See the Alternative Explanations section of the Discussion for details.
REFERENCES
- Adamovich S. V., Levin M. F., Feldman A. G. Merging different motor patterns: Coordination between rhythmical and discrete single-joint movements. Experimental Brain Research. 1994;99:325–337. doi: 10.1007/BF00239599. [DOI] [PubMed] [Google Scholar]
- Aiken L. R., Jr. Reaction time and the expectancy hypothesis. Perceptual and Motor Skills. 1964;19:655–661. doi: 10.2466/pms.1964.19.2.655. [DOI] [PubMed] [Google Scholar]
- Amazeen P. G., Amazeen E. L., Turvey M. T. Dynamics of human intersegmental coordination: Theory and research. In: Rosenbaum D. A., Collyer C. E., editors. Timing of behavior: Neural, psychological, and computational perspectives. Cambridge, MA: MIT Press; 1998. pp. 237–260. [Google Scholar]
- Asatryan D. G., Feldman A. G. Functional tuning of the nervous system with control of movements or maintenance of a steady posture: I. Mechanographic analysis of the work of the limb on execution of a postural task. Biophysics. 1965;10:925–935. [Google Scholar]
- Balasubramaniam R., Feldman A. G. Guiding movements without redundancy problems. In: Jirsa V. K., Kelso J. A. S., editors. Coordination dynamics. New York: Springer; 2004. pp. 155–176. [Google Scholar]
- Bizzi E., Chapple W., Hogan N. Mechanical properties of muscles: Implications for motor control. Trends in Neuroscience. 1982;5:395–398. [Google Scholar]
- Bizzi E., Polit A., Morasso P. Mechanisms underlying achievement of final head position. Journal of Neurophysiology. 1976;39:435–444. doi: 10.1152/jn.1976.39.2.435. [DOI] [PubMed] [Google Scholar]
- Brockett R. W. Proceedings of the 1988 Institute of Electrical and Electronics Engineers (IEEE) International Conference on Robotics and Automation. New York: IEEE; 1988, April. On the computer control of movement; pp. 534–540. [Google Scholar]
- Calvert G. A. Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cerebral Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Carson R. G., Riek S. The influence of joint position on the dynamics of perception-action coupling. Experimental Brain Research. 1998;110:103–114. doi: 10.1007/s002210050442. [DOI] [PubMed] [Google Scholar]
- Carson R. G., Riek S., Smethurst C. J., Parraga J. F. L., Byblow W. D. Neuromuscular-skeletal constraints upon the dynamics of unimanual and bimanual coordination. Experimental Brain Research. 2000;131:196–214. doi: 10.1007/s002219900272. [DOI] [PubMed] [Google Scholar]
- Chapman S. Catching a baseball. American Journal of Physics. 1968;36:868–870. [Google Scholar]
- Collet C. Strategic aspects of reaction time in world-class sprinters. Perceptual and Motor Skills. 1999;88:65–75. doi: 10.2466/pms.1999.88.1.65. [DOI] [PubMed] [Google Scholar]
- Davis R. The role of “attention” in the psychological refractory period. Quarterly Journal of Experimental Psychology. 1959;11:211–220. [Google Scholar]
- Engström D. A., Kelso J. A. S., Holroyd T. Reaction-anticipation transitions in human perception-action patterns. Human Movement Science. 1996;15:809–832. [Google Scholar]
- Feldman A. G. Functional tuning of the nervous system with control of movement or maintenance of a steady posture: II. Controllable parameters of the muscles. Biophysics. 1966;11:565–578. [Google Scholar]
- Feldman A. G. Superposition of motor programs: I. Rhythmic forearm movements in man. Neuroscience. 1980a;5:81–90. doi: 10.1016/0306-4522(80)90073-1. [DOI] [PubMed] [Google Scholar]
- Feldman A. G. Superposition of motor programs: II. Rapid flexion of forearm in man. Neuroscience. 1980b;5:91–95. doi: 10.1016/0306-4522(80)90074-3. [DOI] [PubMed] [Google Scholar]
- Feldman A. G., Levin M. F. The origin and use of positional frames of reference in motor control. Behavioral and Brain Sciences. 1995;18:723–806. [Google Scholar]
- Fitts P. M. The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology. 1954;47:381–391. [PubMed] [Google Scholar]
- FitzHugh R. Impulses and physiological states in theoretical models of nerve membrane. Biophysical Journal. 1961;1:445–466. doi: 10.1016/s0006-3495(61)86902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flash T., Hogan N. The coordination of arm movements: An experimentally confirmed mathematical model. Journal of Neuroscience. 1985;5:1688–1703. doi: 10.1523/JNEUROSCI.05-07-01688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M. S. A., Taylor C. S. R., Moore T., Cooke T. F. The cortical control of movement revisited. Neuron. 2002;36:349–362. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- Günther M., Ruder H. Synthesis of two-dimensional human walking: A test of the k-model. Biological Cybernetics. 2003;89:89–106. doi: 10.1007/s00422-003-0414-x. [DOI] [PubMed] [Google Scholar]
- Harris C. M., Wolpert D. M. Signal-dependent noise determines motor planning. Nature. 1998 August 20;394:725–726. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Hatze H. Energy-optimal controls in mammalian neuromuscular system. Biological Cybernetics. 1977;27:9–20. doi: 10.1007/BF00357705. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., Huxley A. F. A quantitative description of membrane current and its application to conduction and excitation. Journal of Physiology. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan N., Sternad D. On rhythmic and discrete movements: Reflections definitions and implications for motor control. Experimental Brain Research. 2007;181:13–30. doi: 10.1007/s00221-007-0899-y. [DOI] [PubMed] [Google Scholar]
- Huys R., Jirsa V. K., Studenka B., Rheaume N., Zelaznik H. N. Human trajectory formation: Taxonomy of motor primitives based on phase flow topology. In: Fuchs A., Jirsa V. K., editors. Coordination: Neural, behavioral and social dynamics. New York: Springer; 2008. pp. 77–92. [Google Scholar]
- Huys R., Studenka B., Rheaume N., Zelaznik H. N., Jirsa V. K. Distinct timing mechanisms produce discrete and continuous movements. Public Library of Sciences Computational Biology. 2008;4:e100061. doi: 10.1371/journal.pcbi.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsa V. K., Kelso J. A. S. The excitator as a minimal model for the coordination dynamics of discrete and rhythmic movements. Journal of Motor Behavior. 2005;37:35–51. doi: 10.3200/JMBR.37.1.35-51. [DOI] [PubMed] [Google Scholar]
- Kelso J. A. S. Motor control mechanisms underlying human movement reproduction. Journal of Experimental Psychology: Human Perception and Performance. 1977;3:529–543. doi: 10.1037//0096-1523.3.4.529. [DOI] [PubMed] [Google Scholar]
- Kelso J. A. S. The informational character of self-organized coordination dynamics. Human Movement Science. 1994;13:393–413. [Google Scholar]
- Kelso J. A. S. Dynamic patterns: The self-organization of brain and behavior. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Kelso J. A. S., Fink P., DeLaplain C., Carson R. G. Haptic information stabilizes and destabilizes coordination dynamics. Proceedings of the Royal Society of London Series B. 2001;268:1207–1213. doi: 10.1098/rspb.2001.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso J. A. S., Holt K. G. Exploring a vibratory systems analysis of human movement production. Journal of Neurophysiology. 1980;43:1183–1196. doi: 10.1152/jn.1980.43.5.1183. [DOI] [PubMed] [Google Scholar]
- Kelso J. A. S., Holt K. G., Kugler P. N., Turvey M. T. Coordinative structures as dissipative structures: II. Empirical lines of convergence. In: Stelmach G. E., Requin J., editors. Tutorials in motor behavior. Amsterdam: North Holland; 1980. pp. 49–70. [Google Scholar]
- Kelso J. A. S., Scholz J. P., Schöner G. Nonequilibrium phase transitions in coordinated biological motion: Critical fluctuations. Physics Letters A. 1986;118:279–284. [Google Scholar]
- Latash M. L. Independent control of joint stiffness in the context of the equilibrium-point hypothesis. Biological Cybernetics. 1992;67:377–384. doi: 10.1007/BF02414893. [DOI] [PubMed] [Google Scholar]
- Latash M. L. Control of human movement. Champaign, IL: Human Kinetics; 1993. [Google Scholar]
- Maninkonda V., Krishnaprasad P. S., Hendler J. Languages, behaviors, hybrid architectures and motion control. In: Baillieul J., Willems J. C., editors. Mathematical control theory. New York: Springer-Verlag; 1998. pp. 199–226. [Google Scholar]
- Mechsner F. A psychological approach to human voluntary movements. Journal of Motor Behavior. 2004;36:355–370. doi: 10.1080/00222895.2004.11007993. [DOI] [PubMed] [Google Scholar]
- Mechsner F., Kerzel D., Knoblich G., Prinz W. Perceptual basis of bimanual coordination. Nature. 2001 November 1;414:69–72. doi: 10.1038/35102060. [DOI] [PubMed] [Google Scholar]
- Morasso P. Spatial control of arm movements. Experimental Brain Research. 1981;42:223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- Mowrer O. H. Preparatory set (expectancy): Some methods of measurement. Psychological Monographs. 1940;52 (Whole No. 233) [Google Scholar]
- Nagumo J., Arimoto S., Yoshizawa S. An active pulse transmission line simulating nerve axon. Proceedings of the Institute of Radio Engineers. 1962;50:2061–2070. [Google Scholar]
- Nelson W. L. Physical principles for economies of skilled movements. Biological Cybernetics. 1983;46:135–147. doi: 10.1007/BF00339982. [DOI] [PubMed] [Google Scholar]
- Plamondon R., Alimi A. M. Speed/accuracy trade-offs in target-directed aiming. Behavioral and Brain Sciences. 1997;20:279–349. doi: 10.1017/s0140525x97001441. [DOI] [PubMed] [Google Scholar]
- Polit A., Bizzi E. Processes controlling arm movements in monkeys. Science. 1978 September 29;201:1235–1237. doi: 10.1126/science.99813. [DOI] [PubMed] [Google Scholar]
- Polit A., Bizzi E. Characteristics of motor programs underlying arm movements in monkeys. Journal of Neurophysiology. 1979;42:183–194. doi: 10.1152/jn.1979.42.1.183. [DOI] [PubMed] [Google Scholar]
- Quesada D. C., Schmidt R. A. A test of the Adams-Creamer decay hypothesis for the timing of motor responses. Journal of Motor Behavior. 1970;2:273–283. doi: 10.1080/00222895.1970.10734885. [DOI] [PubMed] [Google Scholar]
- Rabinovich M. I., Huerta R., Varona P. Heteroclinic synchronization: Ultrasubharmonic locking. Physical Review Letters. 2006;96:014101. doi: 10.1103/PhysRevLett.96.014101. [DOI] [PubMed] [Google Scholar]
- Saltzman E. L., Kelso J. A. S. Skilled actions: A task dynamic approach. Psychological Review. 1987;94:84–106. [PubMed] [Google Scholar]
- Schaal S., Sternad D., Osu R., Kawato M. Rhythmic arm movement is not discrete. Nature Neuroscience. 2004;7:1136–1143. doi: 10.1038/nn1322. [DOI] [PubMed] [Google Scholar]
- Schmidt R. A., Gordon G. B. Errors in motor responding “rapid” corrections, and false anticipations. Journal of Motor Behavior. 1977;9:101–111. doi: 10.1080/00222895.1977.10735099. [DOI] [PubMed] [Google Scholar]
- Schöner G. A dynamic theory of coordination of discrete movement. Biological Cybernetics. 1990;63:257–270. doi: 10.1007/BF00203449. [DOI] [PubMed] [Google Scholar]
- Shaw R., Kinsella-Shaw J. Could optical “pushes” be inertial forces? A geometro-dynamical hypothesis. Ecological Psychology. 2007;19:305–320. [Google Scholar]
- Sternad D., Dean W. J., Schaal S. Interaction of rhythmic and discrete pattern generators in single-joint movements. Human Movement Science. 2000;19:627–664. [Google Scholar]
- Strogatz S. Nonlinear dynamics and chaos: With applications to physics biology, chemistry, and engineering. Reading, MA: Addison-Wesley; 1994. [Google Scholar]
- Teasdale N., Bard C., Fleury M., Young D. E., Proteau L. Determining movement onsets from temporal series. Journal of Motor Behavior. 1993;25:97–106. doi: 10.1080/00222895.1993.9941644. [DOI] [PubMed] [Google Scholar]
- Todorov E. Optimality principles in sensorimotor control. Nature Neuroscience. 2004;7:907–915. doi: 10.1038/nn1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresilian J. R., Plooy A. M. Effects of acoustic startle stimuli on interceptive action. Neuroscience. 2006;142:579–594. doi: 10.1016/j.neuroscience.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Uno Y., Kawato M., Suzuki R. Formation and control of optimal trajectory in human arm movement-minimum torque-change model. Biological Cybernetics. 1989;61:89–101. doi: 10.1007/BF00204593. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J., Rothwell J. C., Goulart F., Cossu G., Munoz E. Patterned ballistic movements triggered by a startle in health humans. Journal of Physiology–London. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J., Sole A., Valldeoriola F., Munoz E., Gonzalez L. E., Tolosa E. S. Reaction time and acoustic startle in normal human subjects. Neuroscience Letters. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]