Abstract
Maize survival under the anaerobic stress due to submergence conditions is dependent on complex metabolic, physiological and morphological adaptation strategies. Here, we focus on gene expression regulation at the transcriptional and post-transcriptional level in submerged maize root cells. Early in progressive oxygen deprivation, root cells sense the low oxygen signal to trigger expressions of TF genes, anaerobic response genes and miRNA genes. The induced TFs, in turn, promote a broad spectrum of responses from morphogenetic to metabolic; these responses occur at later stages of the stress treatment. The selective translation of anaerobically induced transcripts and selective degradation of some APs are also suggested to be an important regulatory mechanism. In addition, miRNAs are possibly transcriptionally regulated in submerged root cells and involved in post-transcriptional control of target genes. Thus, regulation of gene expression in response to low oxygen involves in significant transcriptional and post-transcriptional control.
Key words: maize, Zea mays L., anaerobic stress, adaptation, gene expression, microRNA
Introduction
Anaerobic or low oxygen conditions occur when maize plants are submerged or subjected to flooding of the soil. Maize survival under low oxygen conditions is mainly dependent on metabolic, physiological and morphological adaptation strategies. Under low oxygen conditions, a few ANPs are selectively synthesized, and most of the ANPs identified were found to be enzymes of glycolysis, such as aldolase, enolase, ADH and LDH.1 The synthesis of these enzymes indicated that oxidative phosphorylation of mitochondria is blocked, and cells undergo glycolysis and ethanolic fermentation, thus replacing the Krebs cycle in fulfilling the cellular demand for energy. Especially, during ethanolic fermentation, ADH is responsible for the recycling of the NAD+ needed for the glycolysis process to continue.2 High levels of ADH accumulation and its activity is considered to be positively correlated with the magnitude of the stress injury, but negatively correlated with tolerant to anaerobic stress.3,4 Physiologically, alteration of metabolic pathways results in a change of cytosolic pH, accumulation of ROS and hormone homeostasis.5–8 Adaptation also occurs via a wide variety of morphological and anatomical responses to oxygen limitation of roots or entire plants, including formation of aerenchyma, fast underwater elongation of shoots or leaves, stomatal closure, adventitious root formation and lateral root development.3,9–11 Each of these reactions is mediated by plant hormones, such as ethylene, auxin and ABA.
Our discussion largely centres on recent work in our laboratory carried out on maize. In most of these studies, a progressive depletion of oxygen in roots was carried out by completely submerging seedlings that had developed three leaves, in growth culture buffer. Our results supported the view that submergence results in alterations of gene expression leading to metabolic, physiological and morphological changes, and that transcriptional and post-transcriptional regulation of gene expression is involved these adaptations of maize under submergence conditions.
Transcriptional Regulation of Gene Expression in Submerged Roots of Maize
Previous studies showed that mRNAs encoding ANPs, including important enzymes in glycolysis, accumulated under low oxygen conditions, e.g., pyruvate decarboxylase, ADH, glucose phosphate isomerase, and lactate dehydrogenase.1,12–16 Both high-throughput cDNA microarrays and suppression subtractive hybridization were employed in our laboratory to investigate the gene expression response in root cells of maize, and we found that numerous gene expressions were altered because of submergence treatment. These genes are involved in a broad spectrum of biochemical, cellular, and physiological processes, such as glycolysis, energy metabolism, lipid metabolism, signal conduction, DNA transcription, protein biosynthesis/degradation, and photosynthesis.17–19 The spectrum of genes altered in the first 0.5 h of treatment was significantly different from that of the genes induced later 2–4 h.18 Comparison of the genes expression profile between a tolerant (Hz32) and a sensitive line (Mo17), showed that the response speed was different between the tolerant and sensitive line. The expressions of genes associated with fermentation were higher and earlier in the tolerant line compared to the sensitive line. A similar phenomenon was also revealed for genes involved in signal transduction, protein synthesis, protein trafficking and defense processes.18 Especially, a few TFs were identified to be more highly expressed in the tolerant line than that in the sensitive line. For example, during 0–1 h of treatment, an rs2-like TF, which encodes a type of Myb protein, was induced. Previous results demonstrated that the promoters of anaerobic response genes contain GT and CG motifs. The GT-motif resembles a Myb transcription factor binding site with a 5′-AAC-3′ central motif and Arabidopsis Myb transcription factor AtMyb2 binds specifically to the GT-motif of Adh1.20 Thus, we believed that rs2 plays a key role during Hz32 roots' response to submerging stress. Another TF induced in Hz32 is zm-bRLZ which contains a bRLZ domain, a DNA binding domain and is highly homologous to the rice transcription factor, RISBZ1. Moreover, sequence variation in the promoter region of zm-bRLZ was analyzed, and 2 or 3 deletions in the promoter region of the sensitive line were found by comparison with that of the tolerant line. Similar variations also were discovered in promoters of other genes (unpublished). It is possible to partly elucidate the reason why the expression of zm-bRLZ is higher and earlier in tolerant line than in sensitive. We proposed that maize root cells under submergence firstly sense the low oxygen signal, and then promote the expressions of various TFs, which, in turn, likely regulate the expression of responsive genes to induce diverse mechanism of adaptation.
Post-Transcriptional Regulation of Gene Expression in Submerged Root Cells of Maize
A fact revealed by proteomics is that ANPs are accumulated with a concomitant increase in their transcripts, but some APs did not increase while their mRNAs accumulate in oxygen deprived cells.21–23 Subsequently, the accumulations of ANPs were shown to be tightly associated with efficient loading of anaerobically responsive transcripts with polysomes. Although a large number of aerobic transcripts are also induced in response to oxygen deprivation, fewer aerobic transcripts are loaded with ribosomes.24,25 Comparison of total mRNA populations with polysomal RNA populations reveal that low oxygen reduces the average proportion of individual mRNA species in large polysome complexes, including not only aerobic transcripts, but also a subset of known anaerobic-induced gene transcripts. Moreover, a large number of mRNAs displayed a significant decrease in polysome association without a concomitant decrease in steady-state accumulation. By contrast, a small group of abiotic and biotic stress-induced mRNAs showed a significant increase in polysome association, without a change in abundance.23,26 Evaluation of mRNA sequences demonstrated that a low GC nucleotide content of the 5′-untranslated region provides a selective advantage for translation under low oxygen.23,26 These evidences demonstrate that selected synthesis of anaerobic polypeptides involves transcriptional, as well as significant post-transcriptional, regulation of gene expression.
Another possible cause of this is that APs were selectively degraded by the proteasome. An interesting observation from cDNA microarray data is that a few genes, SKP1/ASK1-like protein and 20S proteasome subunit α-3, which are involved in protein degradation, were also upregulated.17 The SKP1/ASK1-like protein is a subunit of the SKP1, cullin/CDC53, F-box protein (SCF) complex, and plays an important role in selecting substrates for proteolysis by facilitating ubiquitin binding to specific proteins. In plants, the SCF complex can regulate phytohormone responses.27,28 The 20S proteasome subunit α-3, a subunit of a 700-kD multi-subunit protease, facilitates ubiquitin-dependent proteolysis, and participates in the breakdown of some proteins in the absence of ubiquitin.29 At early submergence, the increase in the SKP1/ASK1-like protein implies an increase of the SCF complex. The induced expression of ubiquitin suggests that aerobic proteins bound to ubiquitin are selected as substrates and disassembled by 20S proteasome. The SKP1/ASK1-like protein and the 20S proteasome might play a crucial role in signal transduction by selective degradation of proteins involved in aerobic metabolism, implying that regulatory mechanisms at the translation level also play a crucial role in the low-oxygen response during early submergence.17
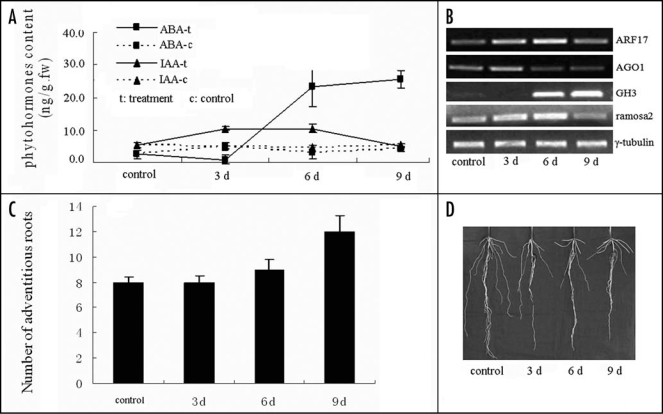
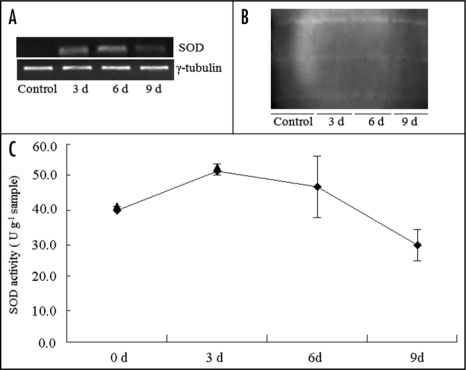
Recently, microRNAs, a class of RNA that target mRNA for degradation, were also shown to be transcriptionally regulated in response to submergence in root cells of maize. We used a miRNA microarray to investigate miRNA populations in maize roots, and showed that the expressions of 39 different miRNAs, falling into four different patterns, are affected by submergence. A group of four miRNAs show early upregulation, another group of four miRNAs show early downregulation and later upregulation under submergence.30 The alteration of the expression levels of miRNAs, in turn, directly regulates cleavage and translation of target mRNAs at the post-transcriptional level. The target mRNAs for the four upregulated miRNAs encode TFs. These TFs, mostly involved in root growth and morphogenesis, are in turn downregulated; most likely leading to an inhibition of elongation and a promotion of adventitious roots. We found that the IAA content increased during progressive submergence for 6 days, whereas ABA content decreased during 3 days of submergence, before rapidly increasing after 3 days of submergence (Fig. 1A). The early upregulated miRNA, miR167 targets auxin response factor 17 (ARF17), which is key factor in the Auxin/IAA signal cascade. ARF17 negatively regulates the expression of GH3 and downstream genes, such as ramosa 2. The RT-PCR profiles of ARF17, GH3 and ramosa 2 were coincident with the expectation mentioned above (Fig. 1B). The changes in expressions of these genes results in a promotion of adventitious roots (Fig. 1C and D). In addition, the miRNA osa-miR528-like, which was downregulated at the early stage and upregulated at the late stage of submergence, can lead to accumulation target mRNAs (SOD and ALDH) and their proteins. The results from RT-PCR of SOD (Fig. 2A), the protein band pattern (Fig. 2B) and SOD enzyme activity (Fig. 2C) support the above hypotheses. Thus, the overall pattern of changes is seen to enable the root to respond appropriately to submergence at all levels from morphogenetic to metabolic.
Figure 1.
Morphological adaptation of maize roots under submergence conditions is regulated at the transcriptional and post-transcriptional level. (A) Hormone homeostasis is disturbed under submergence conditions. Hormone content was measured by enzyme-linked immunosorbent assay (ELISA) (reviewed in ref. 31). (B) Gene expression profiles involved in auxin cascade and regulation of adventitious root development at different time points of submergence treatments. (C and D) the number and conformation of adventitious roots.
Figure 2.
Accumulation of transcript and protein, and enzyme activity of SOD. Activity of SOD was measured by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) (reviewed in ref. 32). (A) SOD expression profile assayed by RT-PCR. (B) SOD isozyme bands on PAGE. (C) SOD activity at different time-points of submergence treatments in roots of maize.
Conclusions
Complex mechanisms are involved in regulation of adaptation to low oxygen in root cells of maize. Under progressive oxygen deprivation, root cells sense the low oxygen signal to trigger expressions of some TF genes, genes encoding ANPs and miRNA genes. The induced ANPs are involved in modulation of glycolysis and energy metabolism. The induced TFs, in turn, promote a broad spectrum of responses from morphogenetic to metabolic, these responses occurring at the later stage of the stress treatment. The selective translation of anaerobically induced transcripts and selective degradation of some APs are also importantly regulation mechanisms. In addition, miRNAs are potentially regulated in submerged root cells at the transcriptional level, and are involved in post-transcriptional control of target genes such as ARF17 and SOD. Thus, regulation of gene expression in response to low oxygen involves significant transcriptional and post-transcriptional control.
Acknowledgements
We are grateful to Dr. Xilin Zou, Wanfu Tang (Huazhong Agricultural University, China) and Liya Wei (Hebei Agricultural University, China) for their contribution to the research. This work was supported by the Hi-Tech Research and Development Program of China (grant numbers 2008AA10Z112), National Natural Science foundation of China grant numbers 30571171 and 30771352.
Abbreviations
- ABA
abscisic acid
- ADH
alcohol dehydrogenase
- ALDH
aldehyde dehydrogenase
- ANPs
anaerobic proteins
- APs
aerobic proteins
- ARF
auxin response factor
- LDH
lactate dehydrogenase
- miRNA
microRNA
- SOD
super oxide dismutase
- ROS
reactive oxygen species
- TF
transcription factor
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7629
References
- 1.Sachs MM, Freeling M, Okimoto R. The anaerobic proteins of maize. Cell. 1980;20:761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- 2.Saglio PH, Raymond P, Pradet A. Metabolic activity and energy charge of excised maize root tips under anoxia. Plant Physiol. 1980;66:1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao CT, Lin CH. Physiological adaptation of crop plants to flooding stress. Proc Natl Sci Counc. 2001;25:148–157. [PubMed] [Google Scholar]
- 4.Tripepi RR, Mitchell CA. Stem hypoxia and root respiration of flooded maple and birch seedlings. Physiol Plant. 1984;60:567–571. [Google Scholar]
- 5.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Nat Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Ann Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Barneo J, del Toro R, Levitsky KL, Chiara MD, Ortega-Saenz P. Regulation of oxygen sensing by ion channels. J Appl Physiol. 2004;96:1187–1195. doi: 10.1152/japplphysiol.00929.2003. [DOI] [PubMed] [Google Scholar]
- 8.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol. 2005;98:404–414. doi: 10.1152/japplphysiol.00722.2004. [DOI] [PubMed] [Google Scholar]
- 9.Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Ann Bot. 1997;79:3–20. [Google Scholar]
- 10.Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot. 2003;91:205–211. doi: 10.1093/aob/mcf116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yordanova RY, Popova LP. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol Plant. 2007;29:535–541. [Google Scholar]
- 12.Dennis ES, Gerlach WL, Walker JC, Lavin M, Peacock WJ. Anaerobic regulated aldolase gene of maize: a chimaeric origin? J Mol Biol. 1988;202:759–767. doi: 10.1016/0022-2836(88)90556-6. [DOI] [PubMed] [Google Scholar]
- 13.Kelley PM. Maize pyruvate decarboxylase mRNA is induced anaerobically. Plant Mol Biol. 1989;13:213–222. doi: 10.1007/BF00016139. [DOI] [PubMed] [Google Scholar]
- 14.Andrews DL, Cobb BG, Johnson JR, Drew MC. Hypoxic and anoxic induction of alcohol dehydrogenase in roots and shoots of seedlings of Zea mays. Adh transcripts and enzyme activity. Plant Physiol. 1993;101:407–414. doi: 10.1104/pp.101.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley PM, Freeling M. Anaerobic expression of maize glucose phosphate isomerase I. J Bio Chem. 1984;259:673–677. [PubMed] [Google Scholar]
- 16.Hoffman NE, Bent AF, Hanson AD. Induction of lactate dehydrogenase by oxygen deficit in barley root tissue. Plant Physiol. 1986;82:658–663. doi: 10.1104/pp.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZX, Tang WH, Tao YS, Zheng YL. cDNA microarray analysis of early response to submerging stress in Zea mays roots. Russ J Plant Physiol. 2005;52:43–49. [Google Scholar]
- 18.Zhang ZX, Zou XL, Tang WH, Zheng YL. Revelation on early response and molecular mechanism of submergence tolerance in maize roots by microarray and suppression subtractive hybridization. Environ Exp Bot. 2006;58:53–63. [Google Scholar]
- 19.Tang WH, Zhang ZX, Zou XL, Zheng YL. Functional genomics of maize submergence tolerance and cloning of the related gene Sicyp51. Science in China. 2005;35:29–36. doi: 10.1360/062004-27. [DOI] [PubMed] [Google Scholar]
- 20.Hoeren F, Dolferus R, Wu Y, Peacoak WJ, Dennis ES. Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase (ADH1) gene by low oxygen. Genetics. 1998;149:479–490. doi: 10.1093/genetics/149.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McElfresh KC, Chourey PS. Anaerobiosis induces transcription but not translation of sucrose synthase in maize. Plant Physiol. 1988;87:542–546. doi: 10.1104/pp.87.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng Y, Wu Y, Avigne WT, Koch KE. Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses. Plant Physiol. 1998;116:573–583. doi: 10.1104/pp.116.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang WWP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment and identification of proteins by mass spectrometry. Plant Physiol. 2000;122:295–317. doi: 10.1104/pp.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fennoy SL, Bailey-Serres J. post-transcriptional regulation of gene expression in oxygen-deprived roots of maize. Plant J. 1995;7:287–295. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- 25.Fennoy SL, Nong T, Bailey-Serres J. Transcriptional and post-transcriptional processes regulate gene expression in oxygen-deprived roots of maize. Plant J. 1998;15:727–735. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- 26.Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J. Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot. 2005;96:647–660. doi: 10.1093/aob/mci217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulman BA, Carrano AC, Jeffrey PD, Bowen Z, Kinnucan ER, Finnin MS, Elledge SJ, Harper JW, Pagano M, Pavletich NP. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- 28.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP. Structure of the Cul1-Rbx1-Skp1-F Box Skp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 29.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ZX, Wei LY, Zou XL, Tao YS, Liu ZJ, Zheng YL. Submergence-responsive microRNAs are potentially involved in the regulation of morphological and metabolic adaptations in maize root cells. Ann Bot. 2008;102:509–519. doi: 10.1093/aob/mcn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S, Chen W, Zhou X. Enzyme linked immunosorbent assay for endogenous plant hormones. Plant Physiol Com. 1988;5:53–57. [Google Scholar]
- 32.Giannopolitis CN, Ries SK. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]