Abstract
The WOX family of transcription factors and polar auxin transport (PAT) are both essential for embryonic patterning and thus normal embryo development in angiosperms. Recent analysis by us of WOX-related genes in Picea and Pinus suggests that they play fundamental roles during embryo development also in conifers.1 It has been proposed that there is a connection between the spatial separation of WOX2 and WOX8, and PAT in the formation of the apical-basal axis in Arabidopsis embryos and that both are involved in the regulation of the auxin efflux carrier PIN1. Auxin also seems to play a crucial role in apical-basal axis formation in conifer embryos based on studies using the polar auxin inhibitor NPA. We recently analyzed the expression of a PIN1-like gene in NPA-treated and untreated precotyledonary somatic spruce embryos and could see a significant upregulation of the PIN1-like gene in the NPA-treated embryos.2 Here we show that PaWOX2 is also significantly upregulated in the same embryos. Taken together, this suggests that PAT is involved in regulating both PIN1 and WOX2 expression in conifers and strengthens the evidence for the proposed connection between WOX and PIN genes in seed plants.
Key words: conifer, Picea abies, embryo development, polar auxin transport, WOX, PIN, NPA, somatic embryogenesis, qRT-PCR
In seed plants, embryogenesis can be divided conceptually into two phases; an early morphogenic phase and a late maturation phase. During morphogenesis, the primary body plan is established with regional specification of apical-basal and radial domains from which morphological structures derive. This process has been termed pattern formation and current knowledge about the genetic regulation of embryonic pattern formation in plants is primarily derived from studies on the model plant Arabidopsis thaliana.3–5 Recently, Haecker et al. identified a group of WUSCHEL-related homeobox (WOX) transcription factors expressed in a lineage-specific manner during early Arabidopsis embryogenesis.6 WOX2 and WOX8 are initially co-expressed in the egg cell and the zygote, and are then specifically expressed in the apical and basal cell lineages after zygotic division, suggesting that the two daughter cells assume different transcriptional profiles. Also, STIMPY/WOX9 is required to maintain cell division in the embryo and the suspensor, and the WOX genes thus appear to be intrinsic determinates in early asymmetric divisions during embryogenesis in Arabidopsis.7,8 Similar transcriptional profiles can also be seen in the monocot Zea mays indicating that the WOX gene family is ancient in the angiosperm lineage.9
Previously, we isolated and analyzed a WOX2 homologous gene from Picea abies (Norway spruce) that we named PaWOX2.1 Quantitative reverse transcription PCR (qRT-PCR) was used to follow the expression pattern of PaWOX2 during somatic embryo development and our results showed that it is highly expressed early during development, but declines as embryos mature. In seedlings there is very low expression of PaWOX2 and this is in line with earlier results from angiosperms.6,10 Furthermore, PaWOX2 expression seems to be linked to the proliferation rate of the embryogenic cell cultures. In addition to WOX2, several genes that cluster to WOX8/WOX9 are expressed in conifer embryos.1 This indicates that WOX genes play fundamental roles also during conifer embryo development, possible with functions related to regulating cell divisions and/or differentiation. It is tempting to consider that several of the WOX genes in Arabidopsis and conifers represent orthologous gene functions based on structural similarities of the genes as well as their expression profile, suggesting that they all have ancient functions associated with embryo development that existed prior to the separation of angiosperms and gymnosperms. Phylogenetic studies based on the homeodomain alone or performed on longer sequences together with other gene features support a distribution of genes into at least three evolutionary lineages with the WOX1 orthologous groups (OG) containing Arabidopsis AtWOX1–7 and AtWUS, WOX8 OG with AtWOX8, 9, 11 and 12 and the WOX13 OG including AtWOX10, 13 and 14.1,11 Genes belonging to WOX1 OG and WOX8 OG have so far only been identified in the seed plants, while the WOX13 gene clade appears to be ancestral to the other WOX gene clades, containing sequences from many different members of the plant kingdom, including Physcomitrella, Selaginella, gymnosperms and angiosperms.1,11
The polar transport of the plant growth factor auxin plays, in addition to the WOX genes, a crucial role in apical-basal axis formation in angiosperms.12–15 This transport is thought to be established and maintained by auxin efflux carriers of the PIN-FORMED (PIN) gene family, with the activity of PIN1, PIN4 and PIN7, leading to auxin gradients and local auxin maxima within the embryo prior to root and cotyledon formation and procambium differentiation.16,17
Recently a connection between the spatial separation of WOX transcription factors and polar auxin transport (PAT) in the formation of the main body axis in Arabidopsis embryos was shown,18 and earlier studies have also shown that auxin induces expression of WOX5 in the root of both Arabidopsis and Medicago truncatula.19,20 Furthermore, the expression of WOX9, a close homolog of WOX8, is altered in Arabidopsis mp mutant embryos and thus appears to be involved in auxin signaling, possibly through the TIR1-Aux/IAA-ARF pathway since MP (MONOPTEROS) is an auxin response factor (ARF).6 Auxin modulates the transcription of multiple PIN proteins through this pathway.21,22 For instance, MP positively regulates PIN1 expression and auxin translocation to the hypophysis, promoting the formation of the embryonic root.23 More recently, it was revealed by Breuninger et al. that WOX2 and WOX8 genes both cooperate with MP in PIN1 regulation.18 Conifer embryos contain several WOX8/9-like genes and, as noted earlier, PaWOX2 seems to have a function related to regulating cell divisions and/or differentiation in the embryos, something that is consistent with earlier results from angiosperms.1
Conclusions
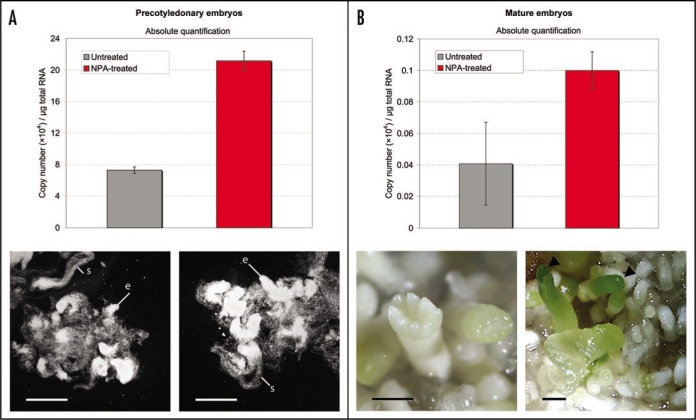
Based on this information we decided to analyze the PaWOX2 expression in Picea abies somatic embryos after treatment with the PAT inhibitor 1-napthylphtalamic (NPA). A recent study by us,2 along with a study by Larsson et al.24 have shown that growth of somatic embryos on medium containing NPA leads to the formation of embryos with poor meristem formation and fused cotyledons, and to a pin-formed phenotype of the regenerated plantlets similar to what is commonly seen on angiosperm embryos.15,25,26 Consequently, PAT seems to, like in angiosperms, play a crucial role in apical-basal axis formation in Picea abies. Furthermore, the most aberrant phenotype was seen if embryos were transferred to NPA-containing medium just before they had initiated cotyledons.2 These embryos also had a significant increase (13.7-fold) in the expression of a PIN1-like gene (accession no. FJ031883) compared to an untreated control. This is consistent with earlier observations in Arabidopsis showing that PIN expression is modified by exogenous application of auxin and auxin transport inhibitors.27 Since NPA had such a dramatic effect on the embryo morphology and PIN expression in somatic embryos we wanted to examine how the PaWOX2 expression was affected by NPA-treatment. When analyzing PaWOX2 expression in NPA-treated (1 µM) precotyledonary and mature embryos, both comparable in developmental stages to the stage 1–2 and stage 4 embryos, respectively, that we previously used in PaWOX2 analysis,1 we found that PaWOX2 was significantly upregulated in NPA-treated precotyledonary embryos (2.9-fold) compared to untreated embryos while we could see no significant difference in expression between NPA-treated and untreated mature embryos (Fig. 1). Taken together, our results show that both a PIN1-like gene and PaWOX2 are significantly upregulated in NPA-treated precotyledonary somatic embryos compared to the untreated control. Thus PAT seems to be involved in regulating both PIN and PaWOX2 expression in Picea abies during early embryo development, strengthening the evidence for the proposed connection between WOX and PIN genes in seed plants.18 This, interestingly, suggests a similar mechanism behind PAT regulation in angiosperms and gymnosperms.
Figure 1.
Absolute expression of PaWOX2 in embryos normalized against µg total RNA in untreated and NPA-treated (1 µM precotyledonary (A) and mature (B) somatic embryos, respectively. The untreated embryos (stage 1–2 that correspond to precotyledonary and stage 4 to mature embryos) along with the absolute qRT-PCR protocol used, including total RNA preparation and reverse transcription have earlier been described by Palovaara and Hakman.1 (A) NPA-treated precotyledonary embryos are comparable to the untreated embryos and no morphological differences can be detected. The suspensor (s) is well formed and the embryo proper (e) is dense in both of them. Dark field. Bars = 1 mm. (B) Mature NPA-treated embryos have fused cotyledons (arrowhead) while the untreated embryos have several cotyledons encircling the shoot apex. Bars = 1 mm. When comparing untreated and NPA-treated somatic embryos, means are statistically significant different (p < 0.05) between untreated and NPA-treated precotyledonary embryos, but not between mature untreated and NPA-treated embryos as evaluated by Student's t-test using STATISTICA v7.1 (StatSoft, Inc.). Each bar is the mean ± SE of triplicate assays.
Research concerning embryonic patterning in plants is advancing rapidly and this work has strengthened past groundwork for the future analysis of the evolutionary development and regulation of embryonic patterning in seed plants.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7684
References
- 1.Palovaara J, Hakman I. Conifer WOX-related homeodomain transcription factors, developmental consideration and expression dynamic of WOX2 during Picea abies somatic embryogenesis. Plant Mol Biol. 2008;66:533–549. doi: 10.1007/s11103-008-9289-5. [DOI] [PubMed] [Google Scholar]
- 2.Hakman I, Hallberg I, Palovaara J. The polar auxin transport inhibitor NPA impairs embryo morphology and increases the expression of an auxin efflux facilitator protein PIN during Picea abies somatic embryo development. Tree Physiol. 2009 doi: 10.1093/treephys/tpn048. (in press) [DOI] [PubMed] [Google Scholar]
- 3.Jürgens G. Apical-basal pattern formation in Arabidopsis embryogenesis. EMBO J. 2001;20:3609–3616. doi: 10.1093/emboj/20.14.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laux T, Würschum T, Breuninger H. Genetic regulation of embryonic pattern formation. Plant Cell. 2004;16:190–202. doi: 10.1105/tpc.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weijers D, Jürgens G. Auxin and embryo axis formation: the ends in sight. Curr Opin Plant Biol. 2005;8:32–37. doi: 10.1016/j.pbi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Chory J, Weigel D. Combinations of WOX activities regulate tissue proliferation during Arabidopsis embryonic development. Dev Biol. 2007;309:306–316. doi: 10.1016/j.ydbio.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ten Hove CA, Heidstra R. Who begets whom? Plant cell fate determination by asymmetric cell division. Curr Opin Plant Biol. 2007;10:1–8. doi: 10.1016/j.pbi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Nardmann J, Zimmermann R, Durantini D, Kranz E, Werr W. WOX gene phylogeny in Poaceae: a comparative approach addressing leaf and embryo development. Mol Biol Evol. 2007;24:2474–2484. doi: 10.1093/molbev/msm182. [DOI] [PubMed] [Google Scholar]
- 10.Malik MR, Wang F, Dirpaul JM, Zhou N, Polowick PL, Ferrie AMR, Krochko JE. Transcript profiling and identification of molecular markers for early microspor embryogenesis in Brassica napus. Plant Physiol. 2007;144:134–154. doi: 10.1104/pp.106.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deveaux Y, Toffano-Nioche C, Claisse G, Thareau V, Morin H, Laufs P, Moreau H, Kreis M, Lecharny A. Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol Biol. 2008;8:291. doi: 10.1186/1471-2148-8-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenik PD, Barton MK. Surge and destroy: the role of auxin in plant embryogenesis. Development. 2005;132:3577–3585. doi: 10.1242/dev.01952. [DOI] [PubMed] [Google Scholar]
- 13.Jenik PD, Gillmor CS, Lukowitz W. Embryonic Patterning in Arabidopsis thaliana. Ann Rev Cell Dev Biol. 2007;23:207–236. doi: 10.1146/annurev.cellbio.22.011105.102609. [DOI] [PubMed] [Google Scholar]
- 14.Schiavone FM. Micromanipulation of somatic embryos of the domesticated carrot reveals apical control of axis elongation and root regeneration. Development. 1988;103:657–664. [Google Scholar]
- 15.Liu C-M, Xu Z-H, Chua N-H. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell. 1993;5:621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 17.Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, Dhonukshe P, Skupa P, Benková E, Perry L, Krecek P, Lee OR, Fink GR, Geisler M, Murphy AS, Luschnig C, Zazímalová E, Friml J. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 18.Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell. 2008;14:867–876. doi: 10.1016/j.devcel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Gonzali S, Novi G, Loreti E, Paolicchi F, Poggi A, Alpi A, Perata P. A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. 2005;44:633–645. doi: 10.1111/j.1365-313X.2005.02555.x. [DOI] [PubMed] [Google Scholar]
- 20.Imin N, Nizamidin M, Wu T, Rolfe BG. Factors involved in root formation in Medicago truncatula. J Exp Bot. 2007;58:439–451. doi: 10.1093/jxb/erl224. [DOI] [PubMed] [Google Scholar]
- 21.Sauer M, Balla J, Lusching C, Wisniewska J, Reinohl V, Friml J, Benkova E. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006;20:2902–2911. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzel CL, Schuetz M, Yu Q, Mattsson J. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J. 2007;49:387–398. doi: 10.1111/j.1365-313X.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- 23.Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jürgens G. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev Cell. 2006;10:265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Larsson E, Sitbon F, Ljung K, von Arnold S. Inhibited polar auxin transport results in aberrant embryo development in Norway spruce. New Phytol. 2008;177:356–366. doi: 10.1111/j.1469-8137.2007.02289.x. [DOI] [PubMed] [Google Scholar]
- 25.Hadfi K, Speth V, Neuhaus G. Auxin-induced developmental patterns in Brassica juncea embryos. Development. 1998;125:879–887. doi: 10.1242/dev.125.5.879. [DOI] [PubMed] [Google Scholar]
- 26.Choi YE, Katsumi M, Sano H. Triiodobenzoic acid, an auxin polar transport inhibitor, suppresses somatic embryos formation and postembryonic shoot/root development in Eleutherococcus senticosus. Plant Sci. 2001;160:1183–1190. doi: 10.1016/s0168-9452(01)00357-0. [DOI] [PubMed] [Google Scholar]
- 27.Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell. 2004;16:1898–1911. doi: 10.1105/tpc.021501. [DOI] [PMC free article] [PubMed] [Google Scholar]