Introduction
Nitro-polycyclic aromatic hydrocarbons (Nitro-PAHs) are believed to account for a significant part of the mutagenicity of air-borne particulate matter1–5. In diesel particulates, approximately 40% of the direct-acting mutagenicity is believed to be due to nitro-PAHs1,6. Nitro-PAHs are formed as a result of incomplete combustion of organic materials both from natural events and human activities7–9. They are also formed in the atmosphere as PAHs react with NO2 or NO3 radicals10–15. Nitro-PAHs undergo photochemical reactions when exposed to sunlight. It has been proposed that the photochemical reaction mechanism for Nitro-PAHs is dependent on the orientation of the nitro group, whether co-planar or perpendicular to the aromatic rings2,11,16–19. A Nitro-PAH with its nitro group situated with two peri-hydrogens is forced into a perpendicular orientation to the aromatic moiety due to steric hindrance. Nitro groups with only one or no peri-hydrogen will maintain a parallel orientation to the aromatic moiety4. Nitro-PAHs with a perpendicular nitro group react faster under light irradiation due to a nitro to nitrite rearrangement as it was proposed20–24. Nitro-PAHs with a perpendicular nitro group are less mutagenic than those with a parallel nitro groups due to their inability to be metabolized into reactive intermediates that form covalent DNA adducts5,25. Nitro-PAHs with parallel nitro groups to the aromatic rings undergo photo-oxidation of the aromatic rings26–28.
In 9-nitroanthracene-like molecules, the nitro group is next to two peri hydrogens and preferentially adopts a perpendicular position to the aromatic rings. Upon absorbing light energy, the nitro group rearranges to a nitrite, which decomposes to a NO radical and phenoxy radical. The phenoxy radical then rearranges to become a carbon centered radical in the anthracene ring20,29,30. The NO radical will react with the most stable carbon radical on the opposite site of the nitro group in a concerted fashion and form a nitroso ketone, which is usually unstable and continues on to become anthraquinone. In the reaction with 10-chloro, cyano, benzoyl, and nitro substituted 9-nitroanthracenes, such a nitroso ketone intermediate was observed21,24,31.
In this report, 9-methyl-10-nitroanthracene and 12-methyl-7-nitrobenz[a]anthracene (Figure 1) were prepared to study the light-induced rearrangement reaction of the nitro group. The presence of the methyl group supposes to stabilize the nitroso ketone and thus to be isolated and characterized.
Figure 1.
Structures of nitroanthracene derivatives used for this study
Materials and Methods
9-Methylanthracene (9-MA), sodium nitrate, acetic anhydride and trifluoroacetic acid were purchased from Aldrich (Milwaukee, WI, USA) and used without further purification. HPLC analysis was carried out using a Shimadzu HPLC system with a RP-18 column (25 cm × 4.0 mm, 5 μm). The eluent used was 90% methanol in water. The flow rate was 1.0 mL/min, and detected at wavelength of 273 nm. Proton NMR was recorded with a Bruker 300 MHz NMR spectrometer as well as a JOEL 400 MHz spectrometer in CDCl3 or acetone-d6. GC-MS instrument used was Hewlett Packard 6890 gas chromatograph coupled to a Hewlett Packard 5973 mass selective detector. The instrument was equipped with a HP-5 MS (30 m × 250 μm × 0.25μm) column: The injector temperature was 250°C; carrier gas He at a constant flow rate of 1 mL/min; the oven temperature was held at 100°C for 2 min, and then heated to 280°C at 10°C/min. MS system: Ionization of the CI reagent gas was performed with 150 eV beam of electrons produced from a heated rhenium filament. Methane served as the reagent gas. The ion source temperature was held at 280°C. UV Absorption: UV spectra for the 9-Methyl-10-nitroanthracene were recorded on a CARY 300E UV-Vis absorption spectrophotometer from Varian Inc. (Houston, TX).
1. Light source
The light source was a 100-W UVA lamp (Type B, UVP, Upland, CA, USA) that produced a main emission band of 365 nm. A stream of cold air was blown across the bottom of the support during irradiation in an effort to displace any heat generated by the lamp.
2. Synthesis of 9-nitroanthracene-like nitro-PAHs
12-Methyl-7-nitrobenz[a]anthracene, 7-nitrobenz[a]anthracene, and 9-methyl-10-nitroanthracene were prepared by nitration of 12-methylbenz[a]anthracene, benz[a]anthracene, and 9-methylanthracene, respectively, with sodium nitrate (1:1 molar ratio) in trifluoroacetic acid/acetic anhydride (1/1, v/v) under argon at ambient temperature with stirring. The reaction products were partitioned between ethyl acetate and water containing a small amount of sulfuric acid (>1%). The organic layer was collected, washed with water, dried over anhydrous MgSO4, and the solvent was removed under reduced pressure. The yellowish residue was column chromatographed over silica gel. Elution with hexane gave the recovered substrate. Elution with hexane/ethyl acetate (2/1 v/v) gave the desired compounds with yields 70–80%. Spectral data of 7-nitrobenz[a]anthracene32,33 and 9-methyl-10-nitroanthracene34 matched the known compounds. 12-Methyl-7-nitrobenz[a]anthracene has not been synthesized before. The spectral data are as follows: MS- M/z: 287 (M+), 257 (M-NO)+ and 241 (M-NO2)+. 1H-NMR (acetone-d6): 3.43 (s, 3, CH3), 7.70 (d, 1, J5,6 = 10 Hz, H6), 7,65–7.88 (m, 4, H2,3,9,10), 8.02 (d, 1, H5), 8.13 (dd, 1, J3,4 = 9.5 Hz, H4), 8.23–8.37 (m, 2, H8,11), and 8.53 ppm (dd, 1, H1).
3. Photolysis of nitro-PAHs and isolation of nitroso ketone
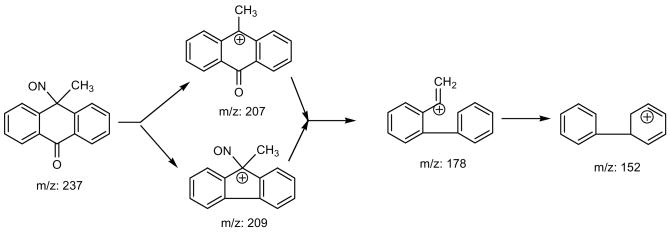
Photolysis of 9-methyl-10-nitroanthracene (0.1 mM) and other nitro-PAHs were carried out in methanol or chloroform for HPLC/TLC analysis. To isolate the photoproduct, 9-methyl-10-nitroanthracene (33 mg) was dissolved in freshly distilled CHCl3 (33 mL) in a Pyrex glass round bottom flask. The solution was irradiated with two UVA lamps for 180 min under stirring. The disappearance of 9-methyl-10-nitroanthracene was monitored by TLC. After the reaction was complete, the solvent was evaporated and the residue dissolved in 1 mL of ethyl acetate and was absorbed onto 41 mg of silica. The ethyl acetate in the silica was evaporated and remaining silica was loaded on the top of a silica gel column prepared with 350 mg of silica. The product was chromatographed using a solvent gradient of 0–5% ethyl acetate in hexane. Fractions containing the desired product were collected and the solvent evaporated at room temperature using a rotary evaporator to obtain a total of 9.7 mg of purified 9-methyl-9-nitrosoanthracene-10-one (yield 30%). 1H-NMR (CDCl3): 8.27 (dd, 2H); 7.97 (dd, 2H); 7.72 (td, 2H); 7.56 (td, 2H); and 1.70 ppm (s, 3H). MS: M+ 237. Fragments: 209 (M-CO), 207 (M-NO), 178 (M-HCONO), and 152 (M-HCONO & C2H2), but no M-NO2 fragment. IR (KBr): cm−1: 2964, 1650, 1600, 1322, 1261, 1095, 1022, 802, 694.
4. Photolysis kinetics
A 0.5 mg of 9-methyl-10-nitroanthracene or 12-methyl-7-nitrobenz[a]anthracene dissolved in 0.5 mL CDCl3 was placed in an NMR tube and kept in the dark until it was to be irradiated. The NMR tube was placed 43 mm above the UVA lamp and irradiated. A stream of cool air was blown across the top of the lamp during irradiation to eliminate heat. After each irradiation interval, an NMR spectrum was recorded. For the same experiments under argon or nitrogen atmosphere, argon or nitrogen gas was used to purge the solution in ice bath for 15 min before the NMR tube was sealed with a cap and rapped tightly with parafilm.
Results and Discussion
1. Photochemical reaction of 9-nitroanthracene-like chemicals and photoproduct purification and characterization
Figure 2 is the HPLC chromatogram of light irradiated 9-methyl-10-nitroanthracene (1 mM) solution in CHCl3 with a UVA lamp. Three photoproducts were detected, and they are numbered P0, P1, and P2. The same three products were also detected if CH3OH was used as solvent. P0 was unstable and difficult to be isolated, and P2 was identified as 9,10-anthraquinone by comparing its UV, NMR and MS spectra with the authentic sample. In order to identify the structure of P1, a preparative photolysis starting with 9-methyl-10-nitroanthracene in CHCl3 was carried out. Upon disappearance of the reactant as shown with TLC, P1 was isolated via silica gel chromatography and the resulting product was subjected to NMR, IR, and MS analysis.
Figure 2.
HPLC profiles of 9-methyl-10-nitroanthracene in CHCl3 (1 mM) irradiated for 30 min with a UVA lamp.
The MS spectrum of P1 has the molecular ion (m/z) of 237, the same molecular mass as the starting material 9-methyl-10-nitroanthracene, but with different ion patterns when compared with the starting material. The electron impact fragmentation of P1 has the following ions: 209 (M-CO), 207 (M-NO), 178 (M-HCONO), and 152 (M-HCONO & C2H2), but no M-NO2 fragment. This indicates that P1 is an isomer of 9-methyl-10-nitroanthracene without the nitro group. The 1H NMR spectra (Figure 3) of the purified P1 has four signals in the aromatic region 8.25, 7.95, 7.73, and 7.53 ppm for the four hydrogen atoms in the benzene ring and a signal resonates at 1.7 ppm for the methyl group. This indicates that there are no substitutions on the two benzene rings of anthracene. Combining the information, we assign P1 to be 9-methyl-9-nitrosoanthracen-10-one. The fragmentation of 209 (M-CO) is also supportive for 9-methyl-9-nitrosoanthracen-10-one. Scheme 1 depicts the pathway by which the respective ions in the mass spectrum are formed.
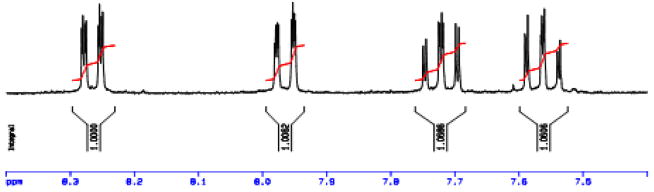
Figure 3.
1H-NMR of the aromatic region of 9-methyl-9-nitrosoanthracen-10-one
Scheme 1.
Possible pathways for the generation of electron impact fragmentations of P1
Photolysis of 7-nitrobenz[a]anthracene produced 7,12-benz[a]anthraquinone, identified through comparison with authentic sample. Irradiation of 12-methyl-7-nitrobenz[a]anthracene in CDCl3 in an NMR tube produced several products. After 30 min of irradiation, >80% of the starting compound disappeared as shown by the disappearance of the methyl signal at 3.4 ppm and other signals in the aromatic region. Several sets of aromatic signals and at least 3 singlet signals at 2–3 ppm (methyl) appeared, indicating that at least three rearrangement products formed. At the same time, a small signal at 9.8 ppm appeared. After 90 min of irradiation, the 3 methyl signals at 2–3 ppm nearly disappeared and the signal at 9.8 ppm increased. This doublet signal is a typical aldehyde proton with one neighboring proton. This indicates that irradiation of 12-methyl-7-nitrobenz[a]anthracene first converts it to at least three rearrangement products, which further converts into a 12-carboxaldehyde.
No aldehyde signal was observed when 9-methyl-10-nitroanthracene was irradiated. However, prolonged stay (6 months) of the isolated photoproduct, 9-methyl-9-nitrosoanthracen-10-one, in CDCl3 in an NMR tube wrapped with aluminum foil in a refrigerator did produce an aldehyde singal at 12 ppm. In comparison to the aldehyde formed from the 12-methyl-7-nitrobenz[a]anthracene which has a doublet signal at 9.8 ppm, this aldehyde signal is a singlet and is much further down field at 12 ppm. This indicates that the 12-carboxaldehyde in benz[a]anthracene-7-one adopts a ketone form and the 9-carboxaldehyde in anthracene-10-one adopts a hydroxyl form (Scheme 2). The large down field shift (>2 ppm) is due to the deshielding effect of the anthracene ring.
Scheme 2.
Mechanism of the photoreaction of 9-methyl-10-nitroanthracene and 7-nitro-12-methylbenz[a]anthracene in methanol or chloroform.
2. Kinetic analysis of the photoreaction
Since the photoreaction of 9-methyl-10-nitroanthracene dissolved in CHCl3 produces two main products, the photoreaction was followed by NMR by placing the 9-methyl-10-nitroanthracene solution in CDCl3 in an NMR tube and irradiated. The proton NMR spectrum was recorded at each irradiation time interval. Figure 4 shows the aromatic proton signals at irradiation times of 0, 15, 30, and 45 min, respectively, in ambient air. The majority of the starting material disappeared at 45 min, and was completely gone at 60 min. Meanwhile, 9-methyl-9-nitrosoanthracen-10-one and the 9,10-anthraquinone appeared. The same experiment was carried out for nitrogen and argon gas purged samples.
Figure 4.
1H-NMR spectra of 9-methyl-10-nitroanthracene in CDCl3 in ambient air irradiated for (from bottom to top): (a) 0 min, (b) 15 min, (c) 30 min, (d) 45 min.
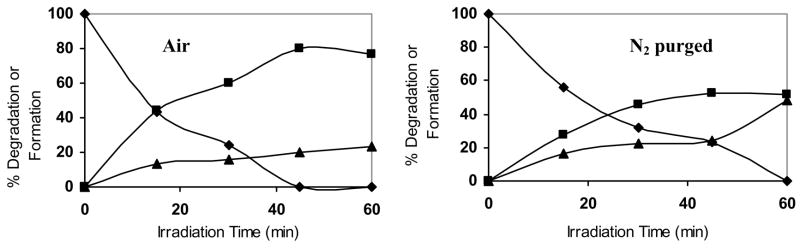
The progress of the photoreaction of 9-methyl-10-nitroanthracene in CDCl3 under ambient air or purged with N2 is plotted in Figure 5. As can be seen, the disappearance of the starting compound was faster in air than purged with N2. Treating the photolysis of 9-methyl-10-nitroanthracene in CDCl3 as a first order reaction (Ln([A]0/[A]t) = kt), the plot Ln([A]0/[A]t) vs irradiation time (t) yielded a straight line (data not shown). Thus the degradation half-life (t1/2 = 0.693/k) was determined. The degradation half-life for 9-methyl-10-nitroanthracene in CDCl3 under ambient air was 14 min, while it was 20 min when purged with N2. At the end of the photolysis (60 min), the ratio of 9-methyl-9-nitrosoanthracene-10-one/9,10-anthraquinone is 80/20 in ambient air and 60/40 when purged with nitrogen. The higher amount of 9,10-anthraquinone formed under nitrogen was surprising since it was thought that the quinone was transformed from the nitroso ketone via further oxidation. It clearly shows here that the 9,10-anthraquinone could be formed even under oxygen free system.
Figure 5.
Photolysis of 9-methyl-10-nitroanthracene (◆) transforms it into 9-methyl-9-nitroso-anthracene-10-one P1 (■) and 9,10-anthraquinone P2 (▲) in ambient air (left) or purged with N2 (right).
3. Mechanism of photoreaction
According to the HPLC, GC-MS and NMR analysis results, the proposed mechanism of photoreaction of 9-methyl-10-nitroanthracene is formulated in Scheme 2. Upon absorption of light energy, the molecule is promoted to excited singlet state, which intersystem crosses to the excited triplet state. The nitro group rearranges to a nitrite in the excited triplet state24,31,35. Breaking of the N-O bond of nitrite forms a nitroso radical and an oxygen-centered radical in the anthracene moiety. The nitroso radical either recombines to go back to nitrite or forms a C-N bond with the carbon on the opposite side of the benzene ring, the most reactive site in anthracene, thus, forming a nitroso ketone (9-methyl-9-nitrosoanthracen-10-one). In the case of 12-methyl-7-nitrobenz[a]anthracene, other carbons are also possible since more than one nitro ketones are seen in the NMR spectra. In the case of non-methyl substituted nitroanthracenes, photolysis of 9-nitroanthracene, and 7-nitrobenz[a]anthracene under the same conditions produced only the respective anthraquinones (data not shown). This is why 9,10-anthraquinone is usually the major photoproduct for the photolysis of 9-nitroanthracene and the nitroso ketone was not isolated. The 9-methyl-9-nitrosoanthracen-10-one is relatively stable and converts to an aldehyde after prolonged stay in the refrigerator without light irradiation. The 10-carboxyanthracene-9-one adopts an enol form since the aldehyde hydrogen is a singlet in proton NMR. In contrast, the 12-methyl-7-nitrobenz[a]anthracene rearrangement product nitroso ketones are not stable and readily converts to the aldehyde (12-carboxaldehyde-benz[a]anthracene-7-one). The doublet of the aldehyde hydrogen indicates that this aldehyde adopts a ketone form due to steric constraint of the extra benzene ring. In the presence of a substituent in the opposite position of the nitro group in anthracene, benzoyl, chloro, cyano, or nitro21,24, the formation of the nitroso ketone was observed. The chloronitroso and nitronitroso ketones were isolated with low yields (12%). The majority of the photoproduct was anthraquinone. In our study with the methyl substituent, more than 80% of the photoproducts were the nitroso methyl ketone when irradiated under air. When bubbled with nitrogen or argon, the amount of the nitroso ketone decreased to about 60%. The 9-methyl-9-nitrosoanthracen-10-one is stable in the solid state. The chloroform solution of 9-methyl-9-nitrosoanthracen-10-one in an NMR tube was relatively stable. However, some of the nitroso ketone converted to anthracene aldehyde after the solution was left in refrigerator for 6 months.
However, prolonged irradiation of the 9-methyl-9-nitrosoanthracen-10-one converted it into the anthraquinone. Therefore, we believe the photochemical reaction of 9-nitroanthracene follows the mechanism above. The conversion of the 9-methyl-9-nitrosoanthracen-10-one to anthraquinone appears to be independent of oxygen. The conversion of the gem-chloronitrosoanthracene or gem-nitronitrosoanthracene to the quinine was also not dependent on oxygen21. It was proposed that gem-chloronitroso or gem-nitronitroso in aliphatic systems readily converts into ketone36, not depending on oxygen.
In conclusion, light irradiation of 9-nitro-substituted anthracene-like molecules causes the nitro group to rearrange to become a nitrite followed by a concerted rearrangement reaction that places the nitroso group on the electron-rich carbon on the opposite end of the six-membered aromatic ring, and at the same time, the phenolic oxygen to become a ketone.
Acknowledgments
This research has been supported by the National Institutes of Health through a generous grant: NIH-RCMI G12 RR13459. This research has also been supported by the “211” program of Central University for nationalities of China. GS wishes to thank the US Department of Education for stipend support through the Title III grant: P031B040101-07.
Footnotes
This article is not an official U.S. Food and Drug Administration guidance or policy statement. No official support or endorsement by the U.S. Food and Drug Administration is intended or should be inferred.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pitts JN, Jr, Lokensgard DM, Harger W, Fisher TS, Mejia V, Schuler JJ, Scorziell GM, Katzenstein YA. Mutagens in diesel exhaust particulate Identification and direct activities of 6-nitrobenzo[a]pyrene, 9-nitroanthracene, 1-nitropyrene, and 5H-phenanthro[4,5-bcd]pyran-5-one. Mutat Res. 1982;103:241–249. doi: 10.1016/0165-7992(82)90049-5. [DOI] [PubMed] [Google Scholar]
- 2.Fu PP. Metabolism of Nito-Polycyclic Aromatic Hydrocarbons. Drug Metab Rev. 1990;22:209–268. doi: 10.3109/03602539009041085. [DOI] [PubMed] [Google Scholar]
- 3.Löfroth G, Nilsson L, Agurell E, Yasuhara A. Salmonella/microsome mutagenicity of 1-nitropyrene-2-ol, a nitropyrene phenol formed in the photolysis of 1-nitropyrene. Z Naturforsch. 1984;39:193–195. doi: 10.1515/znc-1984-1-235. [DOI] [PubMed] [Google Scholar]
- 4.Fu PP, Heflich RH, Unruh LE, Shaikh AU, Wu YS, Lai CC, Lai JS. Relationships among direct-acting mutagenicity, nitro group orientation and polarographic reduction potential of 6-nitrobenzo[a]pyrene, 7-nitrobenz[a]anthracene and their derivatives. Mutat Res. 1988;209:115–122. doi: 10.1016/0165-7992(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 5.Fu PP, Ni YC, Zhang YM, Heflich RH, Wang YK, Lai JS. Effect of the orientation of the nitro substituent on the bacterial mutagenicity of dinitrobenzo[e]pyrenes. Mutat Res. 1989;225:121–125. doi: 10.1016/0165-7992(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 6.Fu PP, Herrero-Saenz D. Nitro-polycyclic aromatic hydrocarbons: A class of genotoxic environmental pollutants. Environ Carcinog & Ecotox Rev. 1999;C17:1–43. [Google Scholar]
- 7.Heeb NV, Schmid P, Kohler M, Gujer E, Zennegg M, Wenger D, Wichser A, Ulrich A, Gfeller U, Honegger P, Zeyer K, Emmenegger L, Petermann JL, Czerwinski J, Mosimann T, Kasper M, Mayer A. Secondary Effects of Catalytic Diesel Particulate Filters: Conversion of PAHs versus Formation of Nitro-PAHs. Environ Sci Technol. 2008;42:3773–3779. doi: 10.1021/es7026949. [DOI] [PubMed] [Google Scholar]
- 8.Howsam M, Jones KC. Sources of PAHs in the environment. In: Neilson AH, editor. PAHs and Related Compounds. Springer-Verlag; Berlin, Germany: 1998. pp. 137–174. [Google Scholar]
- 9.Srogi K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ Chem Lett. 2007;5:169–195. doi: 10.1007/s10311-007-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arey J, Atkinson R. Photochemical reactions of PAHs in the atmosphere. In: Douben PET, editor. PAHs: An Ecotoxicological Perspective. Wiley & Sons Ltd; Chichester, England: 2003. pp. 47–63. [Google Scholar]
- 11.Pitts JN, Jr, Van Cauwenberghe KA, Grosjean D, Schmid JP, Fitz DR. Atmospheric Reactions of Polycyclic Aromatic Hydrocarbons: Facile Formation of Mutagenic Nitro Derivatives. Science. 1978;202:515–519. doi: 10.1126/science.705341. [DOI] [PubMed] [Google Scholar]
- 12.Reisen F, Arey J. Atmospheric Reactions Influence Seasonal PAH and Nitro-PAH Concentrations in the Los Angeles Basin. Environ Sci Technol. 2005;39:64–73. [PubMed] [Google Scholar]
- 13.Fan Z, Chen D, Birla P, Kamens RM. Modeling of Nitro-Polycyclic Aromatic Hydrocarbon Formation and Decay in the Atmosphere. Atmos Environ. 1995;29:1171–1181. [Google Scholar]
- 14.Atkinson R, Arey J. Atmospheric chemistry of gas-phase polycyclic aromatic hydrocarbons: formation of atmospheric mutagens. Environ Health Perspect. 1994;102(suppl 4):117–126. doi: 10.1289/ehp.94102s4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arey J. Atmospheric reactions of PAHs including formation of nitroarenes. In: Neilson AH, editor. PAHs and Related Compounds. Springer-Verlag; Berlin, Germany: 1998. pp. 347–385. [Google Scholar]
- 16.Fu PP, Chou MW, Beland FA. Effects of nitro substitution on the in vitro metabolic activation of polycyclic aromatic hydrocarbons. In: Yang SK, Silverman BD, editors. Polycyclic Aromatic Hydrocarbon Carcinogenesis: Structure-Activity Relationships. II. CRC Press; Boca Raton, Florida: 1988. pp. 37–65. [Google Scholar]
- 17.Yu H. Environmental Carcinogenic Polycyclic Aromatic Hydrocarbons: Photochemistry and Phototoxicity. J Environ Sci Health, Part C. 2002;C20:149–183. doi: 10.1081/GNC-120016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu PP, Herrero-Saenz D. Nitro-polycyclic aromatic hydrocarbons: A class of genotoxic environmental pollutants. J Environ Sci Health: Environ Carcinog Ecotox Rev. 1999;C17:1–43. [Google Scholar]
- 19.Phousongphouang PT, Arey J. Rate constants for the photolysis of the nitronaphthalenes and methylnitronaphthalenes. J Photochem Photobiol A: Chemistry. 2003;157:301–309. [Google Scholar]
- 20.Chapman OL, Heckert DC, Reasoner JW, Thackaberry SP. Photochemical Studies on 9-Nitroanthracene. J Am Chem Soc. 1966;88:5550–5554. [Google Scholar]
- 21.Galiani G, Rindone B. The photolysis of 9-nitroanthracene derivatives. Gazz Chim Ital. 1977;107:435–436. [Google Scholar]
- 22.Ioki Y. Aryloxyl radicals by Photorearrangement of Nitro-compounds. J Chem Soc, Perkin Trans. 1977;2:1240–1242. [Google Scholar]
- 23.Hamanoue K, Amano M, Kimoto M, Kajiwara Y, Nakayama T, Teranishi H. Photochemical Reactions of Nitroanthracene Derivatives in Fluid Solutions. J Am Chem Soc. 1984;106:5993–5997. [Google Scholar]
- 24.Hamanoue K, Nakayama T, Amijima Y, Ibuki K. A rapid decay channel of the lowest excited singlet state of 9-benzoyl-10-nitroanthracene generating 9-benzoyl-10-anthryloxy radical and nitrogen(II)oxide. Chem Phys Lett. 1997;267:165–170. [Google Scholar]
- 25.Umbuzeiro GA, Franco A, Martins MH, Kummrow F, Carvalho L, Schmeiser HH, Leykauf J, Stiborova M, Claxton LD. Mutagenicity and DNA adduct formation of PAH, nitro-PAH, and oxy-PAH fractions of atmospheric particulate matter from Sao Paulo, Brazil. Mutat Res. 2008;652:72–80. doi: 10.1016/j.mrgentox.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Holloway MP, Biaglow MC, McCoy EC, Anders M, Rosenkranz HS, Howard PC. Photochemical instability of 1-nitropyrene, 3-nitrofluoranthene, 1,8-dinitropyrene and their parent polycyclic aromatic hydrocarbons. Mutat Res. 1987;187:199–207. doi: 10.1016/0165-1218(87)90037-1. [DOI] [PubMed] [Google Scholar]
- 27.Stärk G, Stauff J, Miltenburger HG, Stumm-Fischer I. Photodecomposition of 1-nitropyrene and other direct-acting mutagens extracted from diesel-exhaust particulates. Mutat Res. 1985;155:27–33. doi: 10.1016/0165-1218(85)90021-7. [DOI] [PubMed] [Google Scholar]
- 28.Iversen B, Greibrokk T. Identification of the decomposition products of nitrobenzanthracenes in solution. Anal Chim Acta. 1985;174:317–322. [Google Scholar]
- 29.Fukuhara K, Kurihara M, Miyata N. Photochemical Generation of Nitric Oxide from 6-Nitrobenzo[a]pyrene. J Am Chem Soc. 2001;123:8662–8666. doi: 10.1021/ja0109038. [DOI] [PubMed] [Google Scholar]
- 30.Crespo-Hernandez C, Burdzinski G, Arce R. Environmental Photochemistry of nitro-PAHs: Direct observation of ultrafast intersystem crossing in 1-nitropyrene. J Phys Chem A. 2008;112:6313–6319. doi: 10.1021/jp803847q. [DOI] [PubMed] [Google Scholar]
- 31.Hamanoue K, Nakayama T, Ushida K, Kajiwara K, Yamanaka S. Photophysics and Photochemistry of Nitroanthracenes Part 1. - Primary Processes in the Photochemical Reactions of 9-Benzoyl-10-nitroanthracene and 9-Cyano-10-nitroanthracene by Steady-state Photolysis and Nanosecond Laser Photolysis. J Chem Soc Faraday Trans. 1991;87:3365–3371. [Google Scholar]
- 32.Iversen B, Sydnes LK, Greibrokk T. Characterization of nitrobenzanthracenes and nitrodibenzanthracenes. Acta Chem Scandin B: Org Chem Biochem. 1985;39:837–847. [Google Scholar]
- 33.Newman MS, Lilje KC. Synthesis of 7-fluorobenz[a]anthracene. J Org Chem. 1979;44:1347–8. [Google Scholar]
- 34.Armillotta N, Bartoli G, Bosco M, Dalpozzo R. Conjugate addition of Grignard reagents to nitroarenes: a new synthesis of 9-alkylanthracenes, 9-nitro-10-alkylanthracenes, and 10,10-dialkylanthrones. Synthesis. 1982:836–9. [Google Scholar]
- 35.Hamanoue K, Nakayama T, Kajiwara K, Yamanaka S, Ushida K. Photophysics and Photochemistry of Nitroanthracenes Part 2.† Primary Process in the Photochemical Reaction of 9-Nitroanthracene studied by Steady-state Photolysis and Laser Photolysis. J Chem Soc Faraday Trans. 1992;88:3145–3151. [Google Scholar]
- 36.Kornblum N, Blackwood RK, Mooberry DD. The reaction of aliphatic nitro compounds with nitrite esters. J Am Chem Soc. 1956;78:1502–1504. [Google Scholar]