Abstract
Small monomeric RAC/ROP GTPases act as molecular switches in signal transduction processes of plant development and stress responses. They emerged as crucial players in plant-pathogen interactions either by supporting susceptibility or resistance. In a recent publication, we showed that constitutively activated (CA) mutants of different barley (Hordeum vulgare) RAC/ROPs regulate susceptibility to barley fungal leaf pathogens of different life style in a contrasting way. This illustrates the distinctive signalling roles of RAC/ROPs for different plant-pathogen combinations. We also reported the involvement of RAC/ROPs in plant epidermis development in a monocotyledonous plant. Here we further discuss a failure of CA HvRAC/ROP-expressing barley to normally develop stomata.
Key words: Hordeum vulgare, G-proteins, RAC, ROP, cell expansion, stomata, transpiration
Members of the RHO family of small G-proteins in plants (RAC/ROPs) regulate signal transduction processes at the plasma membrane.1 They act as multifunctional signalling switches in plant development and a variety of stress responses. RAC/ROP GTPases play regulatory roles in polar growth and cell morphogenesis in several cell systems including pollen tubes, developing root hairs and leaf pavement cells.2
In a recent publication,3 we showed that constitutively activated (CA) mutants of different barley (Hordeum vulgare) RAC/ROPs support susceptibility to the barley powdery mildew fungus Blumeria graminis f.sp. hordei (Bgh). CA HvRAC1 supported susceptibility to biotrophic Bgh but resistance to hemibiotrophic Magnaporthe oryzae in barley at the penetration level in both cases. Additionally, CA HvRAC1 supported local callose deposition at sites of attack from Bgh and a secondary H2O2 burst in whole non-penetrated epidermal cells. This supports a regulatory function of RAC/ROPs in plant defence1 and the potential corruption of defence pathways in susceptibility to Bgh. Because the rice ortholog of HvRAC1, OsRAC1, can regulate an H2O2 burst via activation of the plasma membrane NADPH oxidase OsRBOHB,4 one can speculate that the secondary H2O2 burst in CA HvRAC1 barley could also be caused by over-activation of an NADPH oxidase. However, CA HvRAC1 barley was also more susceptible to fungal penetration, and penetrated cells did not show an H2O2 burst. Hence, CA HvRAC1 did not contribute to penetration resistance, and the H2O2 burst might have been suppressed by Bgh after successful penetration. Interestingly, Bgh secretes a catalase during interaction with the plant.5
The involvement of RAC/ROPs in plant development has been widely studied in the dicots Arabidopsis and tobacco. In Arabidopsis, CA AtRAC/ROPs disturb root hair tip growth and epidermal cell morphogenesis.6,7 We showed similar developmental aberrations as a result of CA HvRAC/ROP expression in monocotyledonous barley. Root hair polarity disruption and enhanced leaf epidermal cell expansion was observed in CA HvRAC/ROP expressing barley. Here, we further report on reduced or abnormal development of stomata as an effect of CA HvRAC/ROP expression.
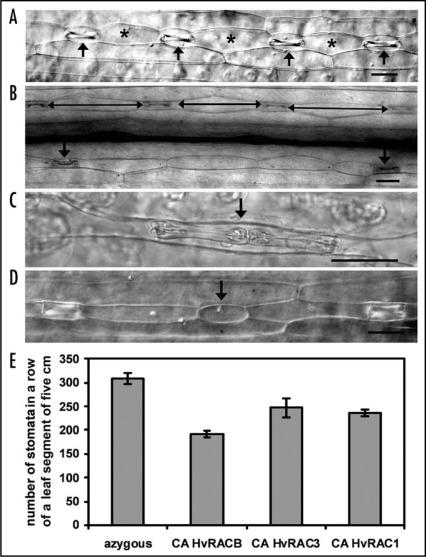
In barley, stomata and short epidermal cells alternate in a row of leaf epidermal cells (Fig. 1A). The number of stomata number was significantly reduced in three CA HvRAC/ROP (CA HvRACB, CAHvRAC3, CA HvRAC1) expressing barley genotypes when compared to azygous controls (barley siblings that lost the transgene due to segregation) (Fig. 1E). In part, this could be explained by enhanced length of epidermal cells intercalated between stomata (Fig. 1B). The presence of longer epidermal cells in all CA HvRAC/ROP-barleys further supports that RAC/ROPs are operating in epidermal cell expansion.3
Figure 1.
Stomatal abnormalities observed in CA HvROPexpressing transgenic barley leaves. (A) Wild type leaf adaxial epidermis with alternating stomata complexes (arrows) and short epidermal cells (asterisk). (B) Presence of more than one short epidermal cell in between two stomata. Arrows point the stomata. Double headed arrows highlight intercalated cells with enhanced cell length (C) Two stomata lacking an intercalated short epidermal cell. (D) Stoma failed to develop and left an abnormal blank cell. (E) Average number of stomata present in 5 cm of a stomatal row in transgenic plants expressing distinct CA barley CA HvRAC/ROPs. For all samples, stomatal rows present on either side of the mid rib were counted in the leaf upper epidermis. Fully expanded leaves of 3-weeks-old barley plants were used for counting stomata. Error bars show 95% confidence intervals. Repetition of the experiment led to similar results. Scale bars = 50 µm.
Previously, we carried out porometry experiments to measure stomata conductivity in CA HvRACB expressing barley leaves.8 The CA HvRACB leaves showed up to 50% less transpiration than azygous controls without any treatment. Additionally, CA HvRACB leaves were less responsive to abscisic acid (ABA) and subsequently they could not effectively reduce transpiration when treated with ABA or when cut-off from water supply.8 Our data on numbers of stomata per leaf segment could now explain the lower rates of transpiration in non-stressed CA HvRACB barley when compared to wild type.
Apart from the stomata number, developmental abnormalities were observed in the arrangement of epidermal cells. Generally, the shape of epidermal cells was less regular in CA HvRAC/ROP barley.3 We also observed the presence of more than one short epidermal cell in between two stomata (Fig. 1B) or two stomata lacking an intercalated short epidermal cell (Fig. 1C), or stomata failed to develop, which ended up in an abnormally short epidermal cell (Fig. 1D). Although such abnormalities were also rarely observed in wild type plants, all three CA HvRAC/ROP-barley leaves exhibited a clearly higher frequency of abnormalities in a given length of a stomata row. Together, CA HvRAC/ROPs had an effect on both the number and development of stomata. These observations suggest that RAC/ROPs are not only operating in cell expansion but also in barley cell differentiation for stomata development.
Acknowledgements
This work was supported by the German Research Foundation (DFG-HU886/1-3), and by the German Academic Exchange Service.
Abbreviations
- RHO
rat sarcome oncogene product (RAS) homologue
- RAC
Ras related C3 botulinum toxin substrate
- ROP
RHO of plants
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7477
References
- 1.Yang Z, Fu Y. ROP/RAC GTPase signaling. Curr Opin Plant Biol. 2007;10:490–494. doi: 10.1016/j.pbi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nibau C, Wu HM, Cheung AY. RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci. 2006;11:309–315. doi: 10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Pathuri IP, Zellerhoff N, Schaffrath U, Hensel G, Kumlehn J, Kogel KH, et al. Constitutively activated barley ROPs modulate epidermal cell size, defense reactions and interactions with fungal leaf pathogens. Plant Cell Rep. 2008;27:1877–1887. doi: 10.1007/s00299-008-0607-9. [DOI] [PubMed] [Google Scholar]
- 4.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Henderson C, Gurr SJ. Blumeria graminis secretes an extracellular catalase during infection of barley: potential role in suppression of host defence. Mol Plant Pathol. 2004;5:537–547. doi: 10.1111/j.1364-3703.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- 6.Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, et al. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20:2779–2788. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultheiss H, Hensel G, Imani J, Broeders S, Kumlehn J, Kogel KH, et al. Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol. 2005;139:353–362. doi: 10.1104/pp.105.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]