Abstract
The modulation of cell adhesion is fundamental to the morphogenesis that accompanies proper embryonic development. Cadherins are a large family of calcium-dependent cell adhesion molecules whose spatial and temporal expression is critical to the formation of the neural crest, a unique, multipotent cell type that contributes to the patterning of the vertebrate body plan. Neural crest cells arise from the embryonic ectoderm through inductive interactions and reside in the dorsal aspect of the neural tube. These cells under go an epithelial-to-mesenchymal transition and migrate to precise destinations in the embryo, where they go on to differentiate into such diverse structures as melanocytes, elements of the peripheral nervous system and the craniofacial skeleton. Distinct cadherins are expressed during the induction, migration and differentiation of the neural crest. With the advent of genomic sequencing, assembly and annotation for various model organisms, it has become possible to elucidate the molecular mechanisms underlying cadherin expression, and how these cadherins function, during neural crest development. This review explores the known roles of cadherins and details, where relevant, how different cadherins are regulated during the formation of the neural crest.
Key words: cadherins, neural crest, EMT, induction, migration, differentiation
Cadherins
Structure.
Cadherins are a large family of calcium-dependent, homophilic-binding cell adhesion molecules that play critical roles in morphogenetic events underlying embryonic development, including cell sorting, cell motility and signaling. These proteins typically span the plasma membrane once, with an extracellular amino (N) terminus and an intracellular carboxy (C) terminus. Structurally, cadherins consist of five extracellular (EC) domain repeats of approximately 110 amino acids containing specific conserved motifs, such as DRE, DXNDNAPXF and DXD, known as the cadherin repeat.1 The EC domains bind three calcium ions and facilitate the formation of parallel or cis homodimers on the cell surface (reviewed in refs 2–4),7 and the presence of calcium ions is critical to enhance the adhesive strength and for cellular signaling.5 The intracellular portion of the cadherin molecule consists of a juxtamembrane domain bound by p120 catenin, a src substrate whose binding to cadherins has been shown to regulate cadherin adhesion and assist in cadherin clustering (reviewed in refs. 1 and 6). Following the juxtamembrane domain is a 30 amino acid binding domain for β-catenin or plakoglobin. These proteins share 65% identity and consist of 12 armadillo repeats comprised of 42 amino acids that mediate binding to the cadherin C terminus. The plakoglobin or β-catenin N terminus interacts directly with α-catenin, which in turn interacts directly with actin, or associates with the actin binding proteins vinculin, talin, α-actinin and possibly formin-1, thus creating a scaffold for an interaction with the actin cytoskeleton.7 Collectively, the association of cadherins to these intracellular proteins results in the formation of the adherens junction, located at the apical surface of polarized epithelial cells.
Regulation.
The initial contacts made by cadherin-containing adherens junctions on neighboring cells allows for the clustering and growth of the adherens junction in order to permit adherens junction maintenance. It has been postulated that cis cadherin dimers that interact with one another through EC1 and EC2 can subsequently interact with cadherins expressed on adjacent cells in trans, particularly through an association with EC1, to make a “zipper-like” structure that increases the overall strength of the adherens junction.8 In addition, these cell-cell junctions are highly dynamic due to the internalization and recycling of cadherins, and disassembly can also occur by attrition or large-scale proteolysis of adherens junction components.1 Regulation of cadherins also occurs intracellularly via the catenins. α-catenin and formin-1 have been shown to regulate actin polymerization at the adherens junction, and similar to the “inside-out” signaling observed with integrins, cytoplasmic signals through the catenins can regulate the dimerization state and/or cadherin clustering independent of their binding to the cytoskeleton, thus resulting in changes in cadherin adhesion.7 Concomitantly, interaction of catenins with the actin cytoskeleton promotes changes in cell shape and/or the onset of cell motility through the production of forces that are transduced to the cadherin, resulting in the establishment of cell polarity. Moreover, it has long been hypothesized that p120 catenin plays an important role in modulating cadherin-based signaling, as dephosphorylated p120 enhances adhesion and phosphorylated p120 promotes cadherin instability.7,9 Specifically, experiments by Davis et al. (2003) and Xiao et al. (2007) demonstrate that p120 catenin binding to cadherin inhibits cadherin degradation and thus regulates the total level of cadherin found in the cell.10,11 p120 catenin is also a Rho GTP dissociation inhibitor, and it has been shown to inhibit Rho GTPase and activate Rac1 and Cdc42 GTPases.12–15 Rho GTPases, particularly RhoA, normally act to negatively regulate cadherins, such as E-cadherin, and p120 catenin counteracts this effect by sequestering RhoA in the cytoplasm.16,17 Conversely, Rac1 and Cdc42 co-localize to the membrane with cadherins, and positively regulate E-cadherin.16–18
Types of cadherins.
Five main cadherin sub-groups have been described and are summarized in Figure 1 (reviewed in refs. 1 and 7). The classical cadherins are those containing 5 EC domains and whose intracellular domain binds p120, and β-and α-catenin in order to attach to the actin cytoskeleton. Type I (E-cadherin, N-cadherin, P-cadherin, R-cadherin) and II (cadherin-5 and beyond) classical cadherins differ from each other by the presence of the HAV tripeptide in the type I EC1 domain.19 This domain is required for adhesion and many function-blocking antibodies map here. The desmosomal cadherins (desmogleins and desmocollins) that comprise desmosomes interact homo-and heterotypically through their N termini, and bind plakoglobin and desmoplakin through their C termini in order to associate with cytoskeletal intermediate filaments in epithelial and cardiac muscle cells. Atypical cadherins, such as T-cadherin and Ll-cadherin, interact homotypically but do not provide a link to the cytoskeleton as they lack the necessary intracellular domain (T-cadherin is GPI-linked, while Ll-cadherin has a short cytoplasmic tail). Found only in vertebrates, protocadherins (α-, β- and γ Pcdhs, PAPC, AXPC) usually contain six to seven EC repeats, and although they localize to sites of cell-cell contact, they do not bind catenins but instead interact with kinases such as Fyn. Finally, the cadherin-like or -related signaling proteins such as FAT, Daschous, Flamingo (all of which contain seven transmembrane domains) and Ret tyrosine kinase share some homology with G protein-coupled receptors, contain an unfixed number of EC cadherin repeats, do not interact with catenins and the actin cytoskeleton, and have diverse roles in development, including cell polarity, patterning and tumor suppression.
Figure 1.
Domain structure of various cadherin sub-groups. Five cadherin sub-groups can be described in the Cadherin family of cell adhesion molecules. All cadherins possess the ability to bind calcium ions and contain a variable number of common extracellular (EC) domains. Most cadherin sub-groups are defined as single-pass transmembrane proteins possessing one intracellular domain capable of binding different proteins (catenins, kinases), with the exception of the atypical cadherins that are membrance-anchored. Cadherin-like (or -related) proteins, however, span the plasma membrane multiple times and share homology with G protein-coupled receptors.
The Neural Crest
Neural crest cells are a transient population of migratory cells that arise from the dorsal neural folds, a region of tissue localized to the border between the non-neural (future epidermis) and neural ectoderm/plate (future central nervous system). During the process of neurulation, the neural plate invaginates and neural folds elevate, forming the neural tube. Premigratory neural crest cells (the precursors to migratory neural crest cells) reside in the dorsal neural folds and, consequently, the dorsal region of the neural tube. These precursor cells are epithelial in character and therefore possess apicobasal polarity, with apically localized adherens junctions and a basal lamina.20–22
Depending upon the vertebrate species, migratory neural crest cells will form either prior to or soon after the fusion of the neural folds.20 These migratory neural crest cells are generated by an epithelial-to-mesenchymal transition (EMT), a process characterized by loss of cell-cell contacts mediated by cell junctions, reorganization of the cytoskeleton, and the subsequent acquisition of a motile phenotype (reviewed in refs. 19 and 23–25). Thus, migratory neural crest cells are mesenchymal in character, as they express the intermediate filament vimentin and possess a flattened morphology with filopodia and lamellipodia in order to facilitate increased spreading.26–28 Importantly, EMTs occur during both normal (e.g., neural crest migration) and aberrant (e.g., tumor cell metastasis) developmental processes that generate motile mesenchymal cells.
After EMT, neural crest cells leave the dorsal neural tube and migrate along stereotypical pathways in the head and trunk regions of the embryo. Cranial neural crest cells invade the surrounding cranial mesenchyme and ultimately cease migration, coalesce, and form the various cranial ganglia and craniofacial cartilages and bones. Migratory neural crest cells in the trunk that follow the dorsoventral path will differentiate to form components of the peripheral nervous system (dorsal root ganglia of the spinal cord, Schwann cells), while those migrating dorsolaterally will become melanocytes.20
Cadherin Expression during Neural Crest Development
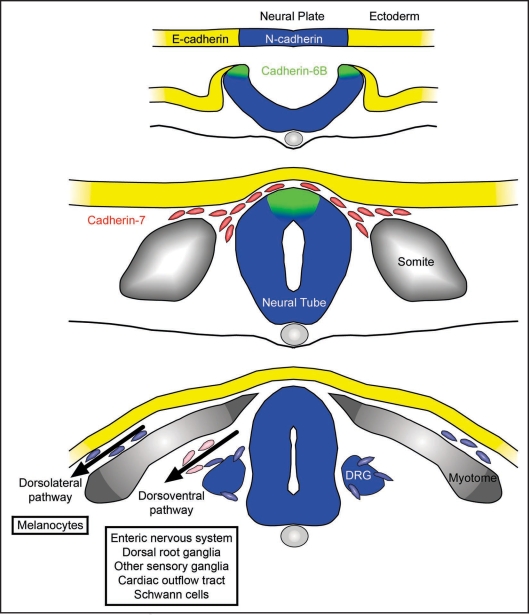
Dynamic spatial and temporal cadherin expression is found throughout embryonic development. Interestingly, the formation of the neural crest provides an excellent paradigm to study changes in cadherin expression and their function. Premigratory neural crest cells initially reside in the dorsal neural tube as adherent epithelial cells, sharing multiple contacts with their neighbors. However, these cells eventually detach themselves from the neuroepithelium during the process of EMT to migrate away to their final destinations. During their induction, migration and subsequent differentiation, neural crest cells express various cadherins at different developmental times, and it has been hypothesized that the distinct repertoire of cadherins expressed by the neural crest during its ontogeny plays a crucial role in segregating these cells from other cells in the neural tube, as well as allowing for specific sub-populations of neural crest cells to interact with one another during migration and subsequently after cessation during the differentiation process.9 See Figure 2 for an overview of cadherin expression during chick neural crest development.
Figure 2.
Cadherin expression during the formation of the neural crest in the avian embryo. Prior to neurulation, E-cadherin (yellow) is expressed throughout the developing embryonic ectoderm. The neural plate is later defined by the acquisition of N-cadherin (blue) and the loss of E-cadherin, which is still maintained in the non-neural ectoderm or future epidermis. During neurulation, N-cadherin expression persists in the invaginating neural plate, while cadherin-6B (green) comes on in the neural folds of the embryo, delineating the future premigratory neural crest cell domain in the dorsal neural tube. Cadherin-6B and N-cadherin are downregulated as neural crest cells undergo EMT, and migratory neural crest cells instead express cadherin-7 (red) as they migrate along stereotypical pathways to their final destinations. During neural crest cell differentiation, N-cadherin expression is detected in the dorsal root ganglia (DRG). Pink cells along the dorsolateral pathway in the bottom panel of the figure indicate migratory neural crest cells expressing multiple cadherins. Structural derivatives of the dorsolateral and dorsoventral pathways are indicated in boxes.
N-cadherin.
Induction. In mouse and chick embryos, initially epithelial cadherin (E-cadherin) or L-CAM, respectively, is expressed throughout the ectoderm.29,30 During neurulation in these vertebrates, as the neural plate invaginates to form the neural tube, E-cadherin expression is initially maintained by the non-neural ectoderm but is later downregulated in the neural plate and replaced by neural cadherin or A-CAM (N-cadherin) expression in this region.19,31 Importantly, N-cadherin expression is absent in the non-neural ectoderm. These authors hypothesize that this “switch” in cadherin expression is important to keep ectodermal and non-ectodermal cell types separate during neural plate invagination. Furthermore, N-cadherin protein is observed throughout the neural tube, particularly distributed apically and laterally, but is reduced in the dorsal-most region.21
EMT/migration. Recent progress has been made towards understanding the molecular regulation of N-cadherin during neural crest EMT/migration. Using trunk neural crest development in 15–18 somite stage chick embryos as a model, Shoval et al. (2007) revealed that neural crest emigration relies upon the downregulation of N-cadherin through the coordinated action of BMP signaling and proteolytic processing of the cell surface N-cadherin protein.32 N-cadherin transcripts are present throughout the dorsal neural tube in the chicken trunk, irrespective of the axial level, whereas N-cadherin protein is distributed in an increasing rostrocaudal gradient, similar to the distribution of the BMP inhibitor noggin. Overexpression of N-cadherin in this region inhibits neural crest emigration in vivo, and similar results are found in in vitro explant cultures of dorsal neural tubes. Conversely, overexpression of different N-cadherin mutants, such as those containing a deletion of the β-catenin binding domain or the extracellular domain, do not alter neural crest emigration, but instead affect the morphology of the neuroepithelium suggesting that these domains are important for potentially modulating adhesion and cell polarity.
These recent results are consistent with previous studies on the effects of N-cadherin overexpression in the chicken embryo. In earlier work in the chick trunk, overexpression of full-length N-cadherin was performed using adenoviral vectors, resulting in a reduction in overall neural crest cell migration (including the loss of melanoblast emigration), accompanied by an accumulation of neural crest cells along the neural tube midline and occasionally the localization of cells in the lumen of the neural tube.21 Under these experimental conditions, those neural crest cells that emigrate normally successfully differentiate into their appropriate derivatives, except for a small decrease in the number of dorsal root ganglia cells. However, adenoviral-mediated overexpression of a construct containing a deletion of the N-cadherin extracellular domain did not have adverse effects on neural crest migration; i.e., it did not act as a dominant negative. Furthermore, overexpression of a catenin binding domain mutant of N-cadherin had variable effects, with some neural crest cells remaining in the neural tube and others migrating normally. Interestingly, those cells entering the lumen of the neural tube expressed markers of melanocytes (e.g., Mitf), suggesting that neural crest cell differentiation is not affected. These authors hypothesize that perhaps cadherins work to directly modulate cell adhesion properties rather than affect cell motility, and that downregulation of cadherins is necessary to facilitate migration. Furthermore, they postulate that the presence of neural crest cells in the neural tube lumen may be due to the disruption of apicobasal polarity exhibited in the neural tubes of embryos overexpressing N-cadherin, implying a role for apicobasal polarity in mediating the direction of neural crest cell migration. Importantly, however, this study could only distinguish effects on the neural crest cell population migrating dorsolaterally. Briefly, the presence and accumulation of N-cadherin protein takes several hours after introduction of the adenoviral N-cadherin transgene construct into the dorsal neural tube due to the fact that the transgene must be introduced into the cell (infected), and properly transcribed and translated using cellular machinery. As dorsoventrally migrating neural crest cells leave the dorsal neural tube earlier than dorsolaterally migrating neural crest cells (the presumptive pigment cells), the only effects apparent on neural crest development will be observed in the population of neural crest cells still remaining in the neural tube (the dorsolaterally migrating population). In order to address effects on dorsoventrally migrating neural crest cells, the construct would need to be introduced into the embryo at an earlier time point.
Previous research has shown that trunk neural crest cell delamination in the chicken occurs via a BMP4-mediated mechanism during the G1-S transition of the cell cycle.33 Interestingly, N-cadherin overexpression reduces DNA synthesis and cyclin D1 expression, providing insight as to why emigration is impaired under these conditions.32 The requisite loss of N-cadherin protein from the membrane of emigrating neural crest cells occurs due to the activity of BMP4, as treatment with BMP4 causes an increase in the number of migratory neural crest cells and the loss of N-cadherin protein from the membrane.32 The mechanism by which membrane-associated N-cadherin protein is removed has been well-documented in other systems and relies upon the proteolytic cleavage of N-cadherin.34,35 Initially, the type I transmembrane protein ADAM10 (A Disintegrin And Metalloproteinase) cleaves the extracellular domain of N-cadherin, leaving behind a 40 kDa membrane-bound C terminal fragment (CTF1). CTF1 then serves as a substrate for PS1-dependent γ-secretase cleavage to produce a 35 kDa intracellular peptide (CTF2). CTF2 subsequently interacts with the transcription factor CBP (CREB binding protein) and promotes its degradation, thus inhibiting the expression of CREB response genes.35 ADAM10 is expressed in the dorsal neural tube and in emigrating avian neural crest cells obtained from in vitro explants,36 and ADAM10 colocalizes with N-cadherin in the mouse neural tube at Embryonic day (E) 9.5.34 As such, treatment of neural explants with an inhibitor of ADAM10 (GI254203X), in the presence or absence of BMP4, leads to an inhibition of neural crest emigration, with N-cadherin protein accumulating on the cell surface. Moreover, overexpression of CTF2 results in the translocation of CTF2 to the nucleus of migrating neural crest cells, an increase in cyclin D1 expression and an increase in neural crest emigration. Thus, downregulation of N-cadherin through proteolytic processing is a critical step in avian neural crest emigration in the trunk.32
In vitro studies performed using quail explants reveal that constitutively phosphorylated N-cadherin protein is located on migratory neural crest cells at the tip of cell processes, but is absent from regions of cell-cell contact.37 These studies demonstrate that cell surface-localized N-cadherin protein is not associated with the cytoskeleton in migratory neural crest cells as evidenced by the presence of only a small fraction of Triton X-insoluble N-cadherin protein. Furthermore, these authors show that N-cadherin protein becomes unstable upon the inactivation of a broad spectrum of protein kinases. Specifically, the addition of protein kinase inhibitors that block serine/threonine kinases, protein kinase C and tyrosine kinases results in the restoration of N-cadherin-mediated cell-cell adhesion in migratory neural crest cell cultures in vitro. These researchers conclude that kinase inhibitors are potentially indirectly modulating N-cadherin activity by affecting other cytoplasmic components in the cell (actin, catenins), leading to an increase in cell-cell adhesion observed on migrating neural crest cells. This conclusion is substantiated by the observation that the total level of N-cadherin phosphorylation does not change in the presence or absence of the different kinase inhibitors.
Although these data differ somewhat with the in vivo data described above, it is known that an N-cadherin function-blocking antibody disrupts avian cranial neural crest cell migratory pathways, with ectopic aggregates of cells found adjacent to the neural tube.38 The discrepancy between the in vitro and in vivo studies described above might be due to the fact that the N-cadherin protein has a relatively long half-life, allowing protein to exist on the surface of migratory neural crest cells concomitant with the loss of mRNA expression. Alternatively, the in vitro culture system may not fully recapitulate the in vivo setting, as there may be a loss of negative regulation of N-cadherin in the in vitro system.
Mice deficient for N-cadherin in the post-otic neural crest migratory pathway show perturbations in neural crest cell motility.39 Normally, N-cadherin protein is present on the surface of cardiac neural crest cells, clustering in a punctate pattern along cell processes, and co-localizes with the gap junction protein connexin43. Loss of N-cadherin leads to a decrease in dye-coupling, both in vitro and in vivo, reflecting an alteration in gap junction communication among migrating neural crest cells. Furthermore, migratory neural crest cells in the N-cadherin knock-out mouse exhibit an increase in speed and persistence of movement, but a decrease in the directionality of cell motility. Thus, N-cadherin appears to control various aspects of neural crest cell movement, a function that is independent of its interaction with connexin43 and potential modulation of gap junctions.39
Differentiation. At the end of dorsoventral migration, N-cadherin is upregulated in aggregating neural crest cells just prior to their differentiation into the dorsal root ganglia (DRG) and sympathetic ganglia.8,40 Following dorsolateral migration, dermal melanocytes express N-cadherin, facilitating contacts with fibroblasts in the skin dermis.8 After ganglion formation, N-cadherin is detected on (1) the apical surface of the neural tube, (2) the basolateral surface of the floorplate, (3) neuronal cells localized ventrally and laterally with in the neural tube, (4) fibrous axonal processes, and (5) ventral root and sympathetic ganglia.40 In adult organisms, N-cadherin is observed in neural tissue, the retina, endothelial cells, fibroblasts, osteoblasts and myocytes.8
As N-cadherin is also expressed in other differentiating tissues, such as in the segmental plate and somites,31 the N-cadherin knockout mouse dies at day 10 of gestation.41 These embryos undergo abnormal heart morphogenesis, resulting in an enlarged heart bulge, and present an undulating neural tube phenotype in which the neural tube still closes normally but is columnar in nature, most likely due to the presence and redundancy with other cadherins. Targeted deletion of N-cadherin from the neural crest in the mouse using a Wnt1-Cre system results in embryonic lethality with cardiovascular defects at E13.42 In wild-type embryos, an increase in N-cadherin expression is observed as neural crest cells elongate and condense to form the cardiac outflow tract. In mutant embryos, neural crest cells migrate and home to the cardiac outflow tract niche normally, but cells do not undergo normal morphogenesis associated with remodeling of the cardiac outflow tract, leading to persistent truncus arteriosus and aorticopulmonary septum formation. A thinned ventricular myocardium is also observed in these animals. Finally, recent work by Kasemeier-Kulesa et al. (2008) demonstrates the importance of N-cadherin in regulating the aggregation of neural crest cells into discrete sympathetic ganglia. Using intravital time-lapse confocal microscopy in the chicken embryo, these authors demonstrated that, upon arrival of neural crest cells to the sympathetic ganglia target site, neural crest cells distribute themselves in an anterior-to-posterior direction, and later coalesce and differentiate into ganglia. Interestingly, the levels of N-cadherin must be tightly controlled, as both excess and reduced N-cadherin disrupt the formation of the sympathetic ganglia, presumably due to the aberrant migratory behavior imparted upon the neural crest cells.43
Cadherin-6B.
Induction. Studies in the chicken embryo have shown that neural fold cells, the precursors to the neural crest, express N-cadherin and another cadherin, cadherin-6B.22 Upon formation of the neural tube, the dorsal-most region contains the premigratory neural crest cells, and these cells express low and high levels of N-cadherin and cadherin-6B, respectively (reviewed in ref. 19). However, N-cadherin expression persists at high levels in the remaining cells of the neural tube, whereas cadherin-6B expression is much weaker. Cadherin-6B expression is first observed in the chick at stage 6 in the neural folds and persists during neural tube formation, and cadherin-6B protein is distributed on the luminal (apical) side of dorsal neural tube cells.21,22 The respective localization of cadherin-6B and N-cadherin to dorsal neural tube cells and other neural tube cells, is suggestive of a possible mechanism by which distinct functional populations of neural tube cells are segregated during development.22
EMT/migration. Cadherin-6B transcripts and protein are absent from migratory and differentiating neural crest cells in the chicken embryo, implicating it in the formation and maintenance of the premigratory neural crest cell domain in the dorsal neural tube.21,22,44 The molecular mechanism by which cadherin-6B transcripts are downregulated prior to neural crest cell emigration has recently been elucidated for the chicken embryo. In both the premigratory neural crest region of the chick midbrain and trunk, relatively non-overlapping expression patterns are observed for the transcriptional repressor protein Snail2 and cadherin-6B transcripts.44 Using a combination of morpholino-mediated knock-down of Snail2 and quantitative PCR (QPCR), the authors showed that cadherin-6B transcripts are de-repressed upon depletion of Snail2. To demonstrate that cadherin6B is a direct target of Snail2 repression, genomic sequence analysis of the cadherin-6B putative regulatory region was performed, revealing the presence of 3 pairs of clustered E boxes (putative Snail2 binding sites) of the sequence CAGGTA. The in vivo functionality of these E boxes was shown using chromatin immunoprecipitation coupled with QPCR in the chicken premigratory midbrain and trunk regions, revealing that Snail2 preferentially associates with different E boxes in the cadherin-6B regulatory region. These in vivo results were also corroborated in vitro by performing luciferase experiments and electrophoretic mobility shift assays. In addition, morpholino-mediated knock-down of Snail2 suppresses neural crest cell migration, and this effect can be alleviated upon the combined knock-down of both Snail2 and cadherin-6B, resulting in an increase in neural crest cell emigration from explants of dorsal neural fold tissue. Notably, this paper was the first to identify a cadherin as a direct, in vivo target for Snail2 during neural crest cell emigration.44
Importantly, cadherin-6B plays a pivotal, temporal role in regulating avian neural crest cell emigration.45 Depletion of cadherin-6B from the chick premigratory midbrain region results in premature neural crest cell emigration, as demonstrated by in situ hybridization and immunohistochemical staining for a battery of neural crest molecular markers. This precocious migration phenotype results in a statistically significant increase in Sox10- and Snail2-positive migratory neural crest cells upon cadherin-6B knockdown. Moreover, in vitro explant cultures of dorsal neural folds following depletion of cadherin-6B reveal an increase in the number of emigrating/migrating neural crest cells compared to control. The migratory neural crest cells in these cultures are bona fide mesenchymal cells, as they have re-arranged their actin cytoskeleton and are positive for the intermediate filament protein vimentin. Conversely, overexpression of cadherin-6B in the premigratory chick midbrain leads to a disruption in neural crest cell emigration/migration, with cells aggregating adjacent to the neural tube and/or failing to exit the dorsal neural tube proper. In some instances, migratory neural crest cells enter the neural tube lumen, reminiscent of the phenotype observed upon overexpression of N-cadherin and cadherin-7.21 This misdirected migration phenotype could perhaps be due to the loss of apicobasal polarity in the neuroepithelium, as cadherin-6B protein is expressed throughout the dorsoventral axis of the neural tube on both the apical and lateral surfaces of the neuroepithelium, pointing to the potential importance of neuroepithelium polarity in directing neural crest cell migration. Interestingly, in vitro explant cultures of dorsal neural folds following overexpression of cadherin-6B revealed no alterations in neural crest cell migration, implying that these cells are indeed still motile in vitro but are perhaps unable to migrate properly in vivo because of their interaction with molecules in the extracellular matrix and/or their increased adhesive state. In summary, the regulatory relationship between cadherin-6B and the Snail2 is critical for proper spatio-temporal control of neural crest cell emigration in the avian embryo.
Cadherin-7.
EMT/migration. The role of cadherin-7 in neural crest cell migration has been studied primarily in the context of avian embryos, as mouse cadherin-7 was initially identified in the embryonic eye46 and is not detected during embryogenesis.47 Cadherin-7 transcripts are initially found in stage 10 chicken embryos in the dorsal midbrain in a sub-population of migratory neural crest cells (less than 50%).22 Expression perdures throughout neural crest cell migration, particularly with hindbrain neural crest cells populating the trigeminal ganglion, and albeit weaker, in cells surrounding various cranial nerves. At stage 15 in chick embryonic development, cadherin-7-positive neural crest cells are observed migrating between the neural tube and dermamyotome, as well as in regions lateral to the neural tube. These cells will eventually populate the proximal dorsal and ventral roots. However, no cadherin-7-positive neural crest cells are observed migrating dorsolaterally between the ectoderm and the somite, and only a subset of migratory neural crest cells are double-positive for the migratory neural crest cell marker HNK1 and cadherin-7 transcripts or protein, particularly melanocyte precursor cells.21 Interestingly, cadherin-7 protein can be detected in the neural tube on newly emigrating neural crest cells, overlapping with cadherin-6B and N-cadherin proteins. Overexpression of cadherin-7 in the chick trunk also results in a similar phenotype to that seen upon overexpression of N-cadherin, with neural crest cells invading the neural tube lumen and clustering adjacent to the neural tube.21
Differentiation. Migratory neural crest cells that are cadherin-7-positive are hypothesized to be fate-restricted to become Schwann cells, as evidenced by their presence in a “ring” structure around the nerve. Cadherin-7 expression is also observed in the neural tube, at the junction of the alar and basal plates, and other trunk-derived tissues.21
Cadherin-6.
The expression pattern of cadherin-6 in the mouse can be simplistically described as a combination of the expression patterns observed for chick cadherin-6B (premigratory neural crest) and cadherin-7 (migratory neural crest) (reviewed in refs. 19 and 48). However, mouse cadherin-6 exhibits a unique metameric expression in the hindbrain, a pattern not observed in the chicken embryo.48 Cadherin-6 is expressed in the neural folds and by migratory neural crest cells, and is later observed briefly in the forming cranial ganglia and persists in some peripheral nerves (Schwann cells) but is absent from others (in the central portions of the dorsal root ganglia and sympathetic ganglia after differentiation).48
Cadherin-11.
Induction. Low levels of mouse cadherin-11 are observed initially in the forming mouse neural plate. However, expression is noted in the cells comprising the cranial dorsal neural folds, both prior to and after neural tube closure, as well as in the trunk dorsal midline.49 Xenopus cadherin-11 is also found in the anterior neural folds of the embryo.50
EMT/migration. Newly emigrating neural crest cells in the frog express cadherin-11,51 as do more mature migratory neural crest cells, particularly the mandibular crest that migrates around the eye, as well as crest cells entering the hyoid and branchial arches.50 Xenopus trunk neural crest cells that populate the dorsal neural fin are also positive for cadherin-11.50 Moreover, overexpression of full-length or a β-catenin binding domain mutant of Xenopus cadherin-11 in grafted cranial neural crest cells abolishes cell migration to the branchial arches, with differentiated cells piling up next to the forming brain, but does not affect the neural crest induction process.51 Interestingly, grafts of cells overexpressing a mutant between EC1 and EC2 of cadherin-11 migrate earlier (up to 18 hrs post-transplantation), possibly due to the loss of adhesion with neighboring cells.51 Thus, the degree of Xenopus cadherin-11-mediated adhesion must be tightly monitored during the migration process.
Differentiation. Xenopus cadherin-11 expression persists during differentiation,50 and mouse cadherin-11 is localized to multiple mesenchymal derivatives, including the cranial mesenchyme and branchial arches, but is absent from the cranial and dorsal root ganglia.49
Cadherin-19.
EMT/migration. Rat cadherin-19 is homologous to human cadherin-19 and it shows the highest homology to chick, human and mouse cadherin-7.52 Cadherin-19 is expressed in migrating cranial neural crest cells in the face and first pharyngeal arch, as well as in ventromedially migrating trunk neural crest cells. Its expression is also coincident with other molecular markers of migratory neural crest, such as Sox10 and AP-2.52
Differentiation. A subpopulation of post-migratory neural crest cells in the rat retains cadherin-19 expression during the formation of the cranial, dorsal root, and sympathetic ganglia.52 Post-migratory trunk neural crest cells differentiating into Schwann cell precursors along the spinal nerve express cadherin-19, while cells contributing to the base of both the dorsal and ventral roots no longer express cadherin-19. Interestingly, rat cadherin-19 appears to have usurped the function normally provided by cadherin-7 in other organisms, such as the chick, as rat cadherin-7 is not expressed in Schwann cell precursors.52
Cadherin-20.
Induction. Although mouse cadherin-20 is initially observed in cells at the anterior neural plate and newly forming cephalic folds, and later in the cephalic neuroectoderm, it is not expressed in the cranial neural crest-forming region.47
EMT/migration. Early emigrating neural crest cells in the mouse express cadherin-20.47 These cells migrate along the dorsolateral pathway, indicating that they are putative melanocyte precursors.
Differentiation. Mosaic expression of mouse cadherin-20 is seen in the vestibulo-cochlear and geniculate cranial ganglia, the latter of which is a neural crest derivative.47
PCNS.
EMT/migration. PCNS, or ProtoCadherin in Neural crest and Somites, is initially expressed in Xenopus in the cranial neural crest and the somites at the neurula stage, followed by expression in the mandibular, branchial and hyoid arches at the tailbud stage.53 In vivo, overexpression of PCNS leads to a failure of gastrulation, whereas knock-down of PCNS using a morpholino results in craniofacial skeleton defects posterior to the mandible, including the loss of hyoid and branchial arch derivatives, due to the inability of neural crest cells to properly migrate to these regions of the embryo.53 Interestingly, neural crest induction still occurs normally in these PCNS-knock-down embryos. In vitro depletion of PCNS results in the rounding up of neural crest cells during EMT, followed by a loss of adhesion and migratory ability on fibronectin. As such, the morphology of the cells no longer appears to be mesenchymal.53
Conclusion
Much progress has been made recently in understanding the role of Cadherin family members during neural crest development. During the process of neurulation, downregulation of E-cadherin in the neural plate occurs, accompanied by the upregulation of N-cadherin, which separates the non-neural from the neural ectoderm. As the neural plate invaginates and pinches off to form the neural tube in the avian embryo, premigratory neural crest cells localized to the dorsal most region of the neural tube express an additional cadherin, cadherin-6B, whereas in the mouse and frog, these cells express cadherin-11 and cadherin-6 (mouse only). Neural crest cells subsequently delaminate from the dorsal neural tube and embark on their migratory pathways. During this process in the avian embryo, they cease to express N-cadherin and cadherin-6B and instead upregulate cadherin-7. In the mouse, delaminating and migratory neural crest cells maintain cadherin-6 expression and also induce expression of cadherin-20, while rat migratory neural crest cells express cadherin-19. In the frog, migratory neural crest cells express cadherin-11 and PCNS. Upon differentiation, neural crest cells in the avian embryo express cadherin-7 and re-express N-cadherin, mouse and rat neural crest cells maintain expression of cadherin-6, -20, and cadherin-19, respectively, and frog neural crest cells express cadherin-11. Thus, the regulated expression of these various cadherins ensures that induction, migration and differentiation of the neural crest occur normally in order to build a functioning embryo.
However, continuing studies aimed at exploring the expression, regulation and function of existing and potentially novel cadherins are necessary to enhance our understanding of how this class of adhesion molecules contributes to the ontogeny of the neural crest. Genomic screening in different organisms, coupled with phylogenetic analyses, will facilitate the identification of orthologs of existing cadherins, as well as potentially novel cadherins, that are involved in various aspects of neural crest development, and will further delineate the level of functional conservation among these genes. The variations currently observed in cadherin deployment during embryogenesis in different species may prove challenging to researchers studying cadherins, yet this knowledge will ultimately advance our understanding of the functional evolution of the cadherin gene family as a whole.
Of equal importance for the cadherin field will be the deciphering of the upstream regulatory pathways that coordinate cadherin gene expression during the formation of the neural crest, and the availability of genomic sequence for multiple organisms will assist in this effort. Cadherin expression (in general) will undoubtedly be regulated directly or indirectly through conventional growth factor signaling pathways (BMPs, Wnts), as well as through the activity of transcriptional regulators and/or chromatin remodeling factors. Post-transcriptional regulation of cadherins may also play an important role during neural crest development, as has recently been shown for N-cadherin. Thus, regulation of the cadherin protein level and/or activity in a premigratory and/or migratory neural crest cell may prove to be crucial for proper neural crest formation. In studying cadherin protein dynamics, it will be necessary to develop microscopy techniques to observe cadherin regulation in real-time. Applications of these methods to various organisms will also permit the identification of relevant cadherin binding partners important in neural crest development. Importantly, elucidating how and when different cadherins are expressed, and discovering cadherin interacting proteins during the formation of the neural crest, will contribute to our overall knowledge of the molecular basis of neural crest formation, and will potentially shed light on the mechanisms underlying human disorders, diseases and syndromes that occur upon aberrant neural crest development.
Acknowledgements
The author would like to thank Ms. Jill Shah for assistance with literature searches and Dr. Edward G. Coles for critical reading of the manuscript and assistance with figures.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6835
References
- 1.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 2.Nagar B, Overduin M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- 3.Overduin M, Harvery TS, Bagby S, Tong KI, Yau P, Takeichi M, et al. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995;267:386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grübel G, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 5.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 6.Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. Journal of Cell Science. 2000;113:1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- 7.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 8.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 9.Takeichi M, Nakagawa S, Aono S, Usui T, Uemura T. Patterning of cell assemblies regulated by adhesion receptors of the cadherin superfamily. Philos Trans R Soc Lond B Biol Sci. 2000;355:885–890. doi: 10.1098/rstb.2000.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. Journal of Cell Biology. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. Journal of Cell Biology. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, et al. Inhibition of RhoA by p120 catenin. Nature Cell Biology. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 13.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. Journal of Cell Biology. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosheva I, Shtutman M, Elbaum M, D BA. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. Journal of Cell Science. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Ji H, Kim SW, Park JI, Vaught TG, Anastasiadis PZ, et al. Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. Journal of Cell Biology. 2004;165:87–98. doi: 10.1083/jcb.200307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. Journal of Cell Biology. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroda S, Fukata M, Nakagawa M, Kalbuchi K. Cdc42, Rac11, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem Biophys Res Commun. 1999;262:1–6. doi: 10.1006/bbrc.1999.1122. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. Journal of Cell Science. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 19.Pla P, Moore R, Morali OG, Grille S, Martinozzi S, Delmas V, Larue L. Cadherins in neural crest cell development and transformation. J Cell Physiol. 2001;189:121–132. doi: 10.1002/jcp.10008. [DOI] [PubMed] [Google Scholar]
- 20.Le Douarin N, Kalcheim C. The neural crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 21.Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Nieto MA. The Snail superfamily of zinc-finger transcription factors. Nature Reviews Mol Cell Biol. 2002;3:155–165. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 25.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Aat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 26.Newgreen DF, Gibbins IL. Factors controlling the time of onset of the migration of neural crest cells in the fowl embryo. Cell Tissue Res. 1982;224:145–160. doi: 10.1007/BF00217274. [DOI] [PubMed] [Google Scholar]
- 27.Nichols DH. Ultrastructure of neural crest formation in the midbrain/rostral hindbrain and preotic hindbrain regions of the mouse embryo. Am J Anat. 1987;179:143–154. doi: 10.1002/aja.1001790207. [DOI] [PubMed] [Google Scholar]
- 28.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelialmesenchymal transition. BioEssays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 29.Schuh R, Vestweber D, Riede I, Ringwald M, Rosenberg UB, Jackle H, et al. Molecular cloning of the mouse cell adhesion molecule uvomorulin: cDNA contains a B1-related sequence. Proc Natl Acad Sci USA. 1986;83:1364–1368. doi: 10.1073/pnas.83.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edelman GM, Gallin WJ, Delouvee A, Cunningham BA, Thiery JP. Early epochal maps of two different cell adhesion molecules. Proc Natl Acad Sci USA. 1983;80:4384–4388. doi: 10.1073/pnas.80.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- 32.Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- 33.Burstyn-Cohen T, Stanleigh J, Sela-Donenfeld D, Kalcheim C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development. 2004;131:5327–5339. doi: 10.1242/dev.01424. [DOI] [PubMed] [Google Scholar]
- 34.Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. Embo J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, et al. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Hall RJ, Erickson CA. ADAM 10: an active metalloprotease expressed during avian epithelial morphogenesis. Dev Biol. 2003;256:146–159. doi: 10.1016/s0012-1606(02)00133-1. [DOI] [PubMed] [Google Scholar]
- 37.Monier-Gavelle F, Duband JL. Control of N-cadherin-mediated intercellular adhesion in migrating neural crest cells in vitro. J Cell Sci. 1995;108:3839–3853. doi: 10.1242/jcs.108.12.3839. [DOI] [PubMed] [Google Scholar]
- 38.Bronner-Fraser M, Wolf JJ, Murray BA. Effects of antibodies against N-cadherin and N-CAM on the cranial neural crest and neural tube. Dev Biol. 1992;153:291–301. doi: 10.1016/0012-1606(92)90114-v. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, et al. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol. 2001;154:217–230. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- 41.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 42.Luo Y, High FA, Epstein JA, Radice GL. N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev Biol. 2006;299:517–528. doi: 10.1016/j.ydbio.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. [DOI] [PubMed] [Google Scholar]
- 44.Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faulkner-Jones BE, Godinho LN, Reese BE, Pasquini GF, Ruefli A, Tan SS. Cloning and expression of mouse Cadherin-7, a type-II cadherin isolated from the developing eye. Mol Cell Neurosci. 1999;14:1–16. doi: 10.1006/mcne.1999.0764. [DOI] [PubMed] [Google Scholar]
- 47.Moore R, Larue L. Cell surface molecules and truncal neural crest ontogeny: a perspective. Birth Defects Res C Embryo Today. 2004;72:140–150. doi: 10.1002/bdrc.20014. [DOI] [PubMed] [Google Scholar]
- 48.Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- 49.Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, et al. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite and limb bud of early mouse embryos. Dev Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 50.Vallin J, Girault JM, Thiery JP, Broders F. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev. 1998;75:171–174. doi: 10.1016/s0925-4773(98)00099-9. [DOI] [PubMed] [Google Scholar]
- 51.Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–3060. doi: 10.1242/dev.128.16.3049. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi M, Osumi N. Identification of a novel type II classical cadherin: rat cadherin19 is expressed in the cranial ganglia and Schwann cell precursors during development. Dev Dyn. 2005;232:200–208. doi: 10.1002/dvdy.20209. [DOI] [PubMed] [Google Scholar]
- 53.Rangarajan J, Luo T, Sargent TD. PCNS: a novel protocadherin required for cranial neural crest migration and somite morphogenesis in Xenopus. Dev Biol. 2006;295:206–218. doi: 10.1016/j.ydbio.2006.03.025. [DOI] [PubMed] [Google Scholar]