Abstract
The photoperiodic flowering of Arabidopsis is shown to be explained in part by the Bünning's external coincidence model in which clock-controlled expression of CO and stabilization of CO protein by light have important roles. The floral activators, GI and CO, together with ZTL, FKF and CDF1 have been shown to be central for the induction of FT expression during evening to promote the photoperiodic flowering of Arabidopsis. Here we discuss a role of diurnal accumulation of a floral repressor SVP protein in the repression of the FT and SOC1 expression during daytime. A punctual coordination of the diurnal regulation of both positive and negative regulators by circadian clock appears to be important for the photoperiodic flowering in Arabidopsis.
Key words: CCA1, circadian clock, FLC, FT, LHY, photoperiodic flowering, SOC1, SVP
Circadian clock generates oscillations in the biochemical, physiological and behavioral functions of organisms that occur with approximate 24-h time periods with no external timing cues. This process enables an organism to phase its biological activities to the correct time of day. In higher plants, the circadian clock affects various processes, including the expression of many genes, leaf movement, petal opening, hypocotyl elongation and photoperiodic flowering time.1
Molecular genetic studies on a Long-day (LD) plant Arabidopsis and a Short-day (SD) plant rice have identified more than two dozens of key factors including both activators and repressors for the photoperiodic flowering.1,2 However, mechanisms on coordination of a balance between floral activators and repressors for the precise control of flowering have been largely unknown. Recent studies by Fujiwara et al., and Li et al., have demonstrated that a floral repressor complex SHORT VEGETATIVE PHASE (SVP)—FLOWERING LOCUS C (FLC) as well as activators FLOWERING LOCUS T (FT), SUPPRESSOR OF CO OVEREXPRESSION 1 (SOC1) and LEAFY (LFY), integrate flowering signals from various genetic pathways.3,4 These studies have also shown that temporal and spatial regulations of these positive and negative integrators of flowering make a signal to fine-tune the appropriate timing for flowering on each species living in their environments.3,4
In Arabidopsis, LATE ELONGATED HYPOCOTYL (LHY) and CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) are two closely related MYB proteins with redundant functions.5,6 LHY, CCA1 and PSEUDO RESPONSE-REGULATOR (PRR) proteins such as PRR1/TOC1, PRR5, PRR7 and PRR9 are thought to be essential clock components.1,2 LHY and CCA1 play important roles in photoperiodic flowering by controlling the rhythmic expression of flowering-time genes such as GIGANTEA (GI), CONSTANS (CO) and FT in light/dark cycles such as LD and SD.7,8 FT is believed to be a part of the floral hormone “florigen”.9,10
GI, FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1) and CYCLING DOF FACTOR 1 (CDF1) are shown to be required for the rhythmic expression of CO mRNA.8,11,12 However, the molecular mechanism underlying the cooperation between the GI and FKF1-CDF1 was less understood.
Recently it has been demonstrated that blue light stabilizes molecular interaction between GI and ZTL proteins.13 ZTL-GI interaction controls accumulation of the clock component TIMING OF CAB EXPRESSION 1 (TOC1), thus allowing robust circadian oscillations in gene expression.13 CDF1 functions as a transcriptional repressor of flowering via repression of CO expression.12 Formation of an FKF1-GI protein complex is also induced by blue light and this in turn targets CDF1 for degradation.14 Degradation of CDF1 protein results in increase of the CO mRNA and CO protein levels to promote FT expression and flowering under LD.14
These recent studies on FKF1-GI and ZEITLUPE (ZTL)-GI have provided us molecular mechanistic views on how the circadian clock controls the upregulation of FT transcription just after evening under the inductive LD condition.13,14 However, these studies raise several further questions. How is the FT expression kept at trough level? FT gene expression does not go up without activation of CO? What roles do the floral repressors play in the photoperiodic flowering, particularly during the light period to keep FT expression from active state? What are happening during the light period just before dusk under LD?
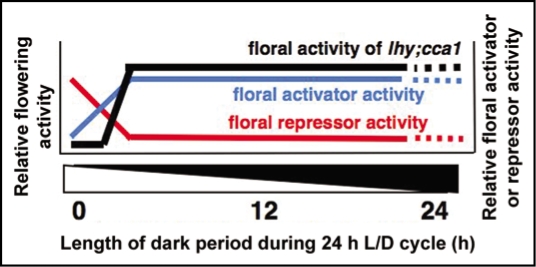
Recently we have found that in addition to repressing the floral transition under SD and LD, the circadian clock proteins LHY and CCA1 also accelerate flowering under continuous light (LL).3,7,8 LHY and CCA1 accelerated flowering in LL by promoting FT expression through a genetic pathway apparently independent of the canonical photoperiodic pathway involving GI and CO proteins.3 The late flowering phenotype of the lhy;cca1 double mutant under LL was suppressed by svp and flc mutations. SVP and FLC are floral repressors of the MADS box protein family.3 Yeast two hybrid analysis demonstrated an interaction between SVP and FLC, and genetic analysis demonstrated that these two proteins act as partially redundant repressors of flowering time.3 Interestingly, we have demonstrated that SVP protein was abundantly accumulated in lhy;cca1 plants grown under LL.3 We proposed a model in which LHY and CCA1 accelerate flowering in part by reducing the abundance of SVP and thereby antagonizing its capacity to repress FT expression under LL (Fig. 1).3
Figure 1.
A model showing a balance between repression of flowering by SVP and FLC in LL and promotion of flowering by altering rhythms of “GI-CO-FT/SOC1” transcription cascade in LD cycles.
Although abnormal, the lhy;cca1 or prr9;prr7;prr5 plants with severe defects in circadian function showed rhythmic expression of clock-controlled genes such as Cab, CCR2, GI and LHYL1 under light/dark cycles.7,8,15 These suggest that external rhythmic conditions such as light and temperature can partially rescue some defects caused by loss of the internal clock function. However, the roles of the circadian clock proteins in long-term developmental control of animals and plants under continuous conditions such as LL and continuous dark (DD) without any rhythmic stimuli are not fully understood. Our results demonstrate that both an internal biological clock and external rhythms are required for proper development of Arabidopsis.3,15
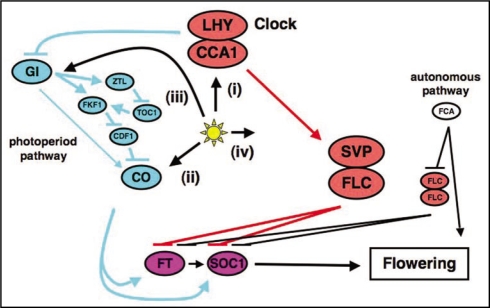
Light appears to affect at least 4 processes involved in the control of flowering (Fig. 2). First, light entrains circadian clock.16 Second, light plays a key role in stabilization of CO protein in the process.17 Third, the light-dependent regulation of CO expression by GI-ZTL/FKF1-CDF1 has recently been demonstrated.13,14 Fourth, we propose a novel role of the LHY and CCA1 in the GI-CO independent process to regulate flowering.3 This pathway probably includes the floral repressors encoding MADS box transcription factors SVP and FLC, and regulates expression of FT and other floral activator gene(s).
Figure 2.
A hypothetical model on the GI-CO independent pathway controlled by LHY/CCA1. For details, see the text.
Not only temporal but also spatial control of floral activator genes have been shown to be crucial for the precise regulation of the photoperiodic flowering.18 Recently, Li et al., have demonstrated that the flowering repressor SVP is controlled by the autonomous, thermosensory, and gibberelic acid (GA) pathways, and suppress directly expression of SOC1 in the shoot apex and leaf.4 FT expression in the leaf is also modulated by SVP. SVP protein associates with the promoter regions of both FT and SOC1 and FLC also binds these regions.4 These findings are consistent with ours and suggest that the interaction between SVP and FLC mediated by various flowering genetic pathways governs the integration of flowering signals.
Li et al. provide mechanistic views on how FLC and SVP precisely regulate expression of two floral activator genes, FT and SOC1, in two different spaces, apical meristems and leaves.4 By contrast, we provide views on how day-night cycles affect SVP protein level to regulate these floral activator genes.3 Accumulation of SVP protein showed diurnal rhythms. It started to accumulate just after dawn, peak in midday, and gradually decreased to dusk, then kept low level during the dark period. Levels of SVP transcripts were, however, not changed during LD-cycle.3 These complementary studies have raised many questions. What factors control accumulation or stability of SVP protein? For example, ZTL-FKF1-LKP2 family members or CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1) are involved in this process?12–14,19 What roles do photoreceptors have in controlling the amount of SVP accumulation? These will help us to understand a whole events occurring during a day to control flowering time.
Acknowledgements
This study was supported in part by the Bilateral Joint-Lab Project between Japan and France of the Ministry of Education, Culture, Sports, Science and Technology (MEXT; T.M.).
Abbreviations
- CCA1
circadian clock associated 1
- CO
constans
- GI
gigantea
- FKF1
flavin-binding, kelch repeat, F-box 1
- FLC
flowering locus C
- FT
flowering locus T
- LHY
late elongated hypocotyl
- LD
long-day
- LL
continuous light
- SD
short-day
- SOC1
suppressor of CO overexpression 1
- SVP
short vegetative phase
- ZTL
zeitlupe
and
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7517
References
- 1.Mizoguchi T, Putterill J, Ohkoshi Y. Kinase and phosphatase: The cog and spring of the circadian clock. Int Rev Cytol. 2006;250:47–72. doi: 10.1016/S0074-7696(06)50002-6. [DOI] [PubMed] [Google Scholar]
- 2.Izawa T. Daylength measurements by rice plants in photoperiodic short-day flowering. Int Rev Cytol. 2007;256:191–222. doi: 10.1016/S0074-7696(07)56006-7. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara S, Oda A, Yoshida R, Niinuma K, Miyata K, Tomozoe Y, Tajima T, Nakagawa M, Hayashi K, Coupland G, Mizoguchi T. Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell. 2008;20:2960–2971. doi: 10.1105/tpc.108.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell. 2008;15:110–120. doi: 10.1016/j.devcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, Coupland G. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–1229. doi: 10.1016/s0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZY, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- 7.Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, Carré IA, Coupland G. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell. 2002;2:629–641. doi: 10.1016/s1534-5807(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 8.Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, Coupland G. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell. 2005;17:2255–2270. doi: 10.1105/tpc.105.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- 11.Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 12.Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309:293–297. doi: 10.1126/science.1110586. [DOI] [PubMed] [Google Scholar]
- 13.Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han L, David K, Putterill J, Nam HG, Somers DE. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature. 2007;449:356–360. doi: 10.1038/nature06132. [DOI] [PubMed] [Google Scholar]
- 14.Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–265. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niinuma K, Nakamichi N, Miyata K, Mizuno T, Kamada H, Mizoguchi T. Roles of Arabidopsis PSEUDO-RESPONSE REGULATOR (PRR) genes in the opposite controls of flowering time and organ elongation under long-day and continuous light conditions. Plant Biotechnol. 2008;25:165–172. [Google Scholar]
- 16.Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- 17.Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 18.Imaizumi T, Kay SA. Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 2006;11:550–558. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Yi C, Deng XW. COP1—From plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]