Abstract
The formation of a nitrogen-fixing nodule involves two diverse developmental processes in the legume root: infection thread initiation in epidermal cells and nodule primordia formation in the cortex. Several plant hormones have been reported to positively or negatively regulate nodulation. These hormones function at different stages in the nodulation process and may facilitate the coordinated development of the epidermal and cortical developmental programs that are necessary to allow bacterial infection into the developing nodule. In this paper, we review and discuss how the tissue specific nature of hormonal action dictates where, when and how a nodule is formed.
Key words: nodulation, hormone regulation, epidermis, cortex
Introduction
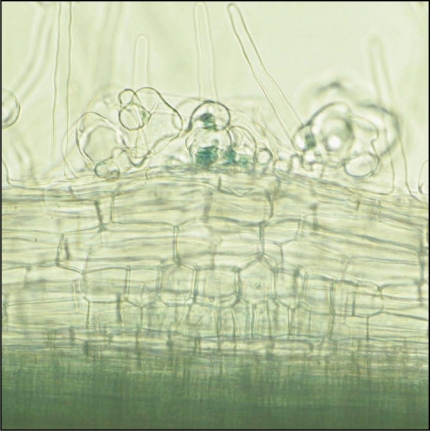
Several genera of plants have developed symbiotic interactions with microorganism that facilitate uptake of mineral nutrients from limited resource environments. A typical symbiosis is the legume-rhizobia symbiosis that benefits the plant through nitrogen acquisition. In this interaction, root hair deformation entraps rhizobial bacteria and infection threads that are formed from these infection sites allow rhizobial invasion into the cells of the root cortex. Meanwhile, cortical cell division is activated and this initiates the nodule primordia at a position below the site of bacterial infection in the epidermis (Fig. 1). Rhizobia are released from infection threads into membrane bound compartments within the cells of the nodule, where they differentiate into a nitrogen fixing form, the bacteroid. The interaction involves multiple levels of communication between the plant and the rhizobial bacteria throughout the process of bacterial colonisation and this optimizes the exchange of sugars for a nitrogen source.
Figure 1.
Root hair deformation in Medicago truncatula entraps Sinorhizobium meliloti to form infection foci, from which will develop infection threads. Immediately below the site of bacterial infection cortical cell divisions are activated to form the nodule primordia.
In this last decade many genetic components in both rhizobia and legumes that control the nodulation process have been defined, providing an approximate mechanism for this interaction. Plants release flavonoid signals to initiate the first communication with rhizobia. Bacterial perception of flavonoids via NodD activates production of Nod factor which consists of a chitin backbone with an N-linked fatty acid moiety attached to the terminal glucosamine.1–3 The perception of Nod factor by Lysine motif (LysM)-receptor-like kinases4–7 in plants induces two different tissue-specific responses, those in the epidermal cells and those in inner cortical cells.
In epidermal cells (and at least outer cortical cells) Nod factor induces calcium oscillations (calcium spiking) in the nuclear associated region.8,9 Two nucleoporins10,11 and two putative cation channels12,13 are required for this Nod factor-induced calcium spiking. The calcium-spiking signal activates a calcium- and calmodulin-dependent protein kinase (CCaMK)14–16 as well as two GRAS family proteins NSP1 and NSP2,17,18 and an ERF transcription factor ERN1.19 This signalling pathway is essential to activate gene expression changes leading to bacterial infection through epidermal cells as well as the initiation of the cortical program leading to a nodule primordia.
There is strong evidence indicating that Nod factor perception at the epidermis leads to localized increases in cytokinin levels that promotes nodule organogenesis in the cortex. Lotus japonicus snf2 which is a gain-of-function mutation in the cytokinin receptor LHK1 forms spontaneous nodules in the absence of rhizobia.20 Plants carrying loss-of-function mutations in the same gene lack the ability to form nodules but show normal infection thread initiation in the epidermis and similar results were observed in RNAi-mediated downregulation of the LHK1 ortholog of Medicago truncatula.21,22 While nodule primordia are initiated in the cortex within approximately 48 hours of rhizobial treatment, responses are observed much earlier. Indeed the first Nod factor induced responses in inner root tissues appear to be cytoskeletal changes in pericycle cells reported as early as 16–18 hours post rhizobial inoculation.23 Nod factor-induced cytoskeletal responses in cortical cells occur 18–24 hours post rhizobial treatment, when gene induction such as ENOD40 is also observed.24,25
While nitrogen fixation in nodules is beneficial to the plant it comes at a high price in the form of demand for photosynthates. The plant must balance its differential nutritional needs with other environmental stresses it may encounter. It is clear that the plant carefully regulates nitrogen fixation and does so at multiple levels, most strikingly through the regulation of nodule number. The plant has well established defence and stress responsive systems and these combined with the interlinking of plant developmental hormones provides mechanisms for the regulation of nitrogen fixation. In this review we take a hormone centric viewpoint of nodulation. We try to integrate the previously reported data to interpret nodulation in the language of plant hormones. From this viewpoint we address two key questions: how does Nod factor recruit different hormones to regulate both bacterial infection and nodule initiation and how does Nod factor coordinate two different developmental processes in these two different tissues?
The Regulation of Rhizobial Infection by Plant Stress Hormones
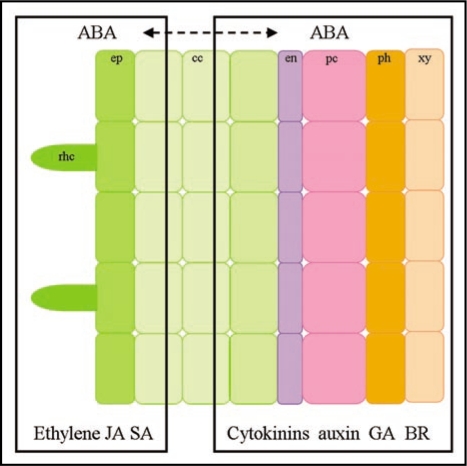
Several defence/stress hormones such as ethylene, salicylic acid (SA), jasmonic acid (JA) and abscisic acid (ABA) play important roles in controlling the epidermal responses during the nodulation process (Fig. 2).26 These hormones negatively regulate multiple epidermal responses in order to inhibit rhizobial infection. Ethylene is the best studied of these hormones. Use of ACC (the precursor of ethylene) and AVG (an inhibitor of the ethylene response) in wild type plants indicates that ethylene inhibits rhizobial induced epidermal responses such as root hair deformation, calcium spiking, the expression of early nodulin genes such as RIP1 and ENOD11 and the frequency of infection threads.27,28 Genetic studies with the ethylene insensitive mutant skl (ein2) validate these physiological studies revealing that ethylene inhibits rhizobial infection as well as nodule formation, such that in the absence of ethylene signalling, excessive numbers of nodules are formed.29,30 These studies reveal that ethylene suppresses Nod factor signal transduction and this reduces the levels of rhizobial infection.28
Figure 2.
In the epidermis, defence hormones such as ethylene, JA and SA as well as the stress hormone ABA negatively regulate Nod factor induced calcium spiking, early nodulin gene expression and infection thread initiation. In the cortex and in pericycle cells, the balance of cytokinins, ABA and auxin dictate whether lateral roots or nodules will be initiated. GA and BR are also involved in the formation of the nodule primodia. ABA has dual roles in regulating epidermal and cortical nodulation processes and as such may facilitate the coordination of the epidermal and cortical programs. rhc, root hair cell; ep, epidermis; cc, cortical cell; en, endodermis; pc, pericycle cell; ph, pholem; xy, xylem; JA, jasmonic acid; SA, salicylic acid; ABA, abscisic acid; GA, gibberellic acid; BR, brassinosteroid.
A synergistic partner to ethylene in defence systems is JA. Previous studies showed that exogenous JA could inhibit the transcription of ENOD11 and RIP1 which are induced by Nod factor in epidermal cells as well as inhibiting calcium spiking.31 Methyl jasmonate (MeJA), JA's fragrant methyl ester, shows a similar action resulting in the suppression of nodulation.32 Surprisingly, although JA and ethylene show a synergistic interaction on nodulation inhibition, there is an antagonistic interaction on regulating calcium spiking. This implies dynamic interactions between JA and ethylene on the negative regulation of nodulation.
ABA regulates the water status of the plant as well as regulating a number of plant developmental processes. Previous studies on ABA has shown that it regulates rhizobial responses including root hair deformation and nodulation in L. japonicus.33 Recent work has shown that ABA functions in the regulation of rhizobial induced epidermal responses in a manner similar to ethylene and JA, inhibiting the number of infection threads, Nod factor induced calcium spiking as well as early nodulin gene induction. Despite the similarities between ABA and ethylene, their regulation of nodulation appears to be independent.34 Dominantly suppressing ABA signalling by overexpressing the Arabidopsis abi1-1 allele in M. truncatula causes enhanced induction of the early nodulin gene, ENOD11, in epidermal cells and increased levels of nodulation.34
Treatment with another well-studied plant defence hormone SA results in both reduced and delayed nodule formation on alfalfa roots inoculated with Sinorhizobium meliloti and the inhibition of the early nodulin RIP1.35 SA levels can be suppressed by the bacterial enzyme Salicylate hydroxylase (NahG) and expression of NahG in L. japonicus results in a marked reduction of endogenous SA levels leading to an increase in the number of rhizobial infections and the mean nodule number.36 Interestingly, there are several pathogenesis-related proteins such as PR2 and PR10 that are downregulated after inoculation with rhizobia.37,38 Taken together this work implies that rhizobial colonisation involves the suppression of plant defences possibly by modifying the levels of SA.
These four hormones, ethylene, JA, SA and ABA have been shown to negatively regulate epidermal Nod factor responses including root hair deformation, calcium spiking and early nodulin gene expression and this regulation is likely to lead to the inhibition of rhizobial infection. But how do these four hormones integrate together to reduce Nod factor signalling? The decision to respond to Nod factor appears to involve a balance between Nod factor concentrations and the concentrations of JA, ethylene and ABA, such that high concentrations of Nod factor can overcome the suppressive nature of these hormones.28,31,34 Since all these four hormones directly or indirectly enhance the production of reactive oxygen species (ROS),9,39–42 it is possible that ROS might be a key mediator in this hormonal regulation of Nod factor signal transduction.
Nodule Primodia Initiation Competes with Lateral Root Emergence
Nodules and lateral roots are different organs that form from different founder cells. Nodule primodia result from cortical cell divisions while lateral root primodia initiate from pericycle cell divisions. In 1948, Nutman recorded the numbers of both lateral roots and nodules and proposed that a balance existed between nodule formation and lateral root formation,43 with nodule primodia initiation dependent on the suppression of lateral root emergence. Some of the first responses to rhizobia in inner root tissues occur in pericycle cells, with alterations in the cytoskeleton, which are followed by similar responses in cortical cells a few hours later.23
In Arabidopsis cytokinins mediate cell cycle arrest in pericycle cells, thus inhibiting lateral root formation.44,45 This is also true in legumes. Lohar et al. made use of ARR5 (a cytokinin-induced response regulator from Arabidopsis) in L. japonicus to reveal a specific absence of cytokinin signalling in those pericycle cells undergoing division to initiate the lateral root primordium.46 Suppression of cytokinin signalling by silencing the cytokinin receptor (CRE1) in M. truncatula caused increased lateral root formation. In contrast, cytokinins play a positive role in cortical cells for the initiation of the nodule primordium. ARR5 expression revealed cytokinin signalling in cortical cells undergoing division to form the nodule primordial.46 In addition, external application of cytokinins can induce ENOD40 expression, a gene associated with nodule primodia, with the same timing and location as rhizobial induction of ENOD40.25 More recently, genetic studies have revealed that gain of function mutations in the L. japonoicus cytokinin receptor LHK1 cause spontaneous formation of nodules, while loss of function mutations of LHK1 or silencing of CRE1 cause an inability to initiate nodule primordia.20–22 Therefore, cytokinins have the ability to initiate nodule organogenesis in the cortex while suppressing lateral root formation in pericycle cells.
During root development, auxin is generally an antagonistic partner to cytokinins. In contrast to cytokinins auxin accumulates in the pericycle cells to initiate lateral root primodia.25,44 While in nodule formation, the initiation of the nodule is associated with the inhibition of auxin flow. Direct measurements of auxin transport using radiolabeled auxin showed that rhizobia locally inhibit acropetal auxin transport capacity in Vicia sativa roots.47 Nod factor and flavonoids were also shown to inhibit auxin flow in the root, similar to the effects of synthetic auxin transport inhibitors.48 The effect of this altered auxin flow is localized suppression of auxin levels associated with the initiation of nodule primordia, as evidenced by studies with the auxin-responsive promoter GH3.48 These localized regions of reduced auxin levels are transitory, since GH3 expression is dramatically increased in nodule primordia.25,44 Moreover, in the sunn mutant, high levels of endogenous auxin are correlated with increased numbers of nodules.49 The ethylene insensitive supernodulation mutant skl also has an exaggerated increase of IAA accumulation.50 These results suggest a complicated role for auxin in nodule initiation. It appears that localized suppression of auxin may be necessary to initiate a nodule primordium, but once initiated high auxin levels are essential for nodule primordia function. This hypothesis implies an early suppressive role for auxin during nodule initiation followed by a positive function for auxin during nodule organogenesis.
In addition to auxin, ABA is also an antagonistic partner to cytokinins. Compared with cytokinins, ABA promotes lateral root formation and inhibits nodulation, thus having opposite effects to cytokinins on cell division in the pericycle and cortex.33,34,51,52 Suppressing ABA signalling by overexpressing the Arabidopsis abi1-1 allele in M. truncatula shows an enhanced nodulation phenotype as well as enhanced ENOD40 expression induced by cytokinins.34 ABA also can inhibit spontaneous nodulation in the LHK1 gain-of-function mutants of L. japonicus.34 Thus the initiation of lateral roots from pericycle cells and nodules from cortical cells may require specific ABA/cytokinin ratios. Strikingly, the ABA to cytokinin ratio in the phloem is higher in wild type soybean compared to the supernodulation mutant nark (the soybean ortholog of SUNN).53
The initiation of a nodule involves the entire collection of plant hormones, with roles also for GA (Gibberellic Acid) and BR (Brassinosteroid) in the initiation of nodule primordia.54 As well as regulating bacterial infection at the epidermis, ethylene also appears to play a role in directing the infection thread through the cortex29,30 and in positioning nodule initiation, opposite protoxylem poles.27 Taken together, the cortical program leading to nodule initiation is predominantly regulated by cytokinins, that function in combination with auxin, ABA. GA, BR and ethylene to dictate the number and position of nodules (Fig. 2). We propose that the balance of auxin, cytokinins and ABA decides the fate of pericycle cells and cortical cells, dictating whether lateral roots or nodules will be initiated.
Coordination between Epidermal Responses and Cortical Responses
As described above, hormones regulate nodulation through the negative regulation of epidermal responses as well as dictating the nature of cortical and pericycle cell divisions. Considering that bacterial infection and nodule primordia initiation occur concurrently in distant tissues, it seems likely that a mobile signal must coordinate these two developmental programs. It is possible that such a mobile signal is a plant hormone and those hormones with dual roles in regulating infection processes and nodule organogenesis may provide the mechanism for the coordination of these two processes. ABA is a hormone which is involved in both epidermal and cortical nodulation responses. It regulates Nod factor signalling in the epidermis as well as cytokinin signalling in the cortex. These two modes of action can be separated since the sta1 mutant of M. truncatula shows reduced sensitivity to ABA for the regulation of Nod factor signalling in epidermal cells and hypersensitivity to ABA for the regulation of cytokinin induced nodulation processes.34 Thus the modulation of ABA signalling can dictate the nature of both the epidermal and cortical nodulation processes. While it is tempting to suggest that ABA, or other hormones, act as the mobile signal(s) that co-ordinately regulate epidermal and cortical nodulation responses, further proof is necessary to support such a hypothesis.
Outlook
The evolution of nodulation has recruited the entire collection of plant hormones to activate or regulate this process. In the epidermis, defence hormones such as ethylene, JA and SA as well as the stress hormone ABA negatively regulate Nod factor induced calcium spiking, early nodulin gene expression and infection thread initiation. In the cortex and in pericycle cells, the balance of cytokinins, ABA and auxin dictate whether lateral roots or nodules will be initiated. Considering the importance of tissue specific responses during nodulation it is important to define the tissue specific nature of hormone action during nodulation. Different hormone responsive genes need to be carefully analysed in specific cell layers through time post rhizobial inoculation. In addition the mechanisms by which hormones activate nodulation or regulate Nod factor signal transduction still remains to be defined. Reverse genetic and forward genetic screens should provide new components in the hormonal regulation of nodulation pathways and may provide insights into the mechanisms of coordination of these fascinating processes.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7693
References
- 1.Denarie J, Debelle F, Prome JC. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu Rev Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- 2.Long SR. Rhizobium symbiosis: nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perret X, Staehelin C, Broughton WJ. Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev. 2000;64:180–201. doi: 10.1128/mmbr.64.1.180-201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrighi JF, et al. The Medicago truncatula lysin motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006;142:265–279. doi: 10.1104/pp.106.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limpens E, et al. LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science. 2003;302:630–633. doi: 10.1126/science.1090074. [DOI] [PubMed] [Google Scholar]
- 6.Madsen EB, et al. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature. 2003;425:637–640. doi: 10.1038/nature02045. [DOI] [PubMed] [Google Scholar]
- 7.Radutoiu S, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003;425:585–592. doi: 10.1038/nature02039. [DOI] [PubMed] [Google Scholar]
- 8.Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 9.Shaw SL, Long SR. Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 2003;132:2196–2204. doi: 10.1104/pp.103.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanamori N, et al. A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA. 2006;103:359–364. doi: 10.1073/pnas.0508883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito K, et al. NUCLEOPORIN85 is required for calcium spiking, fugal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell. 2007;19:610–624. doi: 10.1105/tpc.106.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ane JM, et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science. 2004;303:1364–1367. doi: 10.1126/science.1092986. [DOI] [PubMed] [Google Scholar]
- 13.Edwards A, Heckmann AB, Yousafzai F, Duc G, Downie JA. Structural implications of mutations in the pea SYM8 symbiosis gene, the DMI1 ortholog, encoding a predicted ion channel. Mol Plant Microbe Interact. 2007;20:1183–1191. doi: 10.1094/MPMI-20-10-1183. [DOI] [PubMed] [Google Scholar]
- 14.Gleason C, et al. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature. 2006;441:1149–1152. doi: 10.1038/nature04812. [DOI] [PubMed] [Google Scholar]
- 15.Levy J, et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science. 2004;303:1361–1364. doi: 10.1126/science.1093038. [DOI] [PubMed] [Google Scholar]
- 16.Mitra RM, et al. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning. Proc Natl Acad Sci USA. 2004;101:4701–4705. doi: 10.1073/pnas.0400595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalo P, et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science. 2005;308:1786–1789. doi: 10.1126/science.1110951. [DOI] [PubMed] [Google Scholar]
- 18.Smit P, et al. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–1791. doi: 10.1126/science.1111025. [DOI] [PubMed] [Google Scholar]
- 19.Middleton PH, et al. An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell. 2007;19:1221–1234. doi: 10.1105/tpc.106.048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirichine L, et al. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315:104–107. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rizzo S, Crespi M, Frugier F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell. 2006;18:2680–2693. doi: 10.1105/tpc.106.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray JD, et al. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–104. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- 23.Timmers AC, Auriac MC, Truchet G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development. 1999;126:3617–3628. doi: 10.1242/dev.126.16.3617. [DOI] [PubMed] [Google Scholar]
- 24.Gronlund M, et al. Analysis of promoter activity of the early nodulin Enod40 in Lotus japonicus. Mol Plant Microbe Interact. 2005;18:414–427. doi: 10.1094/MPMI-18-0414. [DOI] [PubMed] [Google Scholar]
- 25.Mathesius U, Charon C, Rolfe BG, Kondorosi A, Crespi M. Temporal and spatial order of events during the induction of cortical cell divisions in white clover by Rhizobium leguminosarum bv. trifolii inoculation or localized cytokinin addition. Mol Plant Microbe Interact. 2000;13:617–628. doi: 10.1094/MPMI.2000.13.6.617. [DOI] [PubMed] [Google Scholar]
- 26.Oldroyd GE, Downie JA. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 27.Heidstra R, et al. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development. 1997;124:1781–1787. doi: 10.1242/dev.124.9.1781. [DOI] [PubMed] [Google Scholar]
- 28.Oldroyd GE, Engstrom EM, Long SR. Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell. 2001;13:1835–1849. doi: 10.1105/TPC.010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penmetsa RV, Cook DR. A Legume ethylene-insensitive mutant hyperinfected by its Rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- 30.Varma Penmetsa R, et al. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008;55:580–595. doi: 10.1111/j.1365-313X.2008.03531.x. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, et al. Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 2006;46:961–970. doi: 10.1111/j.1365-313X.2006.02751.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa T, Kawaguchi M. Shoot-applied MeJA suppresses root nodulation in Lotus japonicus. Plant Cell Physiol. 2006;47:176–180. doi: 10.1093/pcp/pci222. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki A, et al. Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 2004;45:914–922. doi: 10.1093/pcp/pch107. [DOI] [PubMed] [Google Scholar]
- 34.Ding Y, et al. Abscisic acid coordinates Nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell. 2008 doi: 10.1105/tpc.108.061739. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Abarca F, et al. Involvement of salicylic acid in the establishment of the Rhizobium meliloti—alfalfa symbiosis. Molec Plant Microb Interact. 1998;11:153–155. [Google Scholar]
- 36.Stacey G, McAlvin CB, Kim SY, Olivares J, Soto MJ. Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula. Plant Physiol. 2006;141:1473–1481. doi: 10.1104/pp.106.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godiard L, et al. Identification of new potential regulators of the Medicago truncatula—Sinorhizobium meliloti symbiosis using a large-scale suppression subtractive hybridization approach. Mol Plant Microbe Interact. 2007;20:321–332. doi: 10.1094/MPMI-20-3-0321. [DOI] [PubMed] [Google Scholar]
- 38.Mitra RM, Long SR. Plant and bacterial symbiotic mutants define three transcriptionally distinct stages in the development of the Medicago truncatula/Sinorhizobium meliloti symbiosis. Plant Physiol. 2004;134:595–604. doi: 10.1104/pp.103.031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson BJ, Mathesius U. Signaling interactions during nodule development. J Plant Growth Regul. 2003;22:47–72. [Google Scholar]
- 40.Ramu SK, Peng HM, Cook DR. Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol Plant Microbe Interact. 2002;15:522–528. doi: 10.1094/MPMI.2002.15.6.522. [DOI] [PubMed] [Google Scholar]
- 41.Oldroyd GE, Downie JA. Calcium, kinases and nodulation signalling in legumes. Nat Rev Mol Cell Biol. 2004;5:566–576. doi: 10.1038/nrm1424. [DOI] [PubMed] [Google Scholar]
- 42.Cook D, et al. Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell. 1995;7:43–55. doi: 10.1105/tpc.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nutman PS. Physiological studies on nodule formation I. The relation between nodulation and lateral root formation in red clover. Ann Bot. 1948;12:81–96. [Google Scholar]
- 44.Laplaze L, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Mo X, Shou H, Wu P. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physiol. 2006;47:1112–1123. doi: 10.1093/pcp/pcj082. [DOI] [PubMed] [Google Scholar]
- 46.Lohar DP, et al. Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. Plant J. 2004;38:203–214. doi: 10.1111/j.1365-313X.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- 47.Boot KJM, van Brussel AAN, Tak T, Spaink HP, Kijne JW. Lipochitin oligosaccharides from Rhizobium leguminosarum bv. viciae reduce auxin transport capacity in Vicia sativa subsp. nigra roots. Molec Plant Microbe Interact. 1999;12:839–844. [Google Scholar]
- 48.Mathesius U, et al. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 49.van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol. 2006;140:1494–1506. doi: 10.1104/pp.105.075879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prayitno J, Rolfe BG, Mathesius U. The Ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiol. 2006;142:168–180. doi: 10.1104/pp.106.080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y, Harris JM. Response of root branching to abscisic acid is correlated with nodule formation both in legumes and nonlegumes. Am J Bot. 2005;92:1675–1683. doi: 10.3732/ajb.92.10.1675. [DOI] [PubMed] [Google Scholar]
- 52.Liang Y, Mitchell DM, Harris JM. Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Dev Biol. 2007;304:297–307. doi: 10.1016/j.ydbio.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 53.Caba JM, Centeno ML, Fernandez B, Gresshoff PM, Ligero F. Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type. Planta. 2000;211:98–104. doi: 10.1007/s004250000265. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson BJ, Ross JJ, Reid JB. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol. 2005;138:2396–2405. doi: 10.1104/pp.105.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]