Abstract
The α subunit of heterotrimeric G-proteins (Gα) is involved in a broad range of aspects of the brassinosteroid (BR) response, such as the enhancement of lamina bending. However, it has been suggested from epistatic analysis of d1 and d61, which are mutants deficient for Gα and the BR receptor BRI1, that Gα and BRI1 may function via distinct pathways in many cases. In this study, we investigated further the genetic interaction between Gα and BRI1. We report the analysis of transformants of T65d1 and T65d1/d61-7 into which were introduced a constitutively active form of Gα, Q223L. The application of 24-epi-brassinolide (24-epiBL) to T65d1 expressing Q223L still resulted in elongation of the coleoptile and, in fact, it was enhanced over the wild-type plant (WT) level in a concentration dependent manner. In T65d1/d61-7 expressing Q223L, the seed size was enlarged over that of d61-7 due to activation of Gα. These results suggest that Q223L is able to augment the BR response in response to 24-epiBL and also that Q223L functions independently of BRI1 in the process of determining seed morphology, given that Q223L was functional in the BRI1-deficient mutant, d61-7.
Key words: brassinosteroid, BRASSINOSTEROID INSENSITIVE1 (BRI1), genetic interaction, G-protein α subunit, rice plants, seed morphology, transgenic plants
Introduction
Heterotrimeric G-proteins are signaling components which consist of three subunits, namely Gα, Gβ and Gγ. In mammals, these signaling components form a multi-gene family and the signaling system is well studied.1 In contrast, the repertoire of heterotrimeric G-protein subunits in plants is quite limited, for example, Gα is coded by only a single gene in rice plants and Arabidopsis. Despite the simplified number of genes, plant Gα influences a diverse range of physiological events.2 Recently, a number of papers have elucidated the importance of Gα in brassinosteroid (BR) responses.3–5 The mutants of Gα in the rice plant, d1, have been shown to have a reduced BR sensitivity. The most typical BR signaling system is composed of BR receptor, BRI1 and its downstream components such as BSK1, BIN2, BSU1 and BZR1/BES1.6 Based on the analyses of double mutants of Gα and BRI1, Gα functions in a distinct manner from BRI1 in many cases examined.3,7 It is therefore pertinent to investigate possible interactions between Gα and BRI1.
Function of Constitutively Active Gα, Q223L in Response to 24-epiBL
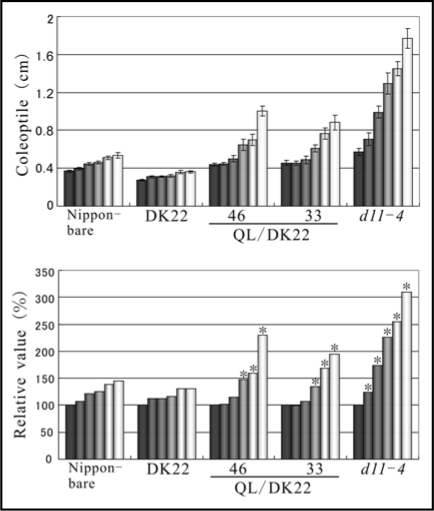
To examine the relationship between Gα and BRI1, we took advantage of the Q223L mutant that is a constitutively active form of rice Gα.8 The Q223L gene was introduced into a d1 allele containing strain, DK22, as previously described using the same binary vector.8 The recurrent parent of DK22 was Nipponbare and the obtained transformants were named QL/DK22. 24-epibrassinolide (24-epiBL) supplemented in agar medium causes coleoptile elongation in Nipponbare as in wild type plants (WT) (up to 145.4% at 10−6 M compared to treatment with 0 M (Fig. 1)). In the case of lines No. 46 and No. 33 of QL/DK22 transformants, the relative elongation rate was greater than observed in WT, namely 229.6% and 194.1%, respectively (Fig. 1). d11-4 was used as a BR-hypersensitive mutant and coleoptile length of d11-4 was elongated to 310.0% at 10−6 M (Fig. 1). With the activation of Gα in QL/DK22, these transgenic plants could still extensively elongate coleoptiles in a 24-epiBL dependent manner. Therefore, it was suggested that the Gα is not capable of triggering a BR response by itself but actually potentiates BR signaling and/or functions together with the BR cascade, to promote coleoptile elongation (Fig. 3; left model).
Figure 1.
Effect of the activation of rice Gα in the BR response. The seeds of Nipponbare, DK22 (an allele of d1), QL/DK22 and d11-4 (a mutant of the BR-biosynthetic enzyme, CYP724B1) were sown onto agar medium supplemented with or without the indicated concentration of 24-epiBL. Each set of columns from dark gray to white represent the values from plants grown at 0, 10−8, 10−7, 2 × 10−7, 5 × 10−7 and 10−6 M of 24-epiBL respectively. The upper and lower panels are the scores expressed in actual values and relative values. The relative values are expressed in terms of the increase in length relative to treatment with 24-epiBL-free medium (defined as 100%). Each value is the mean of 12 seedlings. Experiments were repeated at least 3 times. Asterisks denote statistically significant p-value for the differences measured by a two-tailed t-test, with regard to greater scores than those in WT. (Error bars = SE).
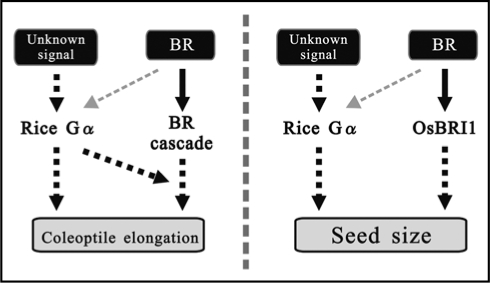
Figure 3.
The relationship between Gα and BRI1. The possible interaction between Gα and BRI1 with regard to promotion of coleoptile elongation by 24-epiBL (left) and control of seed size in the developmental process (right).
Function of Q223L in a BRI1-Deficient Genetic Background
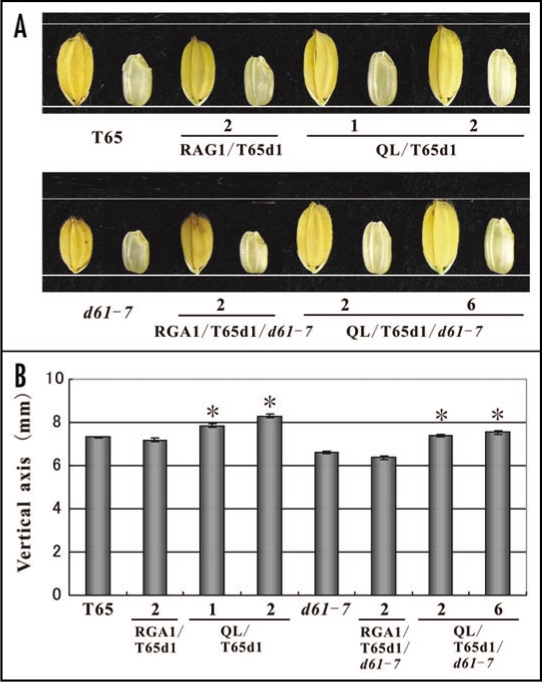
The seed sizes of d1 and a OsBRI1 mutant, d61, were smaller than those of WT.9,10 The expression of Q223L in d1 (DK22) resulted in the enlargement of seed size over WT.8 Here, we used plant materials derived from the T65 rice variety. We introduced Q223L into the T65d1 mutant, which has a d1 null allele, and the T65d1/d61-7 double mutant and resultant transformants were named QL/T65d1 and QL/T65d1/d61-7, respectively. We also introduced RGA1, the native rice Gα gene into T65d1 and the T65d1/d61-7 double mutant and named them RGA1/T65d1 and RGA1/T65d1/d61-7, respectively. Subsequently, the hulled and unhulled seeds were photographed (Fig. 2A) and their lengths measured (Fig. 2B). The seed length of QL/T65d1 was increased by up to 113.6% in the vertical axis over seeds of T65. Likewise, seeds of QL/T65d1/d61-7 were increased by up to 114.3% over those from d61-7. Despite the impaired BRI1 action in QL/T65d1/d61-7, the function of Q223L was well expressed even in the d61-7 background. This clearly indicates that Gα does not require BRI1 for the rice seed developmental process. Gα and BRI1 may function independently in this case (Fig. 3; right model).
Figure 2.
Effect on seed morphology of Q223L in BRI1-deficient genetic background. The effect of RGA1 and Q223L on seed morphology in the T65d1 mutant and the T65d1/d61-7 double mutant was assessed. RGA1 was introduced to T65d1 and T65d1/d61-7, and the resultant transgenic plants were designated as RGA1/T65d1 and RGA1/T65d1/d61-7. Q223L was also introduced into T65d1 and T65d1/d61-7, and designated as QL/T65d1 and QL/T65d1/d61-7. The vector construct used in RGA1 transgenic plants was the same as that used in Q223L transgenic plants except that the cDNA of Q223L was replaced by RGA1. (A) Photographs of the representative seeds of T65, RGA1/T65d1, and QL/T65d1 (Upper) and d61-7, RGA1/T65d1/d61-7 and QL/T65d1/d61-7 (Lower). (B) The vertical length of the seeds. Each value is the mean of 10 seeds. Asterisks denote statistically significant p-values for the differences measured by a two-tailed t-test. (Error bars = SE).
Disscussion
Gα influences the BR response in plants and this property is conserved among monocot and dicot plants. One question is as to whether Gα participates in the BRI1 cascade. The previous studies with the double mutants have suggested that Gα may mainly function via a mechanism distinct from BRI1.3,7 This idea is a promising concept and will guide the course of future study of Gα in the BR response. However, it must be unequivocally tested by another strategy. In this report, we show that Gα can transmit Gα dependent signals without proper BRI1 function, e.g., in seed morphology (Fig. 2). Furthermore, constitutive activation of Gα alone cannot drive a potent BR response, even though Gα certainly promotes the BR response in the presence of 24-epiBL (Fig. 1). These results support the model that Gα mainly functions separately from the BRI1 cascade and enhances some of the BR response via an unidentified signaling pathway.
Abbreviations
- 24-epiBL
24-epi-brassinolide
- BR
brassinosteroid
- Gα
G-protein α subunit
- SE
standard error
- WT
wild-type plants
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7627
References
- 1.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JG. Heterotrimeric G-proteins in plant development. Front Biosci. 2008;13:3321–3333. doi: 10.2741/2928. [DOI] [PubMed] [Google Scholar]
- 3.Oki K, Inaba N, Kitagawa K, Fujioka S, Kitano H, Fujisawa Y, et al. Function of the a subunit of rice heterotrimeric G protein in brassinosteroid signaling. Plant Cell Physiol. 2009;50:161–172. doi: 10.1093/pcp/pcn182. [DOI] [PubMed] [Google Scholar]
- 4.Ullah H, Chen JG, Wang S, Jones AM. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 2002;129:897–907. doi: 10.1104/pp.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Xu YY, Ma QB, Li D, Xu ZH, Chong K. Heterotrimeric G protein α subunit is involved in rice brassinosteroid response. Cell Res. 2006;16:916–922. doi: 10.1038/sj.cr.7310111. [DOI] [PubMed] [Google Scholar]
- 6.Tang W, Kim TW, Oses-Prieto JA, Sun Y, Deng Z, Zhu S, et al. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008;321:557–560. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y, Wang S, Asami T, Chen JG. Loss-of-function mutations in the Arabidopsis heterotrimeric G-protein α subunit enhance the developmental defects of brassinosteroid signaling and biosynthesis mutants. Plant Cell Physiol. 2008;49:1013–1024. doi: 10.1093/pcp/pcn078. [DOI] [PubMed] [Google Scholar]
- 8.Oki K, Fujisawa Y, Kato H, Iwasaki Y. Study of the constitutively active form of the a subunit of rice heterotrimeric G proteins. Plant Cell Physiol. 2005;46:381–386. doi: 10.1093/pcp/pci036. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, et al. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Natl Acad Sci USA. 1999;96:7575–7580. doi: 10.1073/pnas.96.13.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell. 2005;17:776–790. doi: 10.1105/tpc.104.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]