Abstract
Objectives
Aims were to investigate 1) relationships between serum ST2 levels and hemodynamic/neurohormonal variables; 2) myocardial ST2 production; 3) expression of ST2, membrane-anchored ST2L, and its ligand, IL-33, in myocardium, endothelium and leukocytes from patients with LV pressure overload and congestive cardiomyopathy.
Background
Serum levels of ST2 are elevated in heart failure. Relationship of ST2 with hemodynamic variables, source of ST2, and expression of ST2L and IL-33 in the cardiovascular system are unknown.
Methods
Serum ST2 (pg/mL; median [25th-75th]) was measured in patients with LV hypertrophy (aortic stenosis, AS, N=45), congestive cardiomyopathy (CCM N=53), and Controls (N=23). ST2 was correlated to NT-pro BNP, CRP and hemodynamic variables. Coronary sinus and arterial blood sampling determined myocardial gradient (production) of ST2. ST2, ST2L and IL-33 were measured (RT-PCR) in myocardial biopsies and leukocytes; ST2 protein production was evaluated in human endothelial cells. IL-33 protein expression was determined (immunohistochemistry) in coronary artery endothelium
Results
ST2 was elevated in AS (103[65-165], p<0.05) and CCM (194[69-551], p<0.01) vs. Controls (49[4-89]) and correlated with BNP (r=0.5; p<0.05), CRP (r=0.6; p<0.01) and LV EDP (r=0.38, p<0.03). LV ST2 mRNA was similar in AS and CCM vs. Control (NS). No myocardial ST2 protein gradient was observed. Endothelial cells secreted ST2. IL-33 protein was expressed in coronary artery endothelium. Leukocyte ST2L and IL-33 levels were highly correlated (r=0.97, p<0.001).
Conclusions
In human hypertrohy and failure, serum ST2 correlates with diastolic load. Though the heart, endothelium, and leukocytes express components of ST2/ST2L/IL-33 pathway, the source of circulating serum ST2 is extra-myocardial.
Keywords: interleukins, ST2, hypertrophy, heart failure, natriuretic peptides, endothelium-derived factors
Introduction
Inflammatory/innate immune signaling contributes to pathophysiology of human hypertrophy and heart failure. Recently, serum ST2 protein has emerged as a new prognostic biomarker in patients with recent myocardial infarction and congestive heart failure(1-6). ST2, and its membrane-anchored counterpart, ST2L, belong to the Toll-Interleukin-1 receptor family and production of both isoforms from a single mRNA is transcriptionally regulated(7,8). Extracellular engagement of ST2L with its ligand, IL-33, leads to the induction of Th2 cytokines in T cells(9) and downregulation of Toll-like receptor 4 signaling(10-12). Soluble ST2 protein is secreted in response to inflammatory signals. It inhibits the binding of IL-33 to ST2L(13) and has also been shown to bind to macrophages leading to downregulation of proinflammatory cytokines(14-16). Separate studies identified IL-33 as an intracellular protein localizing to the nucleus in inflamed high endothelial venules(17,18)
Apart from prognostic information obtained from soluble ST2 serum levels, little is known about myocardial production of ST2 or regulation and expression of ST2, ST2L, and IL-33(9), in the cardiovascular system. Serum ST2 was strongly correlated with serum B-type natriuretic peptide (BNP) and proANP(3), which are released from myocardium in response to pressure or volume overload and relate to indices of hemodynamic load(19). ST2 levels were also correlated with serum levels of norepinephrine(3), a marker of systemic neurohormonal activation in the failing heart. However, whether hemodynamic factors are directly related to serum ST2 levels in human hypertrophy and failure is unknown.
We tested the hypothesis that serum ST2 levels correlate to hemodynamic variables in patients with pressure overload hypertrophy and congestive cardiomyopathy. Both conditions represent two distinct pathophysiological mechanisms of heart failure allowing to pin down hemodynamic variables related to elevated serum ST2. Furthermore, we evaluated the myocardium as the origin of serum ST2 by investigating expression levels in myocardial biopsies as well as protein production of ST2. Finally, we report new information on secretion of ST2 protein in endothelial cells, and expression of ST2L and IL-33 in the myocardium and peripheral leukocytes from patients with pressure overload hypertrophy and congestive cardiomyopathy.
Methods
Patients
A total of 223 patients were studied. Serum ST2 levels were determined in 163 patients: 50 consecutive patients with symptomatic aortic valve stenosis (AS), 87 patients with congestive heart failure referred for elective diagnostic left-right heart catheterization (CCM), and a control group consisted of 26 subjects in whom diagnostic cardiac catheterization showed normal coronary angiogram and LV function (Control). In addition, 14 patients presenting with diastolic heart failure defined by elevated filling pressures and preserved LV systolic function were included for ST2 determination. Patients presenting with acute coronary syndrome, systemic inflammation or active infection were excluded. For determination of myocardial BNP and ST2 production, coronary sinus and arterial blood were sampled during cardiac catheterization from 24 patients with advanced congestive heart failure. In 38 patients (details below) LV myocardial gene expression for ST2, ST2L, IL-33, and BNP was performed. The study was approved by the institutional ethics committee and all patients gave informed consent.
Hemodynamics
At catheterization, pulmonary capillary wedge pressure was measured by a Swan-Ganz catheter whereas LV pressure was recorded with a catheter positioned in the LV cavity. LV volumes and ejection fraction (EF) were derived from the single plane angiogram using the area-length method. Aortic valve area was calculated using the Gorlin formula.
Echo-Doppler was performed immediately before or after cardiac catheterization according to guidelines of the American Society of Echocardiography(20). LV end-diastolic and end-systolic meridional wall stress (WS) were calculated from M-mode in combination with pressure data as previously described(21). LV mass was calculated by equation of Devereux(22). Increased diastolic load is defined as LV end-diastolic pressure (EDP) > 16 mmHg(23).
Serum Measurements
ST2 was measured by ELISA (MBL International, Inc, Woburn, MA). The ELISA coefficient of variation and stability of ST2 have been described(1,4-6). N-terminal-pro-brain natriuretic peptide (NT-pro-BNP) was measured using automated enzyme immunoassay (Roche Diagnositcs, Basel, Switzerland). High sensitive-CRP (hs-CRP) was determined using immunoturbidimetric method with latex beads coated with anti-CRP-specific antibody (Roche Diagnostics, Basel, Switzerland).
LV biopsies
LV endomyocardial biopsies were obtained from 38 patients (9 Control, 12 AS and 17 CCM). Control biopsies were obtained from patients with normal LV function and stable angina pectoris referred to elective cardiac bypass surgery. In Control and AS, LV biopsies were obtained during cardiac surgery and in patients with congestive CCM during routine cardiac catheterization.
Cultured Cells
Human saphenous vein endothelial cells were cultured in endothelial media at passage 3-5. Human coronary artery endothelial cells were obtained from Lonza (Allendale, NJ). Culture supernatant was assayed for ST2 16 hours after treatment, or, for time course, when indicated. Phorbol ester (200 nmol/L), H2O2 (50 μmol/L), and brefeldin A (5 μmol/L) were obtained from Sigma (St. Louis, MO); IL-1β (5 ng/mL), and TNF-α (10 ng/mL) were obtained from R and D Systems (Minneapolis, MN).
RNA extraction
RNA was extracted using RNeasy Mini Kit (Qiagen, Venlo, Netherlands) and was DNase digested and reverse transcribed using High-Capacity cDNA Archive System (Applied Biosystems, Foster City, CA).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Real-time RT-PCR on cDNAs was performed in triplicate using Assay-on-Demand (ST2) or specific primers and TaqMan MGB probes (ST2L-F: 5’-CAGGCGGCACATTTTCATC-3’, ST2L-R 5’-TGCTCGTAGGCAAACTCCTTATT-3’, ST2L-probe 6-FAM-ACCCCTCAGATCACTC-MGB and IL-33-F: 5’-CCAGGCCTTCTTTGTCCTTCATAAT-3’, IL-33-R 5’-CTCCAGGATCAGTCTTGCATTCAA-3’, IL-33-probe 6-FAM-CACTCCAACTGTGTTTCAT-MGB) (ABI Prism 7000 Sequence Detection System, Applied Biosystems, Foster City, CA). Expression of ST2, ST2L and IL-33 was normalized to GAPDH (Applied Biosystems, Foster City, CA) in the same sample.
Immunohistochemistry
Immunohistochemistry for IL-33 was performed on a human coronary artery using affinity purified rabbit polyclonal antibody against an IL-33 peptide antigen (Affinity Bioreagents, Golden, CO) and DAB staining with antigen retrieval (Vector Laboratories, Burlingame, CA).
Statistical analyses
Data were analyzed using GraphPad Prism (La Jolla, CA) and are presented as mean±SD for normally distributed variables and as median [25th-75th] when non-Gaussian distributed. For three groups, data were analyzed by ANOVA for parametric analysis or Kruskal-Wallis for nonparametric analysis and, when significant (P<0.05), was followed by Dunn’s or Bonferroni multiple comparison testing, respectively. For two groups, data were analyzed using Student’s t-test with p<0.05 considered significant. When variables displayed heterogeneity of variance, data were transformed and are presented on a log scale. Pearson correlation was used for normally distributed variables and Spearman correlation was used for nonparametric variables.
Results
Patient characteristics
Baseline characteristics of Control, AS and CCM patients undergoing serological analyses are shown in Table 1. LV mass index was higher in AS and CCM patients compared to Controls. CCM patients had larger end-diastolic volumes (EDVI), lower ejection fractions (LV EF), and higher diastolic and systolic wall stress compared to Controls. LV EDP was elevated in AS and CCM groups compared to Controls. LV developed pressure was increased in the AS group and decreased in the CCM group compared to Controls. There were no differences in clinical or hemodynamic characteristics between both subpopulations. In both populations, 64% and 52% of CCM patients, respectively, presented with acute decompensation or worsening of congestive heart failure symptoms.
Table 1.
Clinical and Hemodynamic parameters of patients with serological analyses.
| Control (n=23) | AS (n=45) | CCM (n=53) | |
|---|---|---|---|
| Age | 60±15 | 73±8 | 65±14 |
| Female/Male | 10/13 | 24/21 | 20/33 |
| NYHA class (% I/II/III/IV) | 100/0/0/0 | 17/36/47/0 | 2/48/31/19 |
| Acute heart failure | NA | 6 (13%) | 34 (64%) |
| Coronary artery disease | 0 | 7 (15%) | 13 (24%) |
| Betablockers | 5 (22%) | 20(45%) | 40 (75%) |
| ACE/AT1 Blockers | 3 (13%) | 19 (42%) | 46 (87%) |
| Diuretics | 2 (9%) | 22 (49%) | 50 (94%) |
| LV mass index (g/m2) | 75±28 | 128±59** | 136±67** |
| EDVI (mL) | 80±24 | 79±30 | 127±56***,§ |
| LV ejection fraction (%) | 74±10 | 68±21 | 27±11***,# |
| LV developed pressure (mmHg) | 133±31 | 174±44** | 97±30*,# |
| LVEDP (mmHg) | 12±4 | 18±8** | 23±8*** |
| Diastolic wall stress (kdyn/cm3) | 14±7 | 21±10 | 40±22***,# |
| Systolic wall stress (kdyn/cm3) | 93±25 | 74±35 | 120±58*,# |
Values are Mean±SD. Data were analyzed by ANOVA followed by post-hoc analysis. AS – aortic stenosis; CCM – cardiomyopathy;
p<0.05 vs Control,
p<0.01 vs. Control,
p<0.001 vs. Control,
p<0.01 vs AS,
p<0.001 vs AS.
LV – left ventricular; EDVI – end-diastolic volume index; EDP – end-diastolic pressure.
Serum ST2, hs-CRP and NT-pro BNP levels
Serum levels of ST2 (Figure 1A, left) were significantly increased in patients with AS (103[65-165] pg/mL, p<0.05) and CCM (194[69-551] pg/mL, p<0.01) compared to Controls (49[4-89] pg/mL). NT-pro-BNP (Figure 1A, middle) was significantly increased in AS (537[253-1936] pg/mL, p<0.05) and CCM patients (3088[629-7846] pg/mL, p<0.01) vs. Controls (43[28-100] pg/mL). Hs-CRP (Figure 1A, right) was significantly increased in patients with AS (6.1[1.3-15] mg/L, p<0.05) and CCM (12.0[3.3-29.2] mg/L, p<0.01) vs. Controls (2.2[1.2-3.0]). In the entire population, a significant positive relationship was noted between ST2 and NT-pro-BNP (Figure 1B, left, r=0.47, p <0.05) and between ST2 and hs-CRP (Figure 1B, right, r=0.55, p<0.001).
Figure 1. Serum levels of humoral markers.

A. Serum levels of ST2, CRP and BNP in AS, CCM and control patients. Box-plots show median [25th-75th]. B. Scatterplot between NT-pro-BNP and ST2 (left) and CRP and ST2 (right) in all patients.
Serum ST2 and hemodynamic parameters
No significant relationship was noted between serum ST2 levels and indices of LV remodelling including LV end-diastolic or end-systolic volume, LV mass index and LV relative wall thickness. Furthermore, ST2 levels did not correlate with LV systolic developed pressure or systolic wall stress. However, in the entire study population, a weak but significant, inverse relationship was noted between ST2 levels and LV ejection fraction(r= -0.21,p<005). On the other hand, ST2 levels showed stronger relationship with LV EDP(r= 0.37, p<0.01).
ST2 and Diastolic Load
In the entire cohort (Figure 2, left), serum ST2 levels were higher in patients with moderately or severely elevated LV EDP as compared to patients with normal or mildly increased LV EDP. ST2 levels were increased proportionally with extent of diastolic load as assessed from elevated levels of LV EDP (Figure 2, right panel).
Figure 2. Relationship between serum ST2 levels and extent of diastolic load as assessed from left ventricular end diastolic pressure (LVEDP).
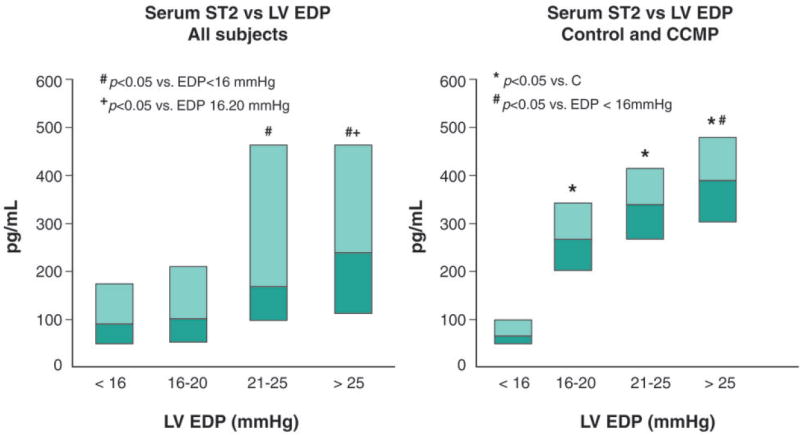
Serum ST2 levels according to LVEDP in all study subjects(left panel), and in Controls and in CCM patients(right panel). Box-plots show median [25th-75th].
To further determine whether diastolic load is associated with ST2 levels independent of LV systolic function (ejection fraction), ST2 was examined in patients (N=14) presenting with isolated diastolic heart failure (HF) and normal LV systolic function (Figure 3A, left). Serum ST2 was higher in these patients compared to Controls (383±149 vs. 75±12 pg/mL, p<0.001). Likewise, NT-pro-BNP was also increased compared to Controls (901±293 vs. 88±31 pg/mL,p=0.001, Figure 3A, right). The corresponding LV EDP (18±10 vs. 12±4 mmHg,p=0.03) and ejection fraction (73±13 vs. 74±7%,NS) are shown (Figure 3B). These results suggest that diastolic load is a hemodynamic factor that modulates ST2 production.
Figure 3. Serum ST2 and BNP in patients with isolated diastolic heart failure.

A. Serum ST2 (left) and BNP (right) levels in patients with isolated diastolic failure. Data were transformed due to heterogeneity of variance into the log scale. B. LVEDP (left) and LV ejection fraction in patients with diastolic failure compared to control patients.
Myocardial production of BNP and ST2
To determine myocardial production of BNP and ST2, the coronary sinus minus arterial blood concentration gradient was determined in 24 patients with chronic heart failure undergoing implantation of biventricular pacemakers. Hemodynamic characteristics of these patients with corresponding LV EDP (22±9 vs. 23±9 mmHg, p=NS) and ejection fraction (27±9 vs. 27±9%, NS) were similar to CCM group undergoing serological analyses. Arterial and coronary sinus levels of NT-pro-BNP were 1069±933 and 1798±1178 pg/mL respectively, with a difference of 729±410 pg/mL, p<0.0001 sinus vs. arterial (Figure 4A, left). Arterial and sinus levels of ST2 were 275±195 and 264±171 pg/mL respectively, with a differences of −11±39 pg/mL, p=NS (Figure 4A, right). These results demonstrate myocardial production of BNP but not of ST2. In LV myocardial biopsies (characteristics in Table 2), BNP mRNA levels were 4.4-fold increased in AS (log AU, 0.98±1.16) and 43 fold in CCM (log AU, 2.71±0.41) compared to Control (log AU, 0.27±1.11, p<0.001) (Figure 4B, left). Serum NT-pro-BNP levels correlated with their corresponding myocardial BNP mRNA levels (samples from the same patients, r = 0.54,p=0.01). ST2 mRNA levels were not significantly increased in AS (log AU 0.73±0.66) and CCM patients (log AU, 0.85±0.39) compared to Controls (log AU, 0.55±0.20, p=NS) (Figure 4B, right) with no correlation between serum ST2 and myocardial ST2 mRNA.
Figure 4. Transmyocardial gradient of and myocardial RNA levels of BNP and ST2.

A. Transmyocardial production of BNP (left) and ST2 (right) in individual patients (N=24) with heart failure. Mean±SD are also shown in addition to individual values. B. LV BNP mRNA levels (left) in Control, AS and CCM cardiac biopsies. LV ST2 mRNA levels (right) are not significantly different in all groups. AU: arbitrary units.
Table 2.
Clinical and hemodynamic parameters of patients with gene expression analysis
| Control (n=9) | AS (n=12) | CCM (n=17) | |
|---|---|---|---|
| Age | 61±13 | 71±9 | 58±16 |
| Female/Male | 1/8 | 6/6 | 4/13 |
| NYHA class | 100/0/0/0 | 25/67/8/0 | 6/42/35/17 |
| Acute heart failure | NA | 2 (12%) | 9 (52%) |
| Coronary artery disease | 6 (66%) | 3 (25%) | 3 (17%) |
| Betablockers | 4 (44%) | 1 (8%) | 10 (57%) |
| ACE/AT1 Blockers | 4 (44%) | 5 (42%) | 17 (100%) |
| Diuretics | 2 (22%) | 5 (42%) | 10 (57%) |
| LV mass index (g/m2) | 52±18 | 117±40** | 120±25** |
| EDVI (mL) | 77±14 | 83±25 | 145±58***,§ |
| LV ejection fraction (%) | 72±11 | 67±18 | 24±11***,# |
| LV developed pressure (mmHg) | 117±26 | 178±46* | 90±25*,# |
| LVEDP (mmHg) | 13±3 | 17±6** | 21±7*** |
| Diastolic wall stress (kdyn/cm3) | 18±9 | 19±9 | 38±15***,# |
| Systolic wall stress (kdyn/cm3) | 70±25 | 69±32 | 134±27*,# |
Values are Mean±SD. Data were analyzed by ANOVA followed by post-hoc analysis. AS – aortic stenosis; CCM – cardiomyopathy;
p<0.05 vs Control,
p<0.01 vs. Control,
p<0.001 vs. Control,
p<0.01 vs AS,
p<0.001 vs AS.
LV – left ventricular; EDVI – end-diastolic volume index; EDP – end-diastolic pressure.
ST2 secretion by venous and arterial endothelial cells
Phorbol-ester induced ST2 secretion in venous endothelial cells compared to Control (299±22 vs. 92±6 pg/mL, p<0.001)) (Figure 5). Interleukin-1β induced ST2 secretion in venous and arterial endothelial cells (venous, 125±6 vs. 92±6 pg/mL, p<0.001); arterial, 103±48 vs. 50±24 pg/mL, p<0.05). TNF-α also induced ST2 secretion (115±4 pg/mL) compared to Control (96±6 pg/mL p<0.001). H2O2 blocked basal ST2 secretion in endothelial cells (16±4 vs. 50±24 pg/mL, p<0.05) demonstrating that local oxidative stress can inhibit ST2 secretion. ST2 secretion was elevated by 3 hours compared to Control (150±20 vs. 52±12 pg/mL, p<0.001), reached peak levels at 6 hours (304±14 vs. 51±10 pg/mL, p<0.001) (Figure 5, right) and declined afterwards (not shown). Brefeldin A, a blocker of protein secretion through the ER/Golgi, in the presence of phorbol ester completely blocked ST2 secretion (Figure 5, right). Data represent 3-4 experiments with duplicate samples.
Figure 5. ST2 protein secretion by human venous and arterial endothelial cells.

Phorbol ester, IL-1β and tumor necrosis factor-1 induced ST2 secretion in venous endothelial cells (left). IL-1β induced secretion while H2O2 blocked secretion in arterial endothelial cells (middle). Time course shows ST2 is rapidly synthesized and secreted with significantly elevated levels at 3 hours and reaches peak values at 6 hours (right). Brefeldin A blocked ST2 secretion (right).
Expression of IL-33 and ST2L in the heart and endothelium
IL-33 mRNA levels were modestly lower in LV biopsies from AS patients (0.80±0.33 AU) compared to CCM patients (1.33±0.48 AU,p=0.005, Figure 6A, left). Levels of membrane-anchored ST2L were significantly decreased in AS compared to Control (0.27±0.22 vs. 1.00±0.60 AU; p<0.05) but levels were similar between CCM (0.92±0.72 AU) and Control (NS,Figure 6A, left). IL-33 protein localizes to endothelial cells in the human coronary artery (Figure 6B). A weak correlation between IL-33 and ST2L mRNA expression in myocardial biopsies was observed (r=0.35, p=0.045).
Figure 6. IL33 and ST2 mRNA levels in myocardial biopsies.
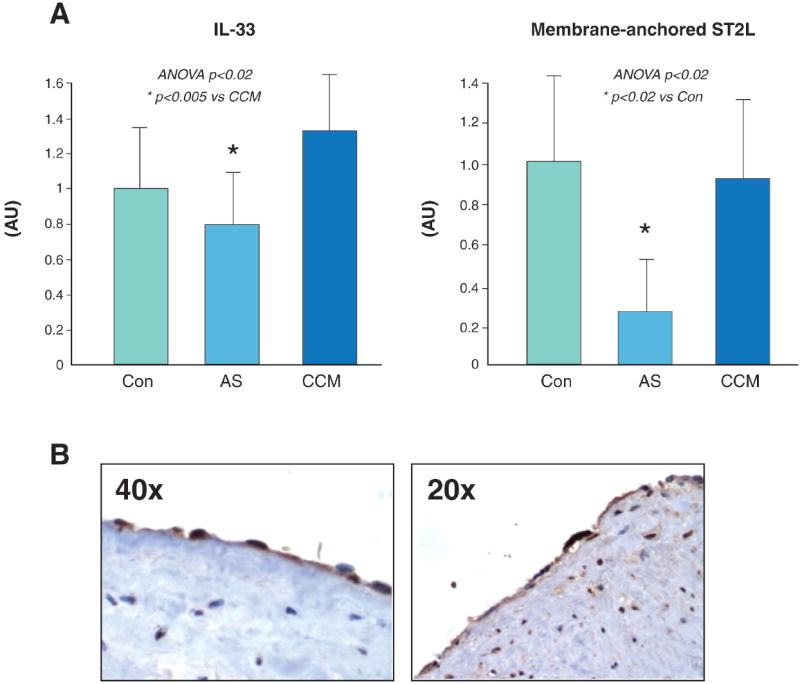
A. IL-33 mRNA levels were decreased in AS hearts compared to CCM hearts. ST2L mRNA levels were significantly decreased in AS hearts compared to Control. ST2L levels were similar in CCM hearts compared to Control (middle). B. IL-33 protein was expressed in human coronary artery endothelium (brown staining).
Peripheral leukocyte expression of ST2, ST2L and IL-33
Levels of soluble ST2 mRNA in peripheral leukocytes were similar in all groups (Control, 2.0±1.4; AS, 4.3±3.6; CCM, 4.2±2.8 AU; NS) as were levels of ST2L (Control, 20.1±15.9; AS, 23.8±17; CCM, 16.0±14 AU, NS) and IL-33 (Control, 0.94±0.84; AS, 1.13±1,1; CCM, 0.63±0.77 AU; NS). However, a strong correlation was noted between IL33 and ST2L mRNA in leukocytes over the range of all values (Figure 7. r = 0.996; p<0.001).
Figure 7. Correlation between ST2L and IL-33 in leukocytes.
Relationship between ST2L vs. IL-33 mRNA levels in leukocytes from Control, AS and CCM patients.
Discussion
The present study shows that serum ST2 protein is elevated in patients with chronic LV pressure overload due to aortic stenosis as well as in patients with congestive heart failure. ST2 positively correlated with NT-pro-BNP and the inflammatory marker CRP. ST2 levels were proportionally related to extent of diastolic load. A dominant role of diastolic load in relationship to ST2 levels is further supported by findings in a subgroup of patients with normal systolic function and isolated diastolic failure. In this group, ST2 levels were increased as were NT-pro-BNP levels. Thus, ST2 induction appears to be sensitive primarily to diastolic load. However, it is interesting that unlike BNP induction(19,23), ST2 production did not show a sigificant relationship with systolic wall stress.
The source of serum ST2 in cardiovascular disease was presumed to be myocardial following in vitro data showing load induction of ST2 mRNA in neonatal rat cardiac myocytes(24). We provide direct evidence that the adult, human myocardium is not the source of increased serum ST2 in pressure overload hypertrophy and congestive cardiomyopathy. First, it is well-established that ST2 is transcriptionally regulated (mRNA induction)(7,8), yet ST2 mRNA levels were not significantly increased in myocardial biopsies. This is in contrast with BNP expression, which was increased in the same RNA pool of LV samples reflecting increased levels of serum NT-pro-BNP in the same patients. Second, direct measurements of the BNP and ST2 protein concentration in coronary sinus and arterial blood samples demonstrated a transmyocardial gradient (production) of BNP, but no transmyocardial gradient of ST2. These surprising findings prompted us to examine ST2 protein production in extra-myocardial, non-myocyte cell types. We present the novel findings that human venous and arterial endothelial cells secrete ST2 protein. ST2 protein synthesis and release was rapid, peaked within 6 hours and was blocked by brefeldin, which blocks intracellular protein transport(25), confirming that ST2 exits endothelial cells through a secretion pathway responsive to inflammatory signals. This finding, together with the relationship between serum ST2 and indices of diastolic load, suggest that the vascular endothelium, sensing hemodynamic and inflammatory status, is a potential source of elevated serum ST2 levels in hemodynamic overload and heart failure.
In this regard, it is noteworthy that ST2 levels failed to provide prognostic information in patients with acute coronary syndrome(26). Interestingly, authors noticed highest serum ST2 levels in chronic alcoholics, patients with systemic sepsis, and concomittant lung diseases, consistent with studies reporting elevated serum ST2 in pulmonary diseases(13,27). Serum ST2 is also elevated in patients with dengue virus infection(28), sepsis and trauma(29), and in CSF fluid following subarachnoid hemorrhage(30). The broad specificity of ST2 induction corroborates its nonmyocardial production and may explain why ST2 performs well as an independent biomarker in multi-marker studies, providing information distinct from BNP in cardiovascular disease.
In addition to a cardiovascular biomarker, ST2/ST2L signaling is likely to be important in cardiovascular disease modulation. At the cellular level, soluble ST2 binds to macrophages and downregulates proinflammatory cytokines, IL-1, IL-6 and TNF-α(14,15). Soluble ST2 can bind to IL-33, the ST2L ligand, to diminish MAP kinase and NFkB signaling(13). Membrane-anchored ST2L inhibits also IL-1 receptor and innate immunity TLR4 signaling (11,12,31). Hence, both ST2L and soluble ST2 negatively regulate IL-1 receptor and TLR4 signaling(12,32). We present the novel findings that ST2L and IL-33 mRNA are expressed in normal and diseased human myocardium and both are modestly but significantly decreased in patients with aortic stenosis. The mechanism is unknown, although it is recognized that soluble vs. membrane-anchored ST2L are regulated separately(7,8). In addiiton, we found that IL-33 protein is expressed in endothelial cells within coronary arteries suggesting that IL-33 may have a similar function in vascular endothelium that may be regulated by soluble ST2. We report a remarkable pronounced correlation between ST2L and IL-33 levels in peripheral blood leukocytes, suggesting that they are highly co-regulated in these cells. Further studies are needed to determine the biological relevance of these observations.
Limitations
The present study is limited to patients with chronic aortic stenosis or heart failure. It would be interesting to interrogate myocardial production of ST2 protein in acute myocardial infarction following which serum ST2 levels are also increased. Though the lack of transmyocardial ST2 gradient argues against cardiac production of serum ST2, ST2 protein production in human adult cardiac myocytes was not directly tested.
Implications
This study describes elevated serum ST2 in patients with pressure overload hypertrophy and congestive or diastolic heart failure. Our data support diastolic load as a predominant hemodynamic factor that contributes to ST2 production in heart disease. Note, our data indicate that overloaded myocardium is not a major source of increased serum ST2. We showed that endothelial cells possess a functional ST2 secretory system in vitro, providing proof-of-concept for ST2 production by the vascular endothelium in vivo, which can be investigated in future studies.
Acknowledgments
This study was funded in part by NIH (Bethesda, MD) grant HL069484 (EOW), by 3M Pharma Prize Award (JB and MV), and by Meijer Lavino Foundation for Cardiac Research, Aalst, Belgium.
List of abbreviations
- AS
aortic stenosis
- CCM
cardiomyopathy
- LV
left ventricular
- BNP
B-type natriuretic peptide
- NT-pro-BNP
N-terminal-pro-brain natriuretic peptide
- EDP
end-diastolic pressure
- EF
ejection fraction
- IL
interleukin
- hsCRP
high sensitivity C-reactive protein
- TLR
toll-like receptor
Footnotes
All authors state no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Januzzi JL, Jr, Peacock WF, Maisel AS, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–13. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Shimpo M, Morrow DA, Weinberg EO, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–6. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 4.Mueller T, Dieplinger B, Gegenhuber A, Poelz W, Pacher R, Haltmayer M. Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin Chem. 2008;54:752–6. doi: 10.1373/clinchem.2007.096560. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Morrow DA, Higgins LJ, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–44. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehman SU, Martinez-Rumayor A, Mueller T, Januzzi JL., Jr Independent and incremental prognostic value of multimarker testing in acute dyspnea: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Clin Chim Acta. 2008;392:41–5. doi: 10.1016/j.cca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. Embo J. 1994;13:1176–88. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwahana H, Yanagisawa K, Ito-Kosaka A, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. Lett FEBS. 1993;318:83–7. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- 11.Brint EK, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–9. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 12.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–80. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 14.Sweet MJ, Leung BP, Kang D, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–9. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 15.Takezako N, Hayakawa M, Hayakawa H, et al. ST2 suppresses IL-6 production via the inhibition of IkappaB degradation induced by the LPS signal in THP-1 cells. Biochem Biophys Res Commun. 2006;341:425–32. doi: 10.1016/j.bbrc.2005.12.206. [DOI] [PubMed] [Google Scholar]
- 16.Yanagisawa K, Naito Y, Kuroiwa K, et al. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J Biochem (Tokyo) 1997;121:95–103. doi: 10.1093/oxfordjournals.jbchem.a021577. [DOI] [PubMed] [Google Scholar]
- 17.Baekkevold ES, Roussigne M, Yamanaka T, et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carriere V, Roussel L, Ortega N, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–7. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol. 2004;44:2349–54. doi: 10.1016/j.jacc.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 20.ACC/AHA guidelines for the clinical application of echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures. Circulation. 1990;82:2323–45. doi: 10.1161/01.cir.82.6.2323. [DOI] [PubMed] [Google Scholar]
- 21.Douglas PS, Reichek N, Plappert T, Muhammad A, St John Sutton MG. Comparison of echocardiographic methods for assessment of left ventricular shortening and wall stress. J Am Coll Cardiol. 1987;9:945–51. doi: 10.1016/s0735-1097(87)80253-x. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Alonso DR, Lutas, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 23.Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135:825–32. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg EO, Shimpo M, De Keulenaer GW, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie L, Boyle D, Sanford D, Scherer PE, Pessin JE, Mora S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem. 2006;281:7253–9. doi: 10.1074/jbc.M511313200. [DOI] [PubMed] [Google Scholar]
- 26.Brown AM, Wu AH, Clopton P, Robey JL, Hollander JE. ST2 in emergency department chest pain patients with potential acute coronary syndromes. Ann Emerg Med. 2007;50:153–8. 158 e1. doi: 10.1016/j.annemergmed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun. 2002;299:18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- 28.Becerra A, Warke RV, de Bosch N, Rothman AL, Bosch I. Elevated levels of soluble ST2 protein in dengue virus infected patients. Cytokine. 2008;41:114–20. doi: 10.1016/j.cyto.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunner M, Krenn C, Roth G, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004;30:1468–73. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 30.Kanda M, Ohto-Ozaki H, Kuroiwa K, Tominaga S, Watanabe E, Iwahana H. Elevation of ST2 protein levels in cerebrospinal fluid following subarachnoid hemorrhage. Acta Neurol Scand. 2006;113:327–33. doi: 10.1111/j.1600-0404.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- 31.Dunne A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003. 2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 32.Trajkovic V, Sweet MJ, Xu D. T1/ST2--an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004;15:87–95. doi: 10.1016/j.cytogfr.2004.02.004. [DOI] [PubMed] [Google Scholar]



