Abstract
Day length perceived by a leaf is a major environmental factor that controls the timing of flowering. It has been believed that a mobile, long-distance signal called florigen is produced in the leaf, and is transported to the shoot apex where it triggers floral morphogenesis. Grafting experiments have shown that florigen is transmissible from a donor plant that has been subjected to inductive day length to an un-induced recipient plant. However, the nature of florigen has long remained elusive. Recent studies have provided evidence that the FLOWERING LOCUS T (FT) protein in Arabidopsis and corresponding proteins in other species are an important part of florigen. Our work showed that the FT activity, either from overexpressing or inducible transgenes or from the endogenous gene, to promote flowering is transmissible through a graft junction, and that an FT protein with a T7 tag (FT-T7) is transported from a donor scion to the apical region of recipient stock plants and becomes detectable within a short period of 24–48 h. That the FT-green fluorescent protein (GFP) fusion protein (FT:GFP) retains limited ability for graft-transmissible action was confirmed.
Key words: Arabidopsis, flowering, florigen, FT, graft, long-distance signal
Seasonal flowering is an important adaptive trait in plants for reproductive success. Plants monitor day length in the leaf to anticipate upcoming seasonal changes and initiate floral morphogenesis at the shoot apex by the action of the leaf-generated mobile florigen.1–3 FT, a 20-kDa protein, is a conserved potent promoter of flowering.4–7 In Arabidopsis, transcription of FT is induced by long days in the phloem tissues of cotyledons and leaves,8–10 while FT protein interacts with a transcription factor FD that is expressed in the shoot apex and activates transcription of meristem identity genes such as APETALA1 (AP1).10,11 Recent studies from several laboratories have provided strong evidence for the FT protein in Arabidopsis and corresponding proteins in other species being an important part of the florigen.12–16 These were mainly based on (1) detection in the shoot apex or its vicinity of GFP-fusion or Myc-tagged proteins expressed by promoters with preferential activity in the phloem tissues in Arabidopsis and rice,12–14 (2) detection of FT-like proteins in the phloem sap from cucurbits,16 and (3) transmission of FT:GFP protein through a graft junction from donor transgenic plants expressing the fusion protein in the vasculature to recipient ft plants with concomitant promotion of flowering.12 Because key observations in Arabidopsis12,14 were based on the effect of cumulative expression by SUCROSE TRANSPORTER 2 (SUC2) promoter which has strong activity in the vasculature within a short distance from the shoot,12,14,17 it is difficult to analyze the temporal aspects of the transport and to exclude the possibility of short-distance, cell-to-cell transport without entering into the phloem. In addition, the ability of GFP for long-distance transport via the phloem and a graft junction18,19 makes an independent confirmation based on methods other than GFP-fusion protein desirable.
Our recent work aimed to resolve these difficulties by the combined use of FT-T7 protein, a local transient induction system, and a two-shoot “Y-grafting” technique originally described by Turnbull and colleagues.20 Since graft-transmission has been an important criterion of florigen, we first demonstrated the graft-transmissible action of FT both from the endogenous gene and transgenes under the control of 35S promoter and SULPHATE TRANSPORTER 2;1 (SULTR2;1) promoter with preferential expression in the phloem.19,21 Grafting of a wild-type scion resulted in small but significant reduction in both days to flowering and the number of leaves at flowering of recipient ft-1 stock plants, while grafting of a strong 35S::FT scion caused much greater (more than 20 leaves) reduction (Fig. 1A). Even in the latter case, rescue may sound rather modest, since the recipient ft-1 stock plants still had 20 more leaves than the intact wild-type plants grown in the same conditions.21 However, given that functional connection of phloem capable of trafficking tracer dyes and enhanced GFP (EGFP) were established about 2 weeks after grafting, by which time wild-type plants made floral transition as evidenced by AP1 expression,19 it is difficult to expect the rescue to the wild-type phenotype. Using a transient induction system by a HEAT-SHOCK PROTEIN (HSP) gene promoter, we showed that the FT-T7 protein was transported from the donor scion to the shoot apical region of the recipient stock plants with promotion of flowering of the stock plants, and was detectable within a short period of 24–48 h after induction.21
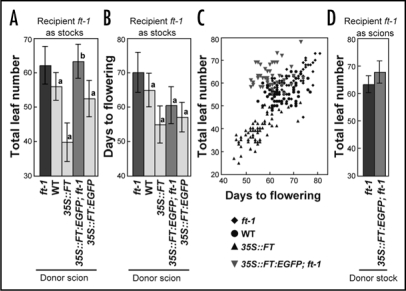
Figure 1.
Flowering time of the recipient ft-1 plants in various grafts. (A and B) Flowering time of the recipient ft-1 stock plants with ft-1, wild-type (WT), 35S::FT, 35S::FT:EGFP; ft-1, and 35S::FT:EGFP scions. Flowering time is expressed as the total leaf number (the number of rosette and cauline leaves at the time of flowering) in (A), and the number of days to flowering (days from germination to the time when the primary inflorescence stem reaches a 1-cm height) in (B). Letters “a” and “b” above the bars designate p < 0.0001 and p > 0.1, respectively, for Student's t-test with ft-1. (C) Relationship between days to flowering and the total leaf number in ft-1 stock plants with 35S::FT, wild-type, ft-1, and 35S::FT:EGFP; ft-1 scions. The linear correlation coefficient (r) for the first three graft combinations is 0.83. (D) Flowering time of the recipient ft-1 scion plants grafted onto ft-1 and 35S::FT:EGFP; ft-1 stocks. (A–C) are based on the same data sets used for Figure 2 and S-Table 1 of Notaguchi et al.21
Because our recent finding that the late-flowering phenotype of the recipient ft-1 stock plants was not rescued by grafting 35S::FT:EGFP; ft-121 or SULTR2;1::FT:EGFP; ft-119 is not in agreement with the previous report of partial rescue of ft-7 by SUC2::FT:GFP; ft-7,12 we tried to resolve the seeming discrepancy by re-examining our data (Fig. 1). In our previous reports, flowering time was measured as the “total leaf number” (the number of rosette and cauline leaves at the time of flowering) which has been commonly used by many researchers, since there generally is a good correlation between “the leaf number” and “days to flowering” in intact plants22 and in grafts (Fig. 1C). Grafting of either 35S::FT:EGFP; ft-1 or SULTR2;1::FT:EGFP; ft-1 did not cause reduction in the number of leaves (Fig. 1A).19,21 Rescue was not observed in an experiment in which recipient ft-1 were grafted onto 35S::FT:EGFP; ft-1 donor stocks as in Corbesier and colleagues12 (Fig. 1D). Therefore, reduction in the leaf number was observed in none of the four experiments. However, we found that if the flowering time was measured by “days to flowering” (days from germination to the time when the primary inflorescence becomes 1-cm height in our experiments), as in the previous report,12 partial rescue of the recipient ft-1 stock plants with a 35S::FT:EGFP; ft-1 becomes discernible (Fig. 1B). For some unknown reasons, ft-1 stock plants with a 35S::FT:EGFP; ft-1 scion had more leaves than expected from the length of vegetative period measured by days (Fig. 1C). Therefore, the discrepancy seems to be due to the different measures of the flowering time used by the two groups. Unfortunately, a similar analysis of data was not available for the other three experiments. It will be interesting to know whether the same holds true for results obtained by Corbesier and colleagues. These results, taken together, indicate that FT:GFP fusion proteins have reduced ability for graft-transmissible action as compared with the intact FT protein or FT-T7 protein.
In conclusion, the long-distance, graft-transmissible action of the FT protein by transport to the shoot apex within a short period of time is well demonstrated by the previous and our recent work.12–16,19,21 However, temporal aspects of the transport and spatial distribution of the FT in the shoot apex remain to be investigated. A local, transient induction system by single-leaf blade heat treatment to express FT-T7 or FT:EGFP proteins21 will provide useful tools.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sport, Science and Technology of Japan and the Mitsubishi Foundation (to T.A.).
Abbreviations
- APETALA1
AP1
- EGFP
enhanced green fluorescent protein
- FT
FLOWERING LOCUS T
- GFP
green fluorescent protein
- HSP
HEAT-SHOCK PROTEIN
- SUC2
SUCROSE TRANSPORTER 2
- SULTR2;1
SULPHATE TRANSPORTER 2;1
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/7558
References
- 1.Imaizumi T, Kay S. Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 2006;11:550–558. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Chailakhyan MK. Concerning the hormonal nature of plant development processes. Dokl Akad Nauk SSSR. 1937;16:227–230. [Google Scholar]
- 3.Zeevaart JAD. Physiology of flower formation. Annu Rev Plant Physiol. 1976;27:321–348. [Google Scholar]
- 4.Kardailsky I, Shukla V, Ahn J, Dagenais N, Christensen S, Nguyen J, et al. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 6.Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, et al. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002;43:1096–1105. doi: 10.1093/pcp/pcf156. [DOI] [PubMed] [Google Scholar]
- 7.Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA. 2006;103:6398–6403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takada S, Goto K. TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005;46:1175–1189. doi: 10.1093/pcp/pci151. [DOI] [PubMed] [Google Scholar]
- 10.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 11.Wigge P, Kim M, Jaeger K, Busch W, Schmid M, Lohmann J, et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- 12.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki S, Matsuo S, Wong H, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu J, Warthmann N, Küttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Lin M, Belanger H, Lee Y, Varkonyi-Gasic E, Taoka K, Miura E, et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the Cucurbits. Plant Cell. 2007;19:1488–1506. doi: 10.1105/tpc.107.051920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131:3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- 18.Imlau A, Truernit E, Sauer N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell. 1999;11:309–322. doi: 10.1105/tpc.11.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notaguchi M, Daimon Y, Abe M, Araki T. Adaptation of a seedling micro-grafting technique to the study of long-distance signaling in flowering of Arabidopsis thaliana. J Plant Res. 2009 doi: 10.1007/s10265-008-0209-1. In press. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull C, Booker J, Leyser H. Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 2002;32:255–262. doi: 10.1046/j.1365-313x.2002.01419.x. [DOI] [PubMed] [Google Scholar]
- 21.Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, et al. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 2008;49:1645–1658. doi: 10.1093/pcp/pcn154. [DOI] [PubMed] [Google Scholar]
- 22.Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]