Abstract
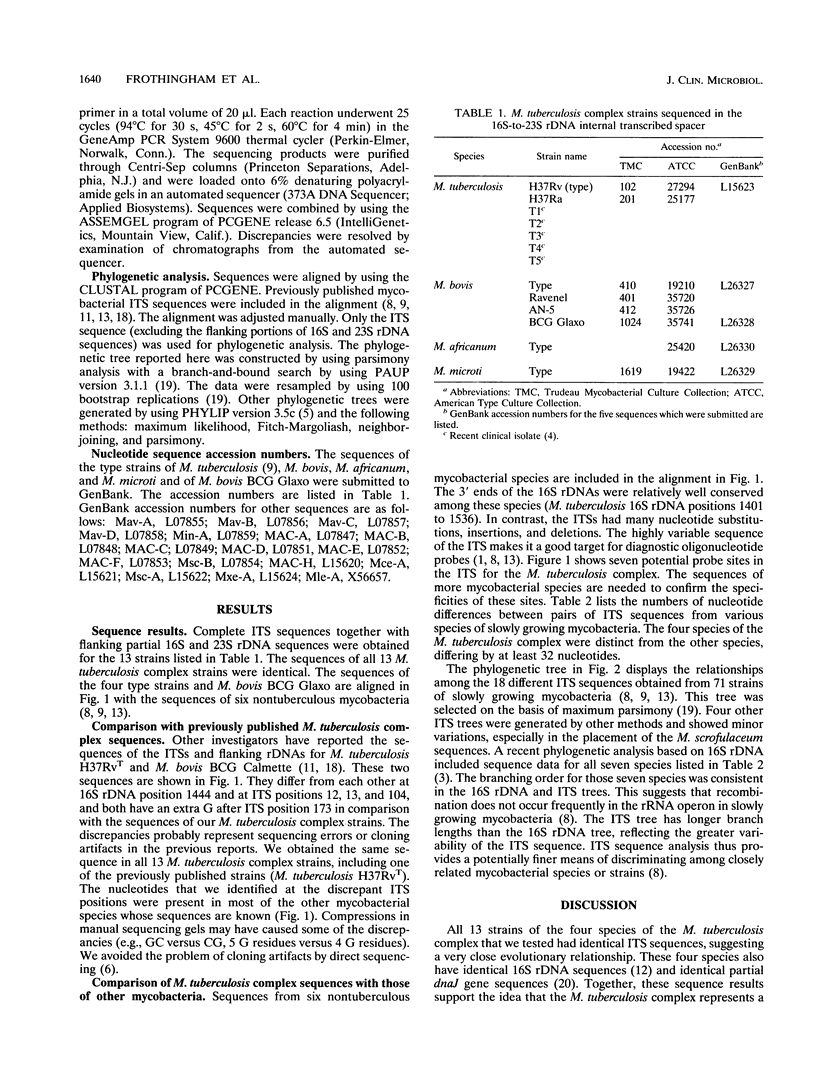
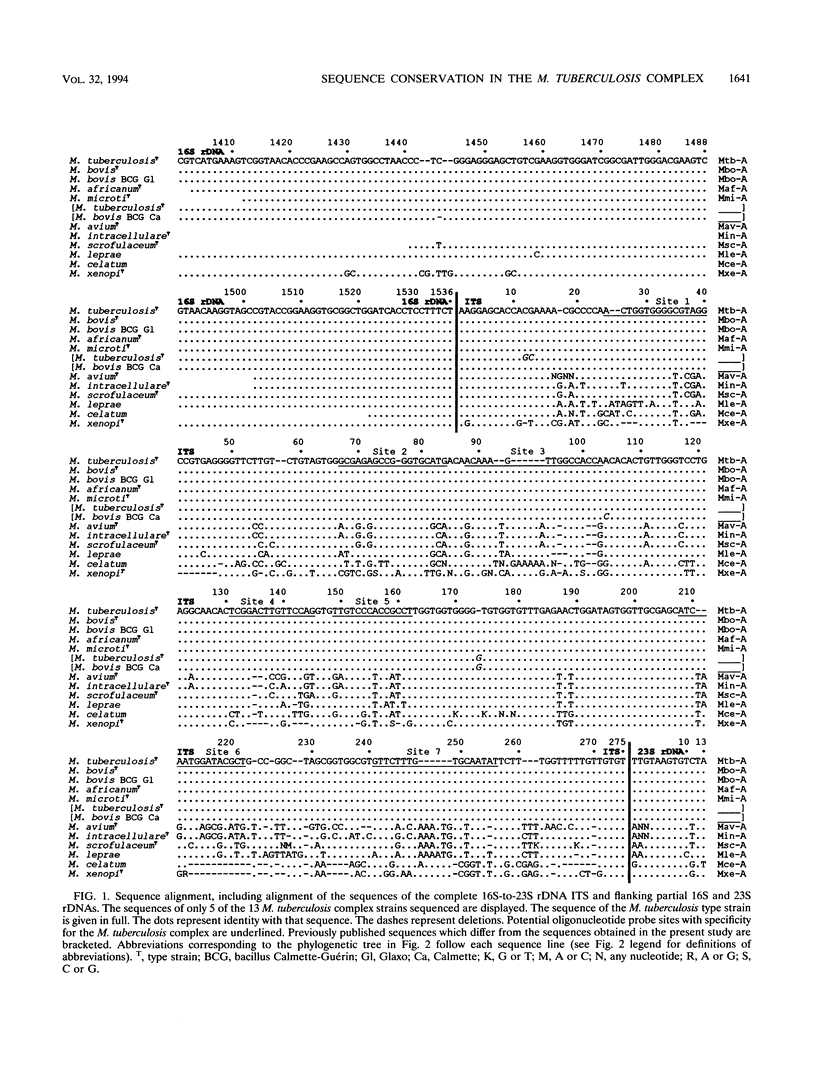
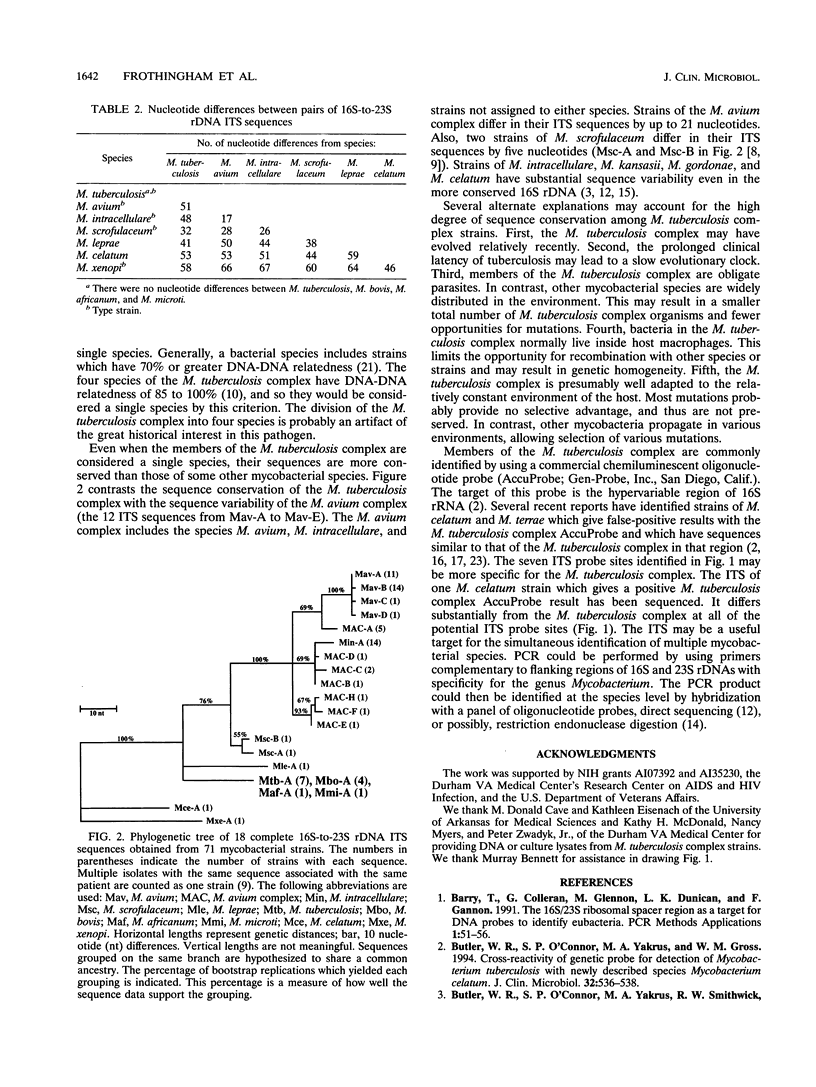
The Mycobacterium tuberculosis complex includes the four species M. tuberculosis, M. bovis, M. africanum, and M. microti. We sequenced 13 M. tuberculosis complex strains in the 16S-to-23S rDNA internal transcribed spacer (ITS). The ITS has a high rate of nucleotide substitution. Previous reports found three nucleotide substitutions in the ITS between two M. tuberculosis complex strains. In contrast, we found the same ITS sequence in all 13 M. tuberculosis complex strains (including all four species and M. bovis BCG). This finding confirms the conservation of 16S rDNA sequence and the high DNA-DNA relatedness found in previous studies. By the usual criteria, the four species of the M. tuberculosis complex would be considered a single species. In a phylogenetic analysis based on the ITS sequence, the four species of the M. tuberculosis complex were distinct from nontuberculous mycobacteria. The ITS contains at least seven potential sites for oligonucleotide probes with specificity for the M. tuberculosis complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry T., Colleran G., Glennon M., Dunican L. K., Gannon F. The 16s/23s ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991 Aug;1(1):51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- Butler W. R., O'Connor S. P., Yakrus M. A., Gross W. M. Cross-reactivity of genetic probe for detection of Mycobacterium tuberculosis with newly described species Mycobacterium celatum. J Clin Microbiol. 1994 Feb;32(2):536–538. doi: 10.1128/jcm.32.2.536-538.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. R., O'Connor S. P., Yakrus M. A., Smithwick R. W., Plikaytis B. B., Moss C. W., Floyd M. M., Woodley C. L., Kilburn J. O., Vadney F. S. Mycobacterium celatum sp. nov. Int J Syst Bacteriol. 1993 Jul;43(3):539–548. doi: 10.1099/00207713-43-3-539. [DOI] [PubMed] [Google Scholar]
- Cave M. D., Eisenach K. D., McDermott P. F., Bates J. H., Crawford J. T. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 1991 Feb;5(1):73–80. doi: 10.1016/0890-8508(91)90040-q. [DOI] [PubMed] [Google Scholar]
- Frothingham R., Wilson K. H. Molecular phylogeny of the Mycobacterium avium complex demonstrates clinically meaningful divisions. J Infect Dis. 1994 Feb;169(2):305–312. doi: 10.1093/infdis/169.2.305. [DOI] [PubMed] [Google Scholar]
- Frothingham R., Wilson K. H. Sequence-based differentiation of strains in the Mycobacterium avium complex. J Bacteriol. 1993 May;175(10):2818–2825. doi: 10.1128/jb.175.10.2818-2825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempsell K. E., Ji Y. E., Estrada I. C., Colston M. J., Cox R. A. The nucleotide sequence of the promoter, 16S rRNA and spacer region of the ribosomal RNA operon of Mycobacterium tuberculosis and comparison with Mycobacterium leprae precursor rRNA. J Gen Microbiol. 1992 Aug;138(Pt 8):1717–1727. doi: 10.1099/00221287-138-8-1717. [DOI] [PubMed] [Google Scholar]
- Kirschner P., Springer B., Vogel U., Meier A., Wrede A., Kiekenbeck M., Bange F. C., Böttger E. C. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol. 1993 Nov;31(11):2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesack W., Sela S., Bercovier H., Pitulle C., Stackebrandt E. Complete nucleotide sequence of the Mycobacterium leprae 23 S and 5 S rRNA genes plus flanking regions and their potential in designing diagnostic oligonucleotide probes. FEBS Lett. 1991 Apr 9;281(1-2):114–118. doi: 10.1016/0014-5793(91)80372-a. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. B., Plikaytis B. D., Yakrus M. A., Butler W. R., Woodley C. L., Silcox V. A., Shinnick T. M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992 Jul;30(7):1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. C., Jackson K., Yang M., Sievers A., Dwyer B. Identification of a genetically distinct subspecies of Mycobacterium kansasii. J Clin Microbiol. 1992 Nov;30(11):2930–2933. doi: 10.1128/jcm.30.11.2930-2933.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L., Springer B., Böttger E. C., Roberts G. D. Mycobacterium tuberculosis nucleic acid probes for rapid diagnosis. Lancet. 1993 Jun 5;341(8858):1486–1486. doi: 10.1016/0140-6736(93)90935-a. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nagata A., Ono Y., Yamada T. Complete nucleotide sequence of the 16S rRNA gene of Mycobacterium bovis BCG. J Bacteriol. 1988 Jun;170(6):2886–2889. doi: 10.1128/jb.170.6.2886-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takewaki S., Okuzumi K., Manabe I., Tanimura M., Miyamura K., Nakahara K., Yazaki Y., Ohkubo A., Nagai R. Nucleotide sequence comparison of the mycobacterial dnaJ gene and PCR-restriction fragment length polymorphism analysis for identification of mycobacterial species. Int J Syst Bacteriol. 1994 Jan;44(1):159–166. doi: 10.1099/00207713-44-1-159. [DOI] [PubMed] [Google Scholar]


