Abstract
To capture patterns of normal age‐associated atrophy, we previously used a multivariate statistical approach applied to voxel based morphometry that identified age‐associated gray and white matter covariance networks (Brickman et al. [2007]: Neurobiol Aging 28:284–295). The current study sought to examine the stability of these patterns by forward applying the identified networks to an independent sample of neurologically healthy younger and older adults. Forty‐two younger and 35 older adults were imaged with standard high‐resolution structural magnetic resonance imaging. Individual images were spatially normalized and segmented into gray and white matter. Covariance patterns that were previously identified with scaled subprofile model analyses were prospectively applied to the current sample to identify to what degree the age‐associated patterns were manifested. Older individuals were also assessed with a modified version of the Mini Mental State Examination (mMMSE). Gray matter covariance pattern expression discriminated between younger and older participants with high optimal sensitivity (100%) and specificity (90.5%). While the two groups differed in the degree of white matter pattern expression (t (75) = 5.26, P < 0.001), classification based on white matter expression was relatively low (sensitivity = 80% and specificity = 61.9%). Among older adults, chronological age was significantly associated with increased gray matter pattern expression (r (32) = 0.591, P < 0.001) but not with performance on the mMMSE (r (31) = −0.314, P = 0.085). However, gray matter pattern expression was significantly associated with performance on the mMMSE (r (31) = −0.405, P = 0.024). The findings suggest that the previously derived age‐associated covariance pattern for gray matter is reliable and may provide information that is more functionally meaningful than chronological age. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
INTRODUCTION
Magnetic resonance imaging (MRI) affords the remarkable capability of visualizing and quantifying age‐associated changes in brain morphology. Using a variety of acquisition and analytic approaches, past research has demonstrated dramatic age‐associated changes in gray matter volume [Allen et al.,2005; Bartzokis et al.,2001; Good et al.,2001; Jernigan et al.,2001; Raz et al.,2005; Zimmerman et al.,2006], white matter volume [Bartzokis et al.,2003; Brickman et al.,2006; Guttmann et al.,1998; Resnick et al.,2000] and the integrity or orientation of white matter fiber tracts [Sullivan and Pfefferbaum,2006]. Consistently, these efforts implicate anterior regions as the most vulnerable to the effects of normal aging, often showing relatively greater age‐associated changes in frontal lobes than in more posterior regions.
Voxel based morphometry (VBM) and manual region‐of‐interest (ROI) techniques are among the most common approaches for the analysis of structural MRI data. In the former, comparisons between or within groups are made on a voxel‐by‐voxel basis to identify regions of volumetric, or density, difference or clinical correlates of individual differences in regional volumetry. In the latter, ROIs are chosen based on a priori hypotheses of group differences or clinical correlates and manually traced to derive volumes for statistical analyses. Despite their ability to identify regions that decline with age, these approaches are, by definition, univariate and do not explicitly test the interrelationship among brain regions. They therefore increase the risk of not capturing age‐associated differences that may be subtle and spatially complex [Davatzikos,2004].
Using a multivariate statistical approach applied to VBM, we recently identified networks of gray and white matter density that reliably distinguished between healthy younger and older adults [Brickman et al.,2007]. The approach, based on the Scaled Subprofile Model (SSM) [Moeller et al.,1987], identified covariance patterns comprising regionally distributed gray or white matter voxels that differed between younger and older adults. Negative loadings, indicating collateral age‐related diminution in voxel density, existed in anterior brain areas, but also throughout the brain in both cortical and subcortical regions. The degree to which each covariance pattern was expressed, quantified by a subject scaling factor (SSF), discriminated between the two groups (i.e., younger and older) with 100% specificity and 97% sensitivity for gray matter and 99% specificity and 93% sensitivity for white matter at an optimum cut‐point. Furthermore, when examining the neurocognitive correlates of pattern expression, SSFs were significantly associated with performance on tasks of memory and executive function, even when chronological age was statistically controlled.
A similar multivariate approach to understanding age‐associated differences in gray matter was recently reported by Alexander et al. [2006]. Also using SSM, the authors identified a distributed pattern of age‐related gray matter atrophy that was regionally similar to the one identified in our study and included frontal, temporal, thalamic, and cerebellar regions. The consistency between the two provides preliminary evidence that the multivariate SSM approach for the identification of age‐associated atrophy is indeed reliable. However, differences between the studies somewhat limit direct comparison. First, Alexander et al. considered chronological age as a continuous variable, whereas we grouped age dichotomously. Second, the association between expression of gray matter patterns and performance on neuropsychological tests was only considered in our study. Finally, Alexander et al. examined gray matter effects, but not white matter effects. This last point is particularly salient, as there are conflicting reports of the existence, distribution, and degree of white matter macrostructure differences across the adult lifespan, with some studies indicating age‐associated white matter atrophy [Bartzokis et al.,2003; Guttmann et al.,1998] that are functionally relevant [Brickman et al.,2006] and others suggesting minimal white matter change [Sullivan et al.,2004].
The purpose of the current study was to examine the stability and reliability of the previously defined age‐related gray and white matter covariance patterns [Brickman et al.,2007] in an independent sample of younger and older neurologically healthy adults. We further sought to determine the predictive utility of the expression of these patterns for cognitive functioning among older adults.
MATERIALS AND METHODS
Subjects
Data for the current study came from ongoing neuroimaging studies in our laboratory. There were 42 younger (mean + SD age = 23.38 + 2.24, range 19–28) and 35 older (mean + SD age = 72.59 + 7.01, range 59–86) neurologically healthy adult participants. The two groups were similar in sex distribution (χ2 (1) = 0.175, P = 0.676); there were 22 women (52.4%) in the younger group and 20 women (57.1%) in the older group. Participants were screened with medical, neurological, psychiatric, and neuropsychological evaluations and excluded if there was evidence of medical or cognitive dysfunction, as previously described [Brickman et al.,2007]. All potential participants in the older age group were evaluated for dementia with the Mattis Dementia Rating Scale [Mattis,1988] and excluded if scored below 135. Written informed consent, approved by the local ethics committee, was obtained from all participants.
Among the older participants, global cognitive ability was evaluated with a modified version of the Folstein Mini Mental State Examination [Folstein et al.,1975]. The modified Mini‐Mental State Examination (mMMSE) [Stern et al.,1987] is a 57 item scale that comprises tasks of attention/calculation, general knowledge, language, and construction, grouped in five cognitive domains: orientation, short‐term memory, long‐term memory, language, and visuoconstruction (see [Mayeux et al.,1983; Sarazin et al.,2005; Stavitsky et al.,2006] for greater detail). In the current study, the primary dependent measure of the mMMSE was the total score, although domain scores were calculated for exploratory analyses following earlier reports from our laboratory [Mayeux et al.,1983; Sarazin et al.,2005].
Image Acquisition and Analysis
T1 weighted spoiled gradient (SPGR) images were acquired with a 1.5 T MR scanner (TE/TR = 5 ms/34 ms; flip angle = 45°; in‐plane resolution of 0.859 mm × 0.859 mm; 256 × 256 matrix; 22 cm2 field of view).
MR images were reviewed by a radiologist, who confirmed that there were no clinically significant findings for any subject. The stages of initial image processing followed the “optimized” voxel based morphometry (VBM) protocol [Good et al.,2001]. Postprocessing and analysis was conducted with SPM99 (Wellcome Department of Imaging Neuroscience, London) and in‐house developed software running on Matlab 5.3. Images were normalized to standardized stereotactic space, defined by the Montreal Neurological Institute template, with 7 × 8 × 7 nonlinear basis functions. Images were segmented into gray, white, and CSF posterior probability images with standardized T1 templates of segmented images provided by SPM. The spatially normalized and segmented gray and white matter images were modulated by the Jacobian determinant to correct for volumetric changes secondary to nonlinear spatial normalization [Good et al.,2001]. The images were smoothed with an isotropic Gaussian kernel of 6 mm full‐width at half‐maximum.
Subprofile Scaling Model
Age group‐associated covariance gray and white matter patterns were defined in an independent sample of 84 younger and 29 older adults and described in detail in a previous report [Brickman et al.,2007]. Briefly, the subprofile scaling model (SSM) was applied on a voxel basis to spatially normalized gray and white matter images, producing a series of principal components (PCs). Positive loadings reflected voxels with concomitant relative increases in density and negative loadings reflected voxels with concomitant relative decreases in density. Next, the degree to which each PC was expressed by each individual was quantified by a subject scaling factor (SSF); higher SSF values indicated greater concomitant increases (i.e., for voxels with positive loadings) or decreases (i.e., for voxels with negative loadings) in voxel density. To identify covariance patterns that best discriminated the two subject groups, or captured patterns of age‐associated atrophy, individuals' SSFs were entered into a linear regression model as predictor variables, with age group (younger vs. older) as the outcome variable. The regression analysis tested linear combinations of the PCs that best discriminated between the two groups, using Akaike's information criterion [Burnham and Anderson,2002] to determine how many PCs should be included. The set of PCs that yielded the model with the lowest value of Akaike's information criterion was selected.
The optimal sets of identified PCs for gray and white matter were 1:7 and 1:6, respectively. The associated “patterns” or “networks,” which refers to voxels with negative loadings implicated in these linearly combined PCs, included wide‐spread areas of cortex and subcortex. For gray matter, these regions included thalamus, cortical and mesial temporal lobe, cortical and subcortical frontal lobe, and parietal lobe. For white matter, areas included the cingulate, corpus callosum, deep frontal lobe white matter, and insula. We plotted the degree to which the patterns were expressed by each individual subject using receiver operating curves (ROC) to derive optimal cut points for between‐group discrimination. Optimal discrimination for gray and white matter patterns yielded 100% specificity and 97% sensitivity and 99% specificity and 93% sensitivity, respectively.
The gray and white matter patterns derived in our previous study were applied prospectively to the magnetic resonance imaging (MRI) data from the current sample. Importantly, no data from participants in the current study were included in the derivation of the age‐associated patterns from our previous study. The forward application operation is mathematically represented by a “dot” product. That is, each gray and white matter voxel value in each participant's segmented image was multiplied by the corresponding voxel weight in the covariance pattern derived in the initial sample and then summed over the entire brain. The resulting single number (i.e., SSF) reflects the degree to which the covariance pattern was manifest in each participant's brain image.
In the current study, we examined how well the forward applied gray and white matter patterns distinguished between the younger and older groups. We plotted ROC curves and examined sensitivity and specificity for classification of age group from the forward applied covariance patterns. Sensitivity/specificity cut‐points were also determined for the forward applied patterns. We further sought to determine the predictive utility of the expression of these patterns for cognitive functioning among older adults by examining the relationship between gray and white matter SSFs and performance on the mMMSE.
RESULTS
The mean expressions of the forward applied gray and white matter patterns were significantly greater in older adults than in younger adults (t (75) = 13.95, P < 0.001 and t (75) = 5.26, P < 0.001, respectively), indicating greater manifestation of the age‐associated pattern among older adults in the current sample. Receiver operating curves, displayed in Figures 1 and 2, showed good classification for gray matter (area under curve = 0.995, SE = 0.005, P < 0.001, null true area = 0.5). Group classification based on the forward applied white matter was more moderate (area under curve = 0.803, SE = 0.049, P < 0.001, null true area = 0.5). The distribution of sensitivity and 1‐specificity at various cut‐points is illustrated in Table I. Examination of this distribution suggests that classification based on the forward applied gray matter pattern yielded an optimal sensitivity of 100% and specificity of 90.5% and an optimal sensitivity of 80%and specificity of 61.9% for white matter. Distributions of forward applied pattern expression are displayed in Figures 3 and 4.
Figure 1.
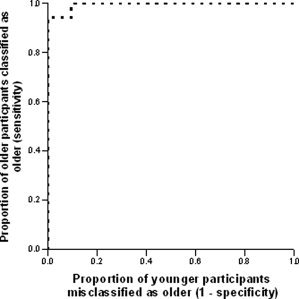
Receiver operating curve demonstrating the classification of participants according to the forward application of gray matter covariance patterns.
Figure 2.
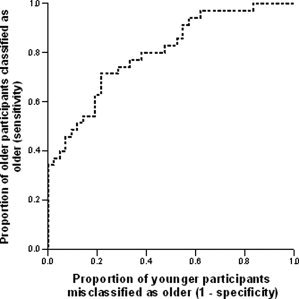
Receiver operating curve demonstrating the classification of participants according to the forward application of white matter covariance patterns.
Table I.
Sensitivity and specificity for classification as young or older as a function of various cut‐offs of gray and white matter pattern expression
| Gray matter pattern expression cut‐off | Sensitivity | 1‐Specificity | White matter pattern expression cut‐off | Sensitivity | 1‐Specificity |
|---|---|---|---|---|---|
| −31.627 | 1.000 | 0.929 | 12.764 | 1.000 | 0.929 |
| −29.522 | 1.000 | 0.810 | 13.366 | 0.971 | 0.833 |
| −28.184 | 1.000 | 0.690 | 13.888 | 0.971 | 0.714 |
| −27.025 | 1.000 | 0.571 | 14.154 | 0.943 | 0.619 |
| −26.461 | 1.000 | 0.452 | 14.630 | 0.886 | 0.548 |
| −24.795 | 1.000 | 0.333 | 15.047 | 0.829 | 0.476 |
| −23.452 | 1.000 | 0.214 | 15.440 | 0.800 | 0.381 |
| −22.707 | 1.000 | 0.095 | 15.633 | 0.743 | 0.310 |
| −20.983 | 0.943 | 0.000 | 15.887 | 0.714 | 0.214 |
| −19.194 | 0.829 | 0.000 | 16.371 | 0.600 | 0.190 |
| −17.876 | 0.686 | 0.000 | 16.755 | 0.514 | 0.143 |
| −15.721 | 0.543 | 0.000 | 17.787 | 0.457 | 0.071 |
| −14.915 | 0.400 | 0.000 | 18.097 | 0.371 | 0.048 |
| −12.846 | 0.257 | 0.000 | 19.467 | 0.286 | 0.000 |
| −11.108 | 0.114 | 0.000 | 22.236 | 0.143 | 0.000 |
| −8.153 | 0.000 | 0.000 | 28.166 | 0.00 | 0.000 |
Figure 3.
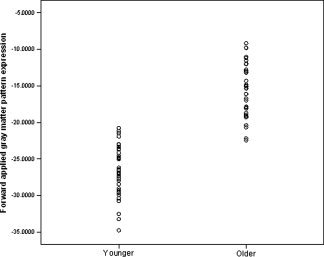
Distribution of the degree to which younger and older participants expressed the forward applied age‐associated gray matter topography.
Figure 4.

Distribution of the degree to which younger and older participants expressed the forward applied age‐associated white matter topography.
Within the older adult group, the association between chronological age and expression of the forward applied patterns was significant for gray matter (r (32) = 0.591, P < 0.001), but not for white matter (r (32) = 0.148, P = 0.419). Although within the older adult group, chronological age was not significantly associated with performance on the mMMSE (r (31) = −0.314, P = 0.085), the expression of the forward applied gray matter pattern was significantly associated with performance on the mMMSE (r (31) = −0.405, P = 0.024), as displayed in Figure 5. The magnitude of the correlations between gray matter pattern expression and mMMSE and between chronological age and mMMSE did not significantly differ from each other with Fisher's Test (P = 0.66). When associations among chronological age, gray matter pattern expression, and subsections of the mMMSE were explored, gray matter pattern expression was selectively and robustly associated with scores on the long term memory subsection (r (31) = −0.503, P = 0.005), whereas chronological age was not (r(31) = −0.256, P = 0.172. Poorer performance on the visuoconstruction items appeared to be selectively associated with increased chronological age (r (31) = −0.513, P = 0.004) and not with gray matter pattern expression (r (31) = −0.254, P = 0.176). Table II displays correlation coefficients among chronological age, pattern expression, and performance on subsections of the mMMSE. White matter pattern expression was not significantly correlated with total mMMSE task performance (r (31) = 0.126, P = 0.501).
Figure 5.
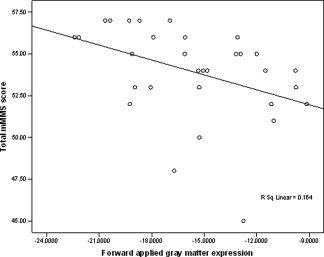
Association between the expression of the forward applied gray matter pattern and performance on the mMMSE among the older participants.
Table II.
Correlation coefficients and P‐values for associations among chronological age, gray and white matter pattern expression, and subtests of the mMMSE
| Chronological age | Gray matter | White matter | |
|---|---|---|---|
| Chronological age | r = 0.591, P < 0.001 | r = 0.148, P = 0.419 | |
| Total mMMSE | r = −0.314, P = 0.085 | r = −0.405, P = 0.024 | r = 0.126, P = 0.501 |
| Short term memory | r = −0.188, P = 0.329 | r = −0.085, P = 0.663 | r = 0.103, P = 0.595 |
| Long term memory | r = −0.294, P = 0.122 | r = −0.532, P = 0.003 | r = −0.004, P = 0.984 |
| Language | r = −0.008, P = 0.966 | r = −0.232, P = 0.227 | r = 0.226, P = 0.237 |
| Visuoconstruction | r = −0.525, P = 0.003 | r = −0.281, P = 0.140 | r = −0.007, P = 0.971 |
Note that these data are for older adults only (n = 35).
DISCUSSION
The current study sought to determine the stability of previously derived age‐associated gray and white matter voxel‐based patterns [Brickman et al.,2007] in discriminating between a sample of younger and older neurologically healthy adults. As predicted, the age‐associated patterns of gray and white matter atrophy were expressed to a significantly higher degree among the older participants in the current independent sample, indicating a greater manifestation of the patterns among the older adults. When we examined the degree to which the patterns could be used to discriminate between the two age groups, gray matter yielded an optimal cut point with high sensitivity and specificity. Although the forward applied expression of white matter patterns also significantly differed between the age groups, greater variability among older adults led to relatively low discriminability. Finally, among older adults, chronological age was associated with increased expression of the gray matter pattern, but was not associated with expression of the white matter pattern or with cognitive test performance. Taken together, the results suggest that the identified gray matter pattern is a reliable estimate of regional age‐associated gray matter loss that may be more biologically relevant than chronological age itself.
Multivariate approaches to the analysis of structural MRI data may have several advantages over univariate approaches. The multivariate approach employed in the current study, SSM, captures a pattern of interrelated regions in a single numeric expression. This representation may reflect uniform or widespread atrophic changes that occur with normal aging. Unlike univariate approaches, which treat each voxel or region as a spatially independent unit, SSM explicitly examines the interrelationship among these units and allows for better inference of the interconnectivity among brain regions. This approach also allows for relatively easy examination of clinical or demographic correlates of the covariance network and for the forward application of the identified pattern, such as in the current project. Prospective application of regionally distributed findings would not be possible with univariate approaches, which would require the derivation of group differences de novo. The identification and forward application of identified covariance patterns with PCs approaches has been successfully implemented in a number of functional neuroimaging studies (e.g., [Devanand et al.,2006; Eidelberg et al.,1991,1997; Moeller and Eidelberg,1997]).
The current study demonstrates the stability of the originally defined gray matter pattern in distinguishing between younger and older adults. A logical future direction would be to forward apply the identified age‐associated gray matter pattern to a clinical population, such as Alzheimer's disease (AD), to understand the extent to which pathological changes overlap with normal age‐associated changes. This type of analysis could potentially identify pathological changes in AD that are independent, or uniquely different, from the changes seen with normal aging by identifying disease‐associated covariance patterns and comparing them to the normal age‐related networks. A similar approach has been successfully applied to the identification of metabolic topographies in clinical populations [Moeller and Eidelberg,1997].
The finding that among the older group the degree of gray matter expression was significantly associated with cognitive function is consistent with our previous report that demonstrated a significant association between the same network pattern expression and performance on a more detailed neuropsychological battery [Brickman et al.,2007]. It also provides some face validation of the functional significance of the covariance pattern. In fact, the correlation between gray matter pattern expression and performance on the mMMSE was stronger than the association between chronological age and mMMSE performance, suggesting that the covariance approach is capturing an aspect of aging that is more biologically meaningful than chronological age. Although the magnitudes of the two coefficients did not significantly differ, the findings suggest that the association between gray matter pattern expression and cognition is more reliable than the association between chronological age and cognition. Post hoc analyses with subsections of the mMMSE suggested that gray matter pattern expression may be most tightly linked with age‐associated changes in long‐term memory, a finding that is also consistent with our earlier report [Brickman et al.,2007]. That the degree to which the forward applied gray matter pattern was expressed was also significantly associated with chronological age provides further face validation for the approach.
Although in our previous report we were able to identify a covariance pattern of white matter that discriminated older from younger adults [Brickman et al.,2007], the forward applied pattern of white matter did not reliably do so in the current study. Age‐associated decline in white matter macrostructure has been reported by some investigators [Bartzokis et al.,2003; Guttmann et al.,1998], but not by others [Sullivan et al.,2004]. The findings from the current study suggest that if gross white matter volumetric changes do occur with normal aging, they are somewhat variable. Indeed, the distribution of white matter pattern expression appeared more variable in the older group than the younger group (see Fig. 4). Age‐associated white matter changes may be best appreciated with MR sequences best suited to detect microstructural abnormalities, such as fractional anisotropy, measured with diffusion tensor imaging (e.g., [Sullivan and Pfefferbaum,2006]), or white matter hyperintensities, measured with T2‐ or FLAIR‐weighted sequences (e.g., [de Leeuw et al.,2001]). Our identified gray matter pattern contained anatomical regions that were distributed similarly as the one reported by Alexander et al. [2006]; however, those authors did not explicitly examine age‐associated white matter patterns. Thus, further studies are necessary to identify a more stable white matter covariance pattern. Age‐associated white matter changes and their clinical impact also may be best appreciated with imaging sequences that measure microstructure.
The current study did not seek to compare explicitly the SSM approach with other multivariate techniques that have been proposed by other research groups. Multivariate pattern classification techniques have indeed been applied previously to structural and functional imaging data with promising results and have included approaches that have exploited machine learning methods [Lao et al.,2004], canonical variance analysis [Almeida and Ledberg,2002], partial least squares [McIntosh and Lobaugh,2004], and multivariate linear modeling [Worsley et al.,1997]. The approach used in the current study, SSM, includes a relatively simple series of computations that yield highly robust findings. Results from the current study, in addition to previous reports with structural and functional data, highlight its potential clinical utility and straightforward application. Nonetheless, future studies should evaluate and compare relative strengths and weaknesses of these varying approaches.
The sensitivity and specificity analyses in the current study were presented as an illustration of how well previously defined covariance patterns can be used to distinguish younger and older participants in a “forward applied” sample for the purpose of examining the stability of the covariance patterns and to evaluate their potential utility as biological markers of normal aging. Typically, sensitivity and specificity analyses are carried out for diagnostic purposes. However, it is unlikely that future work would utilize structural MRI scans to diagnose “young” versus “old.” Thus, pattern expression cut‐points are somewhat less important than the fact that the expression of the gray matter pattern reliably distinguishes younger from older adults. Our study demonstrates that the gray matter pattern expression is useful for future aging studies and may be most illustrative when considered as a continuous variable. It should be noted that the optimal cut‐points identified in the current study were slightly higher than in our previous sample [Brickman et al.,2007]. We attribute this difference to variability in scanner properties, which may have shifted image intensities.
In conclusion, the current study showed that a multivariate‐defined pattern of age‐associated gray matter atrophy can be forward applied to an independent sample and reliably distinguish between younger and older neurologically healthy adults. Multivariate approaches to the analysis of structural MRI captures distributed aging effects that may be more biological meaningful than chronological age itself.
Portions of this work were presented at the annual meeting of the International Neuropsychological Society, Portland, OR, February 2007.
REFERENCES
- Alexander GE,Chen K,Merkley TL,Reiman EM,Caselli RJ,Aschenbrenner M,Santerre‐Lemmon L,Lewis DJ,Pietrini P,Teipel SJ,Hampel H,Rappoport SI,Moeller R ( 2006): Regional network of magnetic resonance imaging gray matter volume in healthy aging. Neuroreport 17(10): 951–956. [DOI] [PubMed] [Google Scholar]
- Allen JS,Bruss J,Brown CK,Damasio H ( 2005): Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging 26(9): 1245–1260; Discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- Almeida R,Ledberg A ( 2002): Exact multivariate tests for brain imaging data. Hum Brain Mapp 16(1): 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G,Beckson M,Lu PH,Nuechterlein KH,Edwards N,Mintz J ( 2001): Age‐related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry 58(5): 461–465. [DOI] [PubMed] [Google Scholar]
- Bartzokis G,Cummings JL,Sultzer D,Henderson VW,Nuechterlein KH,Mintz J ( 2003): White matter structural integrity in healthy aging adults and patients with Alzheimer disease: A magnetic resonance imaging study. Arch Neurol 60(3): 393–398. [DOI] [PubMed] [Google Scholar]
- Brickman AM,Zimmerman ME,Paul RH,Grieve SM,Tate DF,Cohen RA,Williams LM,Clark CR,Gordon E ( 2006b): Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry 60(5): 444–453. [DOI] [PubMed] [Google Scholar]
- Brickman AM,Habeck C,Zarahn E,Flynn J,Stern Y ( 2007): Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging 28(2): 284–295. [DOI] [PubMed] [Google Scholar]
- Burnham KP,Anderson D ( 2002): Model Selection and Multimodel Inference. New York: Springer Verlag. [Google Scholar]
- Davatzikos C ( 2004): Why voxel‐based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage 23(1): 17–20. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE,de Groot JC,Achten E,Oudkerk M,Ramos LM,Heijboer R,Hofman A,Jolles J,van Gijn J,Breteler MM ( 2001): Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70(1): 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP,Habeck CG,Tabert MH,Scarmeas N,Pelton GH,Moeller JR,Mensh BD,Tarabula T,Van Heertum RL,Stern Y ( 2006): PET network abnormalities and cognitive decline in patients with mild cognitive impairment. Neuropsychopharmacology 31(6): 1327–1334. [DOI] [PubMed] [Google Scholar]
- Eidelberg D,Dhawan V,Moeller JR,Sidtis JJ,Ginos JZ,Strother SC,Cederbaum J,Greene P,Fahn S,Powers JM ( 1991): The metabolic landscape of cortico‐basal ganglionic degeneration: Regional asymmetries studied with positron emission tomography. J Neurol Neurosurg Psychiatry 54(10): 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D,Moeller JR,Kazumata K,Antonini A,Sterio D,Dhawan V,Spetsieris P,Alterman R,Kelly PJ,Dogali M, et al. ( 1997): Metabolic correlates of pallidal neuronal activity in Parkinson's disease. Brain 120 (Part 8): 1315–1324. [DOI] [PubMed] [Google Scholar]
- Folstein MF,Folstein SE,McHugh PR ( 1975): ‘Mini‐mental State’: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Good CD,Johnsrude IS,Ashburner J,Henson RN,Friston KJ,Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14 (1 Part 1): 21–36. [DOI] [PubMed] [Google Scholar]
- Guttmann CR,Jolesz FA,Kikinis R,Killiany RJ,Moss MB,Sandor T,Albert MS ( 1998): White matter changes with normal aging. Neurology 50(4): 972–978. [DOI] [PubMed] [Google Scholar]
- Jernigan TL,Archibald SL,Fennema‐Notestine C,Gamst AC,Stout JC,Bonner J,Hesselink JR ( 2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22(4): 581–594. [DOI] [PubMed] [Google Scholar]
- Lao Z,Shen D,Xue Z,Karacali B,Resnick SM,Davatzikos C ( 2004): Morphological classification of brains via high‐dimensional shape transformations and machine learning methods. Neuroimage 21(1): 46–57. [DOI] [PubMed] [Google Scholar]
- Mattis S ( 1988): Dementia Rating Scale (DRS). Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Mayeux R,Stern Y,Rosen J,Benson F ( 1983): Is “subcortical dementia” a recognizable clinical entity? Ann Neurol 14(3): 278–283. [DOI] [PubMed] [Google Scholar]
- McIntosh AR,Lobaugh NJ ( 2004): Partial least squares analysis of neuroimaging data: Applications and advances. Neuroimage 23 ( Suppl 1): S250–S263. [DOI] [PubMed] [Google Scholar]
- Moeller JR,Eidelberg D ( 1997): Divergent expression of regional metabolic topographies in Parkinson's disease and normal ageing. Brain 120 (Part 12): 2197–2206. [DOI] [PubMed] [Google Scholar]
- Moeller JR,Strother SC,Sidtis JJ,Rottenberg DA ( 1987): Scaled subprofile model: A statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab 7(5): 649–658. [DOI] [PubMed] [Google Scholar]
- Raz N,Lindenberger U,Rodrigue KM,Kennedy KM,Head D,Williamson A,Dahle C,Gerstorf D,Acker JD ( 2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15(11): 1676–1689. [DOI] [PubMed] [Google Scholar]
- Resnick SM,Goldszal AF,Davatzikos C,Golski S,Kraut MA,Metter EJ,Bryan RN,Zonderman AB ( 2000): One‐year age changes in MRI brain volumes in older adults. Cereb Cortex 10(5): 464–472. [DOI] [PubMed] [Google Scholar]
- Sarazin M,Stern Y,Berr C,Riba A,Albert M,Brandt J,Dubois B ( 2005): Neuropsychological predictors of dependency in patients with Alzheimer disease. Neurology 64(6): 1027–1031. [DOI] [PubMed] [Google Scholar]
- Stavitsky K,Brickman AM,Scarmeas N,Torgan RL,Tang MX,Albert M,Brandt J,Blacker D,Stern Y ( 2006): The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol 63(10): 1450–1456. [DOI] [PubMed] [Google Scholar]
- Stern Y,Sano M,Paulson J,Mayeux R ( 1987): Modified mini‐mental state examination: Validity and reliability. Neurology 37 ( Suppl): 179.3808297 [Google Scholar]
- Sullivan EV,Pfefferbaum A ( 2006): Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30(6): 749–761. [DOI] [PubMed] [Google Scholar]
- Sullivan EV,Rosenbloom M,Serventi KL,Pfefferbaum A ( 2004): Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging 25(2): 185–192. [DOI] [PubMed] [Google Scholar]
- Worsley KJ,Poline JB,Friston KJ,Evans AC ( 1997): Characterizing the response of PET and fMRI data using multivariate linear models. Neuroimage 6(4): 305–319. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME,Brickman AM,Paul RH,Grieve SM,Tate DF,Gunstad J,Cohen RA,Aloia MS,Williams LM,Clark CR, Whitford TJ, Gordon ( 2006): The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry 14(10): 823–833. [DOI] [PubMed] [Google Scholar]


