Abstract
In tip-growing plant cells such as pollen tubes and root hairs, surface expansion is confined to the cell apex. Vesicles containing pectic cell wall material are delivered to this apical region to provide the material necessarily to build the expanding cell wall. Quantification of wall expansion reveals that the surface expansion rates are not highest at the pole but instead in an annular region around the pole. These findings raise the question of the precise localization of exocytosis events in these cells. Recently, we used spatio-temporal image correlation spectroscopy (STICS) in combination with high temporal resolution confocal imaging to characterize the intracellular movement of vesicles in growing pollen tubes. These observations, together with the analysis of FRAP (fluorescence recovery after photobleaching) experiments, indicate that exocytosis is likely to occur predominantly in the same annular region where wall expansion rates are greatest. Therefore, tip growth in plant cells does not seem to happen exactly at the tip.
Key words: tip growth, pollen tube, exocytosis, cell wall, expansion, root hair, plant cell growth, allometric growth, cytomechanics, cell mechanics, vesicle transport
Mechanics of Allometric Cell Growth
For plant cells to grow the wall surrounding the cell needs to be deformed mechanically, a process that is driven by the internal turgor pressure of the cell. In addition, cell wall material needs to be added to prevent thinning of the stretched wall and subsequent rupture. Most plant cells are characterized by diffuse growth where cell expansion implicates the deformation of the entire cell surface or large portions of it. In such cases, the mechanical anisotropy that oriented cellulose microfibrils confer to the cell surface plays a central role in controlling cell shape. On the other hand, allometric growth relies on local differences in the degree of surface expansion to create complex cell shapes.
An extreme case of allometric growth is realized in tip-growing cells where both surface expansion and delivery of new, primary cell wall material are confined to a very small cellular region. The result is a unidirectionally elongating, cylindrical cell that is capped by an approximately hemispherical apex—the growing region. The cell types that display tip growth—pollen tubes, root hairs and fungal hyphae—have the biological purpose to invade complex substrates—pistillar tissues, soil, or any digestible substance, respectively. Confining surface expansion to a small apical region allows tip-growing cells to negotiate mechanically complex environments by making their growth more manoeuverable and reducing friction with the surrounding substrate. The intense and localized growth activity observed in tip-growing cells make them ideal systems for mechanical and structural analyses of cellular growth processes.
Cell Wall Deformation in Tip-Growing Cells
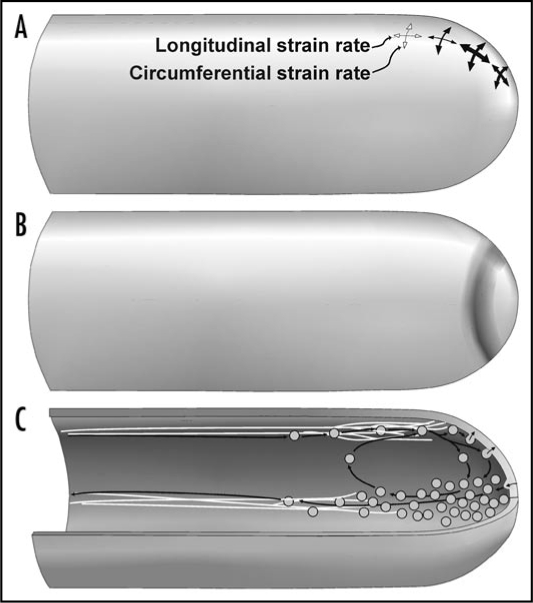
Early measurements of wall expansion in tip-growing cells have all supported the idea that the rate of wall expansion is maximal at the pole of the cell and declines gradually from this location towards the cylindrical portion of the cell.1–4 The first evidence that this expansion pattern might not be universal was obtained for root hairs.5,6 In these cells, the maximal strain rate occurs in an annular region centered at the pole (Fig. 1A). We have now obtained evidence that a similar wall expansion pattern is found in lily pollen tubes (unpublished data). Although structural analyses have failed to reveal any special cellular features that may help maintain this unusual expansion pattern, mechanical analyses do suggest that the apparent wall viscosity or stiffness is reduced in the annulus of high expansion rate.5,7 The reduced wall viscosity could be the result of increased activity of wall loosening agents such as expansins8,9 or reduced activity of pectin methylesterases (PME). An alternative explanation is that the annulus of high surface expansion is experiencing higher rates of secretion of uncross-linked wall material. This last possibility has gained ground following a recent investigation of vesicle dynamics in the tip of pollen tubes.10
Figure 1.
Drawing of the spatial distribution of mechanical features determining tip growth in plant cells. (A) Degree of deformation of the cell wall during its maturation. The thickness of the arrows indicates relative strain rates. (B) Position of the annular ring surrounding the pole of the tip growing cell. (C) Movement patterns of vesicles as observed with FM1–43 label.
Addition of Cell Wall Material
Since surface expansion in tip-growing cells is confined to the apical dome, it is reasonable to assume that addition of new wall material also occurs at this location. Fluorescent labelling experiments11–15 as well as ultrastructural observations16,17 support this conclusion although it has not been possible to map precisely the rates of wall deposition within this region. The unexpected finding that maximum surface expansion does not peak at the pole but in an annular region just below it raises the question of where exactly the addition of new wall material takes place. Mechanically, it is not absolutely necessary that the region of maximum exocytosis coincides with the region of maximum expansion (Fig. 1B). From the dense accumulation of vesicles in the shape of an inverted cone16,17 it was generally presumed that delivery of vesicle contents would occur at the pole. The small size of the vesicles (typically 150 nm in pollen tubes) made it difficult to track them quantitatively in the light or confocal microscope. Also, identifying the exact site of exocytosis in the apical region of the cell was impossible hitherto, even though studies using evanescent wave microscopy observed the dynamics of vesicles in the relatively slowly growing pollen tube of Picea meyeri.18 However, due to technical limitation in combination with cellular geometry, it is unlikely that in this study vesicles in the apical region were observed.
To quantify the movement rates and directions of vesicles in rapidly growing angiosperm pollen tubes, we therefore resorted to high temporal resolution confocal imaging in combination with computational analysis of cytoplasmic movements by spatio-temporal image correlation spectroscopy (STICS). Vesicles were labeled with the lipohilic styryl dye FM1–43 which belongs to a family of dyes that are known to be taken up by endocytosis and to enter the endosome and subsequently the exocytotic pathway.19–22 The resulting vector maps revealed that the source of vesicles streaming into the inverted cone is in a ring-shaped region at the periphery of the dome and that vesicles exit the cone in its tail (Fig. 1C). Computer modeling has shown that the geometry and activity of the actin cytoskeleton lead naturally to this type of vesicle circulation.23 A second remarkable finding of the high temporal resolution confocal microscopical observations was that groups of vesicles seemed to detach from the rearwards streaming tail of the cone and to re-enter the forward traffick in the periphery of the cell (Fig. 1C). This points to the existence of a circulatory delivery system that ensures an efficient target rate by repeated flow of vesicles through the apex.
In the context of cell wall mechanics, the most important finding of this study resulted from photobleaching (FRAP) experiments. We removed any excess dye in the culture medium and photobleached the entire apical region including vesicles and plasma membrane. During the subsequent recovery period any fluorescence appearing in the tip region was transported from the distal cytoplasmic region of the tube and thus represented the population of vesicles circulating through the apical region (and not newly endocytosed vesicles). To our surprise, newly delivered vesicles did not accumulate at the pole, which would have been expected if the peripheral influx of vesicles streamed together at this point. Instead, vesicles seemed to hover in an annular region around the pole before slowly filling the remainder of the inverted cone. Frequently, the cytoplasmic region immediately underlying the pole did not recover full fluorescence intensity after bleaching. The intense concentration of vesicles in this region as shown before bleaching and in transmission electron micrographs,17 is therefore likely to be partly the result of endocytotic activity. This is corroborated by observations using two different endocytosis markers in a pulse-chase type of experiment15 which clearly showed that one region of major endocytotic activity in the growing pollen tube is the pole of the cell.
Conclusions
The emerging picture for tip growth in pollen tubes is therefore the following: The maximum rate of cell wall deformation and deposition of pectic wall material is located in an annular region around the pole of the cell. One might be tempted to conclude that the pole itself represents an almost inert cap of cell wall material that is pushed forward by axially elongating flanks. However, it is important to realize that both exocytotic activity and cell wall deformation are not absent at the pole, their levels are just lower than in the annular region. Therefore, the pole remains a very dynamic region, certainly if one considers that endocytotic vesicles seem to pinch off into the cytoplasm at higher rates there than in the surrounding area.
and
Bernal R, Rojas E, Dumais J. The mechanics of tip growth morphogenesis: What we have learned from rubber balloons. J Mechan Mater Struct. 2007;2:1157–1168.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7633
References
- 1.Castle E. The topography of tip growth in a plant cell. J Gen Physiol. 1958;41:913–926. doi: 10.1085/jgp.41.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J. The kinetics of tip growth in the Nitella rhizoid. Plant Cell Physiol. 1973;14:631–640. [Google Scholar]
- 3.Hejnowicz Z, Heinemann B, Sievers A. Tip growth: Patterns of growth rate and stress in the Chara rhizoid. Zeitschrift für Pflanzenphysiologie. 1977;81:409–424. [Google Scholar]
- 4.Kataoka H. Colchicine-induced expansion of Vaucheria cell apex. Alteration from isotropic to transversally anisotropic growth. Bot Mag Tokyo. 1982;95:317–330. [Google Scholar]
- 5.Dumais J, Long SR, Shaw SL. The mechanics of surface expansion anisotropy in Medicago truncatula root hairs. Plant Physiol. 2004;136:3266–3275. doi: 10.1104/pp.104.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw SL, Dumais J, Long SR. Cell surface expansion in polarly growing root hairs of Medicago truncatula. Plant Physiol. 2000;124:959–969. doi: 10.1104/pp.124.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernal R, Rojas E, Dumais J. The mechanics of tip growth morphogenesis: What we have learned from rubber balloons. J Mech Mater Struct. 2007;2:1157–1168. [Google Scholar]
- 8.Cosgrove DJ. Cell wall loosening by expansins. Plant Physiol. 1998;118:333–339. doi: 10.1104/pp.118.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove DJ. Enzymes and others agents that enhance cell wall extensibility. Ann Rev Plant Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- 10.Bove J, Vaillancourt B, Kroeger J, Hepler P, Wiseman P, Geitmann A. Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy (STICS) Plant Physiol. 2008;147:1646–1658. doi: 10.1104/pp.108.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parton RM, Fischer-Parton S, Trewavas AJ, Watahiki MK. Pollen tubes exhibit regular periodic membrane trafficking events in the absence of apical extension. J Cell Sci. 2003;116:2707–2719. doi: 10.1242/jcs.00468. [DOI] [PubMed] [Google Scholar]
- 12.Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci. 2001;114:2685–2695. doi: 10.1242/jcs.114.14.2685. [DOI] [PubMed] [Google Scholar]
- 13.Camacho L, Malhó R. Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J Exp Bot. 2003;54:83–92. doi: 10.1093/jxb/54.380.83. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro D, Liu Q, Lisboa S, Scherer GEF, Quader H, Malhó R. Phophoinositides and phophatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c, and membrane secretion. J Exp Bot. 2005;56:1665–1674. doi: 10.1093/jxb/eri163. [DOI] [PubMed] [Google Scholar]
- 15.Zonia L, Munnik T. Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot. 2008;59:861–873. doi: 10.1093/jxb/ern007. [DOI] [PubMed] [Google Scholar]
- 16.Derksen J, Rutten T, Lichtscheidl IK, DeWin AHN, Pierson ES, Rongen G. Quantitative analysis of the distribution of organelles in tobacco pollen tubes: implications for exocytosis and endocytosis. Protoplasma. 1995;188:267–276. [Google Scholar]
- 17.Lancelle SA, Hepler PK. Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum. Protoplasma. 1992;167:215–230. [Google Scholar]
- 18.Wang X, Teng Y, Wang Q, Li X, Sheng X, Zheng M, et al. Imaging of dynamic secretory vesicles in living pollen tubes of Picea meyeri using evanescent wave microscopy. Plant Physiol. 2006;141:1591–1603. doi: 10.1104/pp.106.080168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belanger K, Quatrano R. Membrane recycling occurs during asymmetric tip growth and cell plate formation in Fucus distichus zygotes. Protoplasma. 2000;212:24–37. [Google Scholar]
- 20.Fischer-Parton S, Parton R, Hickey P, Dijksterhuis J, Atkinson H, Read N. Confocal microscopy of FM4–64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J Microsc. 2000;198:246–259. doi: 10.1046/j.1365-2818.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 21.Read ND, Hickey PC. The vesicle trafficking network and tip growth in fungal hyphae. In: Geitmann A, Cresti M, IB H, editors. Cell biology of plant and fungal tip growth. Amsterdam: IOS Press; 2001. pp. 137–146. [Google Scholar]
- 22.van Gisbergen PAC, Esseling-Ozdoba A, Vos JW. Microinjecting FM4–64 validates it as a marker of the endocytic pathway in plants. J Microsc. 2008;231:284–290. doi: 10.1111/j.1365-2818.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 23.Kroeger J, Bou Daher F, Grant M, Geitmann A. Microfilament orientation contrains vesicle flow and spatial distribution in growing pollen tubes. Biophys J. doi: 10.1016/j.bpj.2009.07.038. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]