Abstract
Background: ST elevation in precordial leads has been associated with genetic syndromes of arrhythmias and sudden death. ST height data in different ethnic groups are limited.
Methods: ST height was determined in 4612 African‐American, Chinese, Hispanic, and non‐Hispanic white men and women aged 45–84 years in the Multiethnic Study of Atherosclerosis (MESA). For leads I, II, and V1 to V6, ST height, measured at the J point and 60 ms after the J point, adjusted for covariates were compared between non‐Hispanic white and other ethnic groups using analysis of covariance (ANCOVA).
Results: Among men, ST height was significantly different across all ethnic groups at both time points for all leads (P < 0.01), except at the J point for limb lead II (P = 0.2). Among women, differences were also significant at the J point and 60 ms past the J point (P < 0.01). ST height was lowest for non‐Hispanic whites in all leads and at both time points. At the J point, Chinese had the highest ST height for leads V1 and V2, whereas African Americans had the greatest ST height for leads I and V3 to V6. At 60 ms past the J point, Chinese men had the greatest ST height for lead I and V1 to V6; and Chinese women had greatest ST height for leads V1 to V3.
Conclusions: There were significant differences in ST height among ethnic groups in all ECG leads. The physiological mechanisms and clinical significance of these differences and the possible association with arrhythmias require further study.
Keywords: ECG, ethnic, ST height, repolarization
Elevation of ST height in the absence of coronary artery disease was first described in 1936 by Shipley and Hallaran. 1 This phenomenon has been referred to as “unusual RT segment deviation,”“normal RST elevation variant,”“Benign Early Repolarization,” and finally “Early Repolarization.” 2 , 3 Although nonischemic ST elevation had been thought to be “benign,” patients with Brugada syndrome who have right bundle branch block and ST elevation in the right precordial leads are at increased risk for sudden death. 4 In addition, polymorphisms in genes coding for ion channels appear to differ across ethnic groups; some of these polymorphisms might lead to ST elevations associated with sudden death. 5 Finally, ethnic differences in the incidence of sudden death have been described and could be related to ethnic differences in repolarization properties. 6 , 7 Thus, a better understanding of population and ethnic distribution of ST height could provide information as to whether ST elevation is a marker of arrhythmic risk.
Although initially described in non‐Hispanic whites, ST elevation appears to be present in all ethnic groups. In one study, early repolarization had 0.9% overall prevalence; the incidence is 1.8% and 0.2%, respectively, in men and women. 8 We are aware of only two studies that examined racial/ethnic differences in ST height or repolarization. Using data from the Atherosclerosis Risk in Communities Study, Vitelli et al. 9 found that ST height in leads V2 to V5 was statistically significantly higher in African Americans compared to Caucasians. In a population‐based study conducted by Klatsky et al., 8 the incidence of early repolarization, as determined by blinded cardiologists' reading, was assessed. In that study, which contained predominantly African Americans and Caucasians and a limited number of Asians (less than 10%), the incidence of early repolarization was 1.5% in African Americans, 0.6% in Caucasians, and 1.6% in Asians.
To date, there are no studies comparing ST height among African‐Americans, Asians, Hispanics, and non‐Hispanic white men and women. The Multiethnic Study of Atherosclerosis (MESA) is a longitudinal study of the health of older men and women from each of the four ethnic groups with measurements of lifestyle, anthropometric, and coronary heart disease risk factors, and ST height measured at baseline. A comparison of ST height across ethnic groups is of particular interest because of the ethnic differences in inherited arrhythmia syndromes and in sudden death unassociated with the known syndromes noted above.
METHODS
Study Population
MESA is a multicenter, longitudinal cohort study designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease (CVD). Between July 2000 and August 2002, 6814 men and women aged 45–84 years who were free of clinical CVD at baseline and self‐identified as African American, Chinese, Hispanic, or Caucasian were recruited from six US communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota. A detailed description of the design, recruitment, and cohort examination procedures has been published previously. 10 All participants gave informed consent and the MESA protocol was approved by the Institutional Review Boards at each participating site.
For this analysis, we excluded participants who did not have ST heights measured (n = 48), leaving 6766 of the 6814 participants. In addition, exclusions were made for: QRS duration greater than 120 ms (n = 286), weight greater than 300 lbs (n = 11), presence of major ECG abnormalities defined by the Novacode system, 11 left‐ventricular hypertrophy by ECG (n = 580), or missing systolic BP (SBP; n = 1). Patients currently taking the following medications: antiarrhythmics, beta‐blockers, digitalis preparations, diltiazem, or verapamil (n = 705) were excluded. Finally, 538 women who were premenopausal and 33 women who were missing data on the use of hormone therapy (HT) were excluded. The final analytic cohort size was 4612.
Data Collection
Participants were asked to fast for 12 hours and to avoid smoking and heavy physical activity for 2 hours before each exam. Information on age, gender, race/ethnicity, medical conditions, and current use of prescription and nonprescription medications were collected by questionnaire. Weight and height were measured using a balance scale and stadiometer with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as the weight (kg) divided by the height squared (m2). Blood pressure was measured after sitting quietly for 5 minutes using the Dinamap BP Machine (GE Medical Systems, Waukesha, WI). Blood pressure measurements were taken in triplicate and the average of the last two measurements used in analysis.
Cardiac magnetic resonance imaging was performed using scanners with 1.5‐T magnets (Signa LX and CVi, GE Electric Medical Systems, and Somatom Vision and Sonata, Siemens Medical Solutions, Malvern, PA). Imaging was performed with a four‐element, phased‐array surface coil placed anteriorly and posteriorly, electrocardiogram gating, and brachial artery pressure blood pressure monitoring. Imaging consisted of cine images of the left ventricle with time resolution less than 50 ms, as well as phase contrast flow images of the ascending aorta. Readings were performed centrally at the Department of Radiology, Johns Hopkins University School of Medicine. Left ventricular (LV) mass index was determined with a commercially available software package (MASS, version 4.2, Medis, Leesburg, VA).
Ten seconds of simultaneous 12‐lead electrocardiograms (ECGs) were obtained with the participant in the resting supine position using a Marquette MAC‐1200 instrument (GE Medical) and read electronically after transmission over analogue phone lines to a central ECG reading center blinded to all clinical and personal details of the participants. ECG technicians were trained to make a special effort to reduce chest electrode placement errors, thereby reducing interindividual variability and improving the consistency of serial ECG recordings. Careful attention was paid to proper identification of the fourth and fifth intercostal spaces for correct level of the chest electrodes and the left midaxillary line for the V6 electrode location. In addition, a special electrode locator 12 was used for positioning of the V4 electrode at a 45° angle between the midsternal and left midaxillary lines at the fifth intercostal space. Electrodes V3 and V5 were then located in a straight line halfway between electrodes V2 and V4, and V4 and V6, respectively. Limb leads were placed on the wrists and ankles. Abnormalities were elicited by a program for using Novacode criteria 11 and the Minnesota Code criteria. 13 ECG analysis was performed centrally at Wake Forest University. Each ECG was sampled simultaneously with a sampling rate of 500 samples per second. Data on ST height were determined at, the J point, and 60 ms after J point (J60). In this study, we used ST height at the J point and 60 ms past the J point at limb lead I and II and at chest leads V1 to V6, and these represent 8 independent leads. The chest leads were chosen for more detailed analysis because ST height in the chest leads has been associated with inherited syndromes of sudden death and because of the desire to minimize the number of multiple comparisons that were performed. ST height was measured at the J point and 60 ms after the J point because different ion channels may be active at these times. All measurements were automated. Details of the analysis methodology have previously been reported. Measurements later in the ST segment were not analyzed because the focus of this article was on early repolarization.
Statistical Analysis
All continuous variables were normally distributed. Standard errors (SE) rather than standard deviations (SD) are presented because adjusted means were utilized and SD cannot be accurately calculated. Within each gender, age‐adjusted baseline characteristics were compared across the various ethnic groups using analysis of covariance (ANCOVA). Age‐adjusted least‐square mean ST heights were computed for the ethnic groups and compared using ANCOVA. Age‐ and ethnicity‐adjusted Pearson partial correlation coefficients were computed between ST‐height variables and participant characteristics for each gender. ANCOVA was also used to assess overall ethnic differences in ST height adjusted for age, BMI, heart rate, blood pressure, or HT use (in women only).
RESULTS
Demographics
Among the 4612 participants included in this analysis, 51.6% were male and 48.4% were female. For men, 38.7%, 13.3%, 24.1%, and 23.9% were Non‐Hispanic white, Chinese, African American, and Hispanic, respectively. For women, the respective distributions were 37.9%, 12.1%, 27.4%, and 22.6%. As shown in Table 1, for both men and women, non‐Hispanic whites were slightly older and Hispanics younger compared to the other ethnic groups. On average, the age‐adjusted BMI was highest in African‐American and Hispanic participants and lowest in the Chinese. For men, there were small differences in heart rate across ethnic groups that ranged from 62.0 beats per minute for non‐Hispanic white men to 63.2 beats per minute for Chinese men. For women, the age‐adjusted average heart rate was similar for Non‐Hispanic whites, African Americans, and Hispanics; and was slightly lower for Chinese. Among both men and women, SBP was highest for African Americans, and among men it was lowest for Chinese but for women SBP was lowest among non‐Hispanic whites. Among Hispanics, LV mass index was highest and among Chinese LV mass index was lowest.
Table 1.
Characteristics Adjusted for Age of MESA Participants by Sex and Ethnicity
| Non‐Hispanic White | Chinese | African American | Hispanic | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | |
| Men | ||||||||
| N | 922 | 316 | 575 | 569 | ||||
| Age (yrs) | 61.2 | (0.32) | 61.2 | (0.57) | 60.8 | (0.42) | 59.8 | (0.42) |
| BMI (kg/m2) | 27.8 | (0.14) | 23.9 | (0.23) | 28.5 | (0.17) | 28.5 | (0.17) |
| Heart rate (per min) | 62.0 | (0.32) | 63.2 | (0.54) | 62.3 | (0.40) | 63.1 | (0.41) |
| SBP (mmHg) | 122.5 | (0.57) | 121.3 | (0.98) | 127.7 | (0.72) | 123.9 | (0.73) |
| LV mass index (n = 1613) | 36.0 | (0.27) | 34.2 | (0.45) | 38.8 | (0.35) | 40.1 | (0.34) |
| Women | ||||||||
| N | 845 | 270 | 611 | 504 | ||||
| Age (yrs) | 64.2 | (0.31) | 63.9 | (0.54) | 63.6 | (0.36) | 63.1 | (0.43) |
| BMI (kg/m2) | 27.1 | (0.19) | 23.9 | (0.33) | 30.8 | (0.22) | 29.7 | (0.24) |
| Heart rate (per min) | 64.8 | (0.32 | 63.4 | (0.56) | 64.8 | (0.37) | 64.8 | (0.41) |
| SBP (mmHg) | 122.5 | (0.72 | 123.4 | (1.27) | 133.1 | (0.84) | 127.6 | (0.93) |
| LV mass index (n = 1796) | 32.2 | (0.27) | 31.7 | (0.46) | 36.0 | (0.34) | 37.0 | (0.36) |
| Hormone therapy | 48% | 23% | 27% | 23% | ||||
BMI = body mass index; SBP = systolic blood pressure; LV = left ventricular.
Age‐Adjusted ST Height among Ethnic Groups
As shown in Table 2, in general, the age‐adjusted ST height was consistently highest among the Chinese in the right precordial leads (i.e., V1 and V2) while African Americans had a higher ST height in the anteriolateral leads (i.e., leads V3–V6), and Non‐Hispanic whites had the lowest ST height in most leads.
Table 2.
Least Square Mean ST height in μV Adjusted for Age in MESA Participants by Sex and Ethnicity
| Non‐Hispanic White | Chinese | African American | Hispanic | P Value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | ||
| Men | |||||||||
| ST Height at J Point | |||||||||
| Limb lead | |||||||||
| I | 19.9 | (18.4–21.4) | 26.8 | (24.2–29.4) | 32.3 | (30.6–34.2) | 26.2 | (24.3–28.2) | <0.0001 |
| II | 24.5 | (22.5–26.5) | 32.5 | (29.0–35.9) | 24.0 | (21.5–26.6) | 25.8 | (23.2–28.4) | 0.0005 |
| Chest lead | |||||||||
| V1 | 6.4 | (4.3–8.5) | 21.7 | (18.1–25.3) | 13.9 | (11.3–16.6) | 11.6 | (8.9–14.2) | <0.0001 |
| V2 | 49.2 | (45.6–52.7) | 96.4 | (90.4–102.4) | 81.2 | (76.7–85.6) | 60.5 | (56.0–64.9) | <0.0001 |
| V3 | 38.3 | (34.9–41.6) | 78.3 | (72.6–83.9) | 68.3 | (64.1–72.5) | 47.5 | (43.3–51.7) | <0.0001 |
| V4 | 24.1 | (21.2–27.0) | 49.2 | (44.3–54.1) | 48.5 | (44.9–52.2) | 30.4 | (26.8–34.1) | <0.0001 |
| V5 | 19.7 | (17.5–21.8) | 31.0 | (27.2–34.7) | 33.2 | (30.4–35.9) | 24.9 | (22.1–27.7) | <0.0001 |
| V6 | 21.4 | (19.7–23.0) | 27.1 | (24.2–30.0) | 27.5 | (25.4–29.6) | 24.3 | (22.1–26.4) | <0.0001 |
| ST Height at 60 ms | |||||||||
| Limb lead | |||||||||
| I | 29.7 | (28.2–31.3) | 43.7 | (41.4–46.3) | 40.8 | (38.9–42.7) | 37.6 | (35.6–39.5) | <0.0001 |
| II | 30.9 | (29.2–32.6) | 41.3 | (38.4–44.3) | 32.0 | (29.8–34.1) | 33.6 | (31.4–35.9) | <0.0001 |
| Chest lead | |||||||||
| V1 | 43.2 | (40.7–45.7) | 63.2 | (59.0–67.5) | 57.0 | (53.9–60.2) | 54.4 | (51.2–57.6) | <0.0001 |
| V2 | 141.0 | (136.4–145.6) | 211.0 | (203.2–218.9) | 184.8 | (179.0–190.6) | 166.2 | (160.4–172.1) | <0.0001 |
| V3 | 116.8 | (112.7–121.0) | 183.6 | (176.5–190.7) | 154.5 | (149.2–159.7) | 132.5 | (127.2–137.8) | <0.0001 |
| V4 | 76.6 | (73.4–79.9) | 124.4 | (118.8–129.9) | 102.1 | (98.0–106.3) | 86.7 | (82.6–90.9) | <0.0001 |
| V5 | 42.2 | (39.9–44.5) | 70.2 | (66.2–74.1) | 55.3 | (52.4–58.2) | 51.2 | (48.3–54.2) | <0.0001 |
| V6 | 24.2 | (22.5–25.9) | 41.6 | (38.6–44.5) | 30.4 | (28.3–32.6) | 29.4 | (27.2–31.6) | <0.0001 |
| Women | |||||||||
| ST Height at J Point | |||||||||
| Limb lead | |||||||||
| I | 13.7 | (12.2–15.2) | 16.2 | (13.6–18.9) | 28.4 | (26.6–30.2) | 19.3 | (17.3–21.2) | <0.0001 |
| II | 14.8 | (12.9–16.7) | 23.8 | (20.4–27.2) | 19.5 | (17.2–21.8) | 19.5 | (16.9–22.0) | <0.0001 |
| Chest lead | |||||||||
| V1 | 2.0 | (0.2–3.9) | 11.8 | (8.5–15.1) | 3.8 | (1.6–6.0) | 2.7 | (0.3–5.1) | <0.0001 |
| V2 | 23.9 | (20.9–26.9) | 53.6 | (48.4–58.9) | 43.0 | (39.6–46.5) | 29.7 | (25.9–33.5) | <0.0001 |
| V3 | 11.6 | (9.0–14.2) | 34.2 | (29.6–38.8) | 33.5 | (30.4–36.5) | 18.8 | (15.5–22.2) | <0.0001 |
| V4 | 6.2 | (3.9–8.4) | 20.1 | (16.0–24.1) | 28.0 | (25.3–30.7) | 12.6 | (9.6–15.5) | <0.0001 |
| V5 | 6.0 | (4.0–7.9) | 16.2 | (12.7–19.7) | 24.5 | (22.2–26.8) | 14.0 | (11.4–16.5) | <0.0001 |
| V6 | 11.8 | (10.2–13.3) | 17.4 | (14.7–20.2) | 22.9 | (21.1–24.8) | 17.0 | (15.0–19.0) | <0.0001 |
| ST Height at 60 ms | |||||||||
| Limb lead | |||||||||
| I | 11.1 | (9.8–12.4) | 20.4 | (18.1–22.7) | 24.6 | (23.0–26.1) | 17.8 | (16.1–19.5) | <0.0001 |
| II | 18.8 | (17.2–20.3) | 23.3 | (20.6–26.1) | 23.0 | (21.1–24.8) | 22.5 | (20.5–24.5) | 0.0007 |
| Chest lead | |||||||||
| V1 | 34.7 | (32.7–36.7) | 48.1 | (44.6–51.7) | 43.4 | (41.1–45.8) | 40.9 | (38.4–43.5) | <0.0001 |
| V2 | 83.6 | (80.2–87.0) | 124.9 | (118.9–130.9) | 111.9 | (107.9–115.9) | 99.0 | (94.6–103.4) | <0.0001 |
| V3 | 60.3 | (57.4–63.3) | 94.4 | (89.2–99.6) | 82.8 | (79.4–86.3) | 71.4 | (67.6–75.2) | <0.0001 |
| V4 | 36.2 | (33.8–38.6) | 60.2 | (55.9–64.4) | 59.0 | (56.1–61.8) | 46.8 | (43.7–49.9) | <0.0001 |
| V5 | 19.5 | (17.5–21.5) | 35.2 | (31.7–38.7) | 37.5 | (35.2–39.9) | 30.1 | (27.5–32.6) | <0.0001 |
| V6 | 8.8 | (7.4–10.3) | 19.7 | (17.1–22.3) | 18.6 | (16.9–20.3) | 15.7 | (13.9–17.6) | <0.0001 |
*P value for comparison to nonwhite Hispanic Group. Details of intergroup comparisons are included in the test.
Correlations of ST Height with Demographic and CHD Risk Factors
After adjustment for age and ethnicity, ST height was inversely correlated with BMI at the J point and 60 ms past the J point for all leads for men, and results were generally similar for women (Table 3). Heart rate also was inversely correlated with ST height at most leads among men, and among women, the inverse correlations were statistically significant for all leads at the J point, but only for limb leads I and II at 60 ms past the J point. For both men and women, SBP was inversely correlated with ST height at nearly all leads, except at V1 and V2 where there was a positive correlation between SBP and ST height. At both the J point and 60 ms past the J point, LV mass index was inversely correlated with ST height in the limb leads for men and women, and in the chest lead V5 and V6 for men. Conversely, ST height was positively associated with LV mass at chest leads V1 to V3 in both genders at both time points.
Table 3.
Age‐ and Ethnicity‐Adjusted Pearson Partial Correlations between ST Height Variables and MESA Participant Characteristics
| I | II | V1 | V2 | V3 | V4 | V5 | V6 | |
|---|---|---|---|---|---|---|---|---|
| ST height at the J point | ||||||||
| Men (n = 2382) | ||||||||
| BMI | −0.08b | −0.16b | −0.03 | −0.18b | −0.23b | −0.21b | −0.20b | −0.18b |
| Heart rate | −0.14b | −0.08b | 0.01 | −0.10b | −0.16b | −0.17b | −0.16b | −0.17b |
| SBP | −0.15b | −0.16b | 0.14b | 0.06b | −0.03 | −0.12b | −0.18b | −0.21b |
| LV mass index (n = 1796) | −0.04 | −0.11b | 0.16b | 0.13b | 0.09b | −0.00 | −0.10b | −0.12b |
| Women (n = 2230) | ||||||||
| BMI | 0.04 | −0.00 | −0.12b | −0.19b | −0.14b | −0.08b | −0.05a | −0.02 |
| Heart rate | −0.19b | −0.07b | 0.06b | −0.07b | −0.11b | −0.12b | −0.13b | −0.15b |
| SBP | −0.15b | −0.04a | 0.14b | 0.04 | −0.04 | −0.09b | −0.13b | −0.16b |
| LV mass index (n = 1613) | 0.00 | −0.04 | 0.07b | 0.04 | 0.02 | −0.02 | −0.03 | −0.04 |
| ST height at 60 ms past the J point | ||||||||
| Men (n = 2382) | ||||||||
| BMI | −0.03 | −0.25b | −0.07b | −0.19b | −0.25b | −0.20b | −0.16b | −0.16b |
| Heart rate | 0.00 | −0.05a | 0.01 | −0.03 | −0.05a | −0.03 | −0.02 | −0.05b |
| SBP | −0.15b | −0.18b | 0.11b | 0.05b | −0.02 | −0.08b | −0.17b | −0.21b |
| LV mass index | −0.04 | −0.13b | 0.17b | 0.16b | 0.12b | 0.02 | −0.08b | −0.14b |
| Women (n = 2230) | ||||||||
| BMI | 0.02 | −0.08b | −0.10b | −0.17b | −0.11b | −0.03 | 0.02 | 0.02 |
| Heart rate | −0.05a | −0.05a | 0.04 | −0.02 | −0.02 | −0.01 | −0.01 | −0.04 |
| SBP | −0.17b | −0.09b | 0.13b | 0.05a | −0.00 | −0.04 | −0.10b | −0.16b |
| LV mass index | −0.02 | −0.07b | 0.13b | 0.11b | 0.08b | 0.06a | 0.03 | −0.05 |
BMI = body mass index; SBP = systolic blood pressure; LV = left ventricular.
a0.01 < P < 0.05.
bP ≤ 0.01.
Multivariable‐Adjusted ST Height among Ethnic Groups
As shown in Figures 1A through 1D, gender‐specific ST heights were compared across ethnic groups after adjustment for age, BMI, heart rate, blood pressure, or HT use (in women only) (See Appendix for actual multivariable‐adjusted mean ST height values). Among men, ST height was statistically significantly different across all ethnic groups at both time points for all leads (P < 0.01), except at the J point for limb lead II (P = 0.2). Among women, all differences were also statistically significant at the J point and at 60 ms past the J point (P < 0.01). Among men and women, ST height was consistently lowest for non‐Hispanic whites at all leads compared with that of the other ethnic groups. At the J point, the Chinese men and women had the highest ST height for leads V1 and V2, whereas African‐American men and women had the highest ST height for leads I and V3 to V6. At 60 ms past the J point, Chinese men had the highest ST height for leads I and V1 to V6 and Chinese women had highest ST height for leads V1 to V3. Data on LV mass were not available in all patients and thus LV mass was not included in the primary multivariate analysis. However, when univariable analysis was performed, LV mass adjustment did not alter ethnic differences in ST height.
Figure 1.
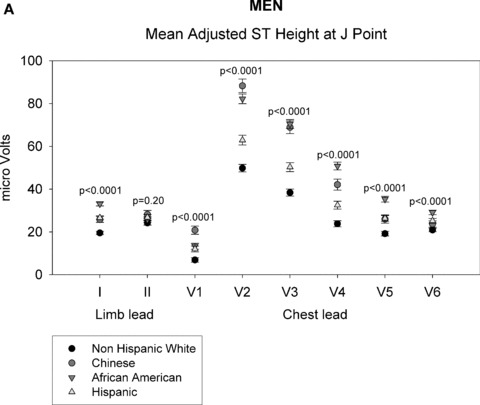
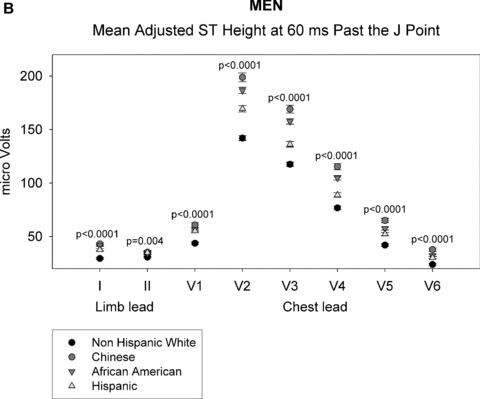
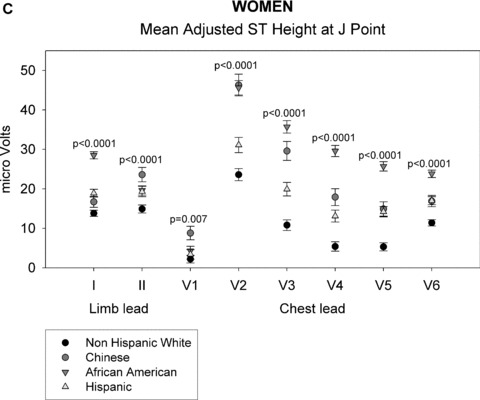
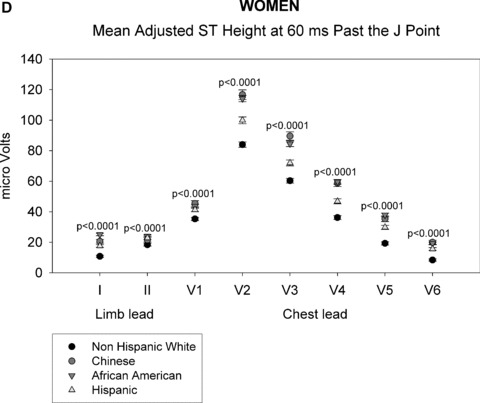
ST height in men (A and B). (A) ST height in μV in men at the J point. ST height was adjusted for age, BMI, heart rate, blood pressure, and hormonal therapy use. The four ethnic groups are shown with symbols denoted by the figure legend. Differences in ST height were seen among the four racial groups in leads V1 through V6. (B) Mean ST height 60 ms after the J point in men, adjusted as described in part A. The figure is organized as in part A. Significant differences among the four ethnic groups were found in all eight leads that were examined. ST height in women (C and D). (C) ST height at the J point in women, adjusted as described in the legend of part A. Significant differences among ethnic groups were seen in all eight leads examined. (D) Mean ST height at 60 ms after the J point in women, adjusted as described in part A. The figure is organized as described in part A. Significant differences among ethnic groups were seen in all eight leads that were examined.
DISCUSSION
The results of this study show that after adjusting for covariates there are significant ethnic differences in early myocardial repolarization. ST height was consistently lowest in non‐Hispanic white men and women in all leads at both time points. At the J point, Chinese had the highest ST height in the right precordial leads whereas African Americans had the highest ST height in mid precordial leads. At 60 ms past the J point, Chinese had the highest ST height in all the leads except for V4 measurements in women. The reasons for the ethnic differences are unknown, but might have important implications for ethnic differences in sudden death and in the response to ion channel blocking drugs. 6 , 7 , 8
Genesis of Nonischemic Myocardial ST Height
The action potentials originating from different areas of the ventricle differ both morphologically and in action potential duration. Transmural differences in action potential dispersion (APD) through the ventricular wall, with APD being shortest in epicardial myocytes, have been reported in different species, including humans. 4 , 14 , 15 Similarly, apicobasal heterogeneity in APD in the ventricular wall and differences in APD between left and right ventricles also have been reported. 16 , 17 These differences are caused by variations in the ion channels in areas across the myocardium. The transmural difference in APD is likely responsible for the genesis of physiologic ST elevation. While not arrhythmogenic under resting conditions, 18 these differences could be arrhythmogenic in the presence of an inherited ion channel abnormality or in response to perturbations.
Ethnic Differences in ST Height
Early repolarization has been shown in a number of studies to be more common in African Americans than other ethnic groups and to be more common in men than women. 8 Our study shows similar results with ST height in men being much higher than women and ST height in African Americans to be higher in the mid and lateral leads (V3 toV6) when compared to other ethnic groups. However, in contrast to prior studies, the right precordial leads (V1 and V2) in Chinese showed the highest ST height when compared to other ethnic groups (V2 had the highest magnitude of ST height of all the leads). These are the same leads that show ST elevation in Brugada syndrome. Abnormalities in the sodium channels have been shown to produce ST elevation in V1 and V2 in Brugada syndrome but only a minority of cases have an ion channel abnormality that has already been defined. 19 There are specific polymorphisms in the sodium channel that have been described and they can be associated with difference in the occurrence of arrhythmias. The results of this study support the concept that other ethnic differences in ion channel polymorphisms exist and could be related to phonotypical differences in presenting arrhythmias. The greater ST height in Chinese in V1 and V2 could be due to a stronger right ventricular epicardial Ito current compared to other racial groups or could be due to differences in the distribution of other ion channels. This strong right ventricular epicardial current in turn may be related to the type of K+ channel (Kv1.4, Kv1.7, Kv4.2, and Kv4.3) 20 , 21 or due to a racial difference in K channel modulators like cytoplasmic proteins, such as KChIP (Kv channel interaction proteins), frequenin, and KChAP (Kv channel‐associated channel). 22 Ethnic differences in the instances of sudden death have been previously described. Zheng et al. showed that African Americans had a higher death rate then Caucasians or other minority groups whereas Hispanics had a lower death rate. Hamaad et al. showed that Indo‐Asians had lower death rates than Caucasians did, but a direct comparison to the study by Zheng et al. is not possible since the studies were conducted in different countries and the ethnic distribution of minority groups may be different in those populations. 6 , 7 A better understanding of ethnic variations in both ST height and in the instance of sudden death will be required to determine whether there is a strong association between these differences.
Physical Factors Associated with ST Height
In our study, BMI, heart rate, and SBP were weakly, and mostly inversely, associated with ST height. In particular, the inverse association of BMI with ST height could be explained by: (1) increase in the distance/impedance in transmission of electrical currents from the heart to the electrodes secondary to fatty tissue, and (2) fatty acids and their metabolites and arachidonic acid inhibits Ito density with prolongation of AP in rat myocytes. 23 , 24 In humans, the relationship between Ito and AP duration is more complex but could still influence APD. In addition to BMI, we also found an inverse relationship of heart rate with ST height. This finding is consistent with that of Lehmann et al. 25 It is important to note that in our study, the association of heart rate with ST height was strongest and most consistent among the leads at the J point, whereas at 60 ms past the J point the relation of heart rate to ST height was smaller and was evident in only a few leads.
Implications
There are ethnic differences in the ST height. African Americans had higher ST height in the anterior and lateral leads while Chinese had a higher ST height in the right precordial leads. This difference in distribution of ST height is possibly due to the difference in the distribution of ion channels, which could explain the difference in the distribution of arrhythmias and the different response to antiarrhythmic drugs in different racial groups. Further studies will be required to establish the mechanism of racial difference in ion channels distribution.
LIMITATIONS
Although significant differences among ethnic groups were seen in almost all leads that were examined, the differences did not exceed 60 μV. The clinical significance of these differences remains to be determined but it should be noted that although some of the differences were small in absolute terms, for comparisons particularly in the chest leads, a 30–40% relative difference among ethnic groups was noted.
Acknowledgments
Acknowledgments: The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions.
Table APPENDIX.
Least square mean ST height in μV adjusted for age, BMI, heart rate, blood pressure or hormone therapy (in women only) in MESA participants by sex and ethnicity
| Non‐Hispanic White | Chinese | African American | Hispanic | P value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | ||
| MEN | |||||||||
| ST Height at J point | |||||||||
| Limb lead | |||||||||
| I | 19.5 | (0.76) | 25.8 | (1.36) | 33.1 | (0.97) | 26.6 | (0.97) | <0.0001 |
| II | 24.2 | (1.01) | 28.2 | (1.82) | 25.8 | (1.29) | 26.8 | (1.30) | <0.20 |
| Chest lead | |||||||||
| V1 | 6.9 | (1.06) | 20.8 | (1.91) | 13.3 | (1.36) | 12.0 | (1.36) | <0.0001 |
| V2 | 49.8 | (1.76) | 88.3 | (3.16) | 82.2 | (2.24) | 62.9 | (2.25) | <0.0001 |
| V3 | 38.4 | (1.64) | 68.9 | (2.95) | 70.4 | (2.09) | 50.3 | (2.10) | <0.0001 |
| V4 | 23.8 | (1.42) | 42.1 | (2.56) | 50.8 | (1.82) | 32.5 | (1.82) | <0.0001 |
| V5 | 19.2 | (1.07) | 26.0 | (1.93) | 35.3 | (1.37) | 26.3 | (1.37) | <0.0001 |
| V6 | 20.9 | (0.83) | 23.7 | (1.49) | 29.1 | (1.06) | 25.2 | (1.06) | <0.0001 |
| ST Height at 60 ms | |||||||||
| Limb lead | |||||||||
| I | 29.5 | (0.77) | 43.0 | (1.38) | 41.6 | (0.98) | 37.6 | (0.98) | <0.0001 |
| II | 30.8 | (0.85) | 35.3 | (1.53) | 34.1 | (1.08) | 35.0 | (1.09) | <0.004 |
| Chest lead | |||||||||
| V1 | 43.7 | (1.26) | 60.7 | (2.27) | 56.8 | (1.61) | 55.2 | (1.61) | <0.0001 |
| V2 | 142.0 | (2.30) | 198.8 | (4.14) | 186.6 | (2.94) | 169.4 | (2.94) | <0.0001 |
| V3 | 117.5 | (2.05) | 169.1 | (3.69) | 157.6 | (2.62) | 136.2 | (2.63) | <0.0001 |
| V4 | 76.8 | (1.63) | 115.3 | (2.92) | 104.8 | (2.07) | 88.8 | (2.08) | <0.0001 |
| V5 | 42.0 | (1.15) | 65.0 | (2.08) | 57.5 | (1.47) | 52.3 | (1.48) | <0.0001 |
| V6 | 23.9 | (0.85) | 37.8 | (1.52) | 32.3 | (1.08) | 30.2 | (1.08) | <0.0001 |
| WOMEN | |||||||||
| ST Height at J point | |||||||||
| Limb lead | |||||||||
| I | 13.8 | (0.77) | 16.7 | (1.38) | 28.5 | (0.91) | 18.9 | (0.98) | <0.0001 |
| II | 14.9 | (1.02) | 23.6 | (1.82) | 19.6 | (1.20) | 19.3 | (1.29) | <0.0001 |
| Chest lead | |||||||||
| V1 | 2.2 | (0.96) | 8.8 | (1.72) | 4.3 | (1.13) | 3.4 | (1.22) | <0.007 |
| V2 | 23.6 | (1.53) | 46.3 | (2.74) | 45.6 | (1.81) | 31.1 | (1.94) | <0.0001 |
| V3 | 10.8 | (1.35) | 29.6 | (2.41) | 35.7 | (1.59) | 19.9 | (1.71) | <0.0001 |
| V4 | 5.4 | (1.19) | 17.9 | (2.13) | 29.6 | (1.41) | 13.1 | (1.51) | <0.0001 |
| V5 | 5.3 | (1.02) | 14.9 | (1.82) | 25.7 | (1.20) | 14.2 | (1.29) | <0.0001 |
| V6 | 11.4 | (0.80) | 16.9 | (1.43) | 23.8 | (0.95) | 17.0 | (1.02) | <0.0001 |
| ST Height at 60 ms | |||||||||
| Limb lead | |||||||||
| I | 10.8 | (0.67) | 20.6 | (1.21) | 25.1 | (0.80) | 17.6 | (0.86) | <0.0001 |
| II | 18.4 | (0.81) | 21.6 | (1.45) | 24.0 | (0.95) | 22.8 | (1.03) | <0.0001 |
| Chest lead | |||||||||
| V1 | 35.3 | (1.03) | 45.4 | (1.84) | 43.6 | (1.22) | 41.3 | (1.31) | <0.0001 |
| V2 | 84.0 | (1.75) | 116.7 | (3.14) | 114.1 | (2.07) | 99.9 | (2.23) | <0.0001 |
| V3 | 60.3 | (1.53) | 89.6 | (2.74) | 84.5 | (1.81) | 72.0 | (1.94) | <0.0001 |
| V4 | 36.2 | (1.27) | 58.8 | (2.27) | 59.6 | (1.50) | 46.8 | (1.61) | <0.0001 |
| V5 | 19.3 | (1.04) | 35.4 | (1.85) | 37.9 | (1.22) | 29.8 | (1.32) | <0.0001 |
| V6 | 8.4 | (0.76) | 20.0 | (1.35) | 19.2 | (0.89) | 15.6 | (0.96) | <0.0001 |
*p‐value compares differences in mean ST Height across all ethnic groups and is computed from ANCOVA.
Supported by contracts N01‐HC‐95159 through N01‐HC‐95165, 1R01 HL075382 01, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org . MESA Coordinating Center, University of Washington, Seattle, WA.
REFERENCES
- 1. Shipley R, Halloran W. The four‐lead electrocardiogram in two hundred men and women. Am Heart J 1936;11:325–345. [Google Scholar]
- 2. Wasserburger RH AW, Lloyd CJ. The normal RS‐T segment elevation variant. Am J Cardiol 1961;8:84–92. [DOI] [PubMed] [Google Scholar]
- 3. Spodick, DH . Early repolarization: An underinvestigated misnomer. Clin Cardiol 1997;20:913–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antzelevitch C FJ. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol 2001;96:517–527. [DOI] [PubMed] [Google Scholar]
- 5. Shin DJ KE, Park SB, Jang WC, et al A novel mutation in the SCN5 A gene is associated with Brugada syndrome. Life Sci 2007;80:716–724. [DOI] [PubMed] [Google Scholar]
- 6. Zheng ZJ CJ, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158–2163. [DOI] [PubMed] [Google Scholar]
- 7. Hamaad A GA, Hirani F, Lip GY, et al Sudden death is less common than might be expected in underprivileged ethnic minorities at high cardiovascular risk. Int J Cardiol 2006;107:235–239. [DOI] [PubMed] [Google Scholar]
- 8. Klatsky AL OR, Cooper RA, Udaltsova N, et al The early repolarization normal variant electrocardiogram: Correlates and consequences. Am J Med 2003;115:171–177. [DOI] [PubMed] [Google Scholar]
- 9. Vitelli LL CR, Shahar E, Hutchinson RG, et al Electrocardiographic findings in a healthy biracial population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Cardiol 1998;81:453–459. [DOI] [PubMed] [Google Scholar]
- 10. Bild DE BD, Burke GL, Detrano R, et al Multi‐ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 11. Rautaharju PM PL, Chaitman BR, Rautaharju F, et al The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol 1998;31:157–187. [PubMed] [Google Scholar]
- 12. Rautaharju PM PL, Rautaharju FS, Crow R. A standardized procedure for locating and documenting ECG chest electrode positions. Consideration of the effect of breast tissue on ECG amplitudes in women. J Electrocardiology 1998;31:17–29. [DOI] [PubMed] [Google Scholar]
- 13. Prineas RJ, Crow RS, Blackburn HW. The Minnesota Code Manual of Electrocardiographic Findings: standards and procedures for measurement and classification. Boston : J. Wright, 1982. [Google Scholar]
- 14. Aiba T, SW, Inagaki M, Hidaka I, et al Transmural heterogeneity of the action potential configuration in the feline left ventricle. Circ J 2003;67:449–454. [DOI] [PubMed] [Google Scholar]
- 15. Drouin E CF, Gauthier C, Laurent K, et al Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: Evidence for presence of M cells. J Am Coll Cardiol 1995;26:185–192. [DOI] [PubMed] [Google Scholar]
- 16. Cheng J, KK, Liu W, Tsuji Y, et al Heterogeneous distribution of the two components of delayed rectifier K+ current: A potential mechanism of the proarrhythmic effects of methanesulfonanilide class III agents. Cardiovasc Res 1999;43:135–147. [DOI] [PubMed] [Google Scholar]
- 17. Di Diego JM SZ, Antzelevitch C. I(to) and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physio 1996;271:H548–H561. [DOI] [PubMed] [Google Scholar]
- 18. Shu J, Zhu T, Yang L, et al ST‐segment elevation in the early repolarization syndrome, idiopathic ventricular fibrillation, and the Brugada syndrome: Cellular and clinical linkage. J Electrocardiol 2005;38:26–32. [DOI] [PubMed] [Google Scholar]
- 19. Letsas KP EM, Pappas LK, Gavrielatos G, et al Early repolarization syndrome: Is it always benign? Int J Cardiol 2006;114:390–392. [DOI] [PubMed] [Google Scholar]
- 20. JM N. Molecular basis of functional voltage‐gated K+ channel diversity in the mammalian myocardium. J Physiol 2000;525:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deal KK ES, Tamkun MM. Molecular physiology of cardiac potassium channels. Physiol Rev 1996;76:49–67. [DOI] [PubMed] [Google Scholar]
- 22. Gavin Y, Oudit ZK, Rajan S, et al The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol 2001;33:851–872. [DOI] [PubMed] [Google Scholar]
- 23. Damron DS VWD, Moravec CS, Bond M. Arachidonic acid and endothelin potentiate Ca2+ transients in rat cardiac myocytes via inhibition of distinct K+ channels. J Biol Chem 1993;268:27335–27444. [PubMed] [Google Scholar]
- 24. Xu ZRG. K+ current inhibition by amphiphilic fatty acid metabolites in rat ventricular myocytes. Am J Physiol 1998;275:C1660–C1667. [DOI] [PubMed] [Google Scholar]
- 25. Lehmann MH YH. Sexual dimorphism in the electrocardiographic dynamics of human ventricular repolarization: Characterization in true time domain. Circulation 2001;104:32–38. [DOI] [PubMed] [Google Scholar]


