Summary
The synthesis of apolipoprotein B (apoB) dictates the formation of chylomicrons and very low density lipoproteins (VLDL), two major lipoprotein precursors in the human plasma. Despite its biological significance, the mechanism of the assembly of these apoB-containing lipoproteins remains elusive. An essential obstacle is the lack of systems that allow fine dissection of key components during assembly, including nascent apoB peptide, lipids in defined forms, chaperones, and microsomal triglyceride transfer protein (MTP). In this study, we use a prokaryotic cell-free expression system to reconstitute early events in the assembly of apoB-containing lipoprotein that involve the N-terminal domains of apoB. Our study shows that the N-terminal domains larger than 20.5% of apoB (B20.5) have an intrinsic ability to remodel vesicular phospholipid bilayers into discrete protein-lipid complexes. The presence of appropriate lipid substrates during apoB translation plays a pivotal role for successful lipid recruitment, and similar lipid recruitment fails to occur if the lipids are added posttranslationally. Cotranslational presence of MTP can dramatically promote the folding of B6.4–20.5 and B6.4–22. Furthermore, apoB translated in the presence of MTP retains its phospholipid recruitment capability posttranslationally. Our data suggest that during the synthesis of apoB, the N-terminal domain has a short window for intrinsic phospholipid recruitment, the timeframe of which is predetermined by the environment where apoB synthesis occurs. The presence of MTP prolongs this window of time by acting as a chaperone. The absence of either proper lipid substrate or MTP may result in the improper folding of apoB and consequently its degradation.
Keywords: apolipoprotein B, microsomal triglyceride transfer protein, cell free, cotranslational, low density lipoprotein
Introduction
Apolipoprotein B (apoB) is the essential protein responsible for the formation of chylomicrons from the small intestine and very low density lipoproteins (VLDL) from the liver. These two types of lipoproteins transform into a series of plasma lipoproteins, which are implicated in both normal lipid homeostasis and a variety of pathological conditions. Full length apoB is a 4563 amino acid glycoprotein, produced primarily in the liver 1. In the small intestine, a tissue specific deaminase generates a stop codon at position 2153 of the nascent mRNA, which gives rise to B48, the N-terminal 48% of apoB, that is present in chylomicrons 2; 3. The translation of apoB begins with a 24 or 27 amino acid signal peptide that targets the synthesis of apoB to the rough endoplasmic reticulum (ER) of hepatocytes and enterocytes, thus committing the protein to a secretory pathway 4.
So far two factors have been found specifically associated with the efficiency of the assembly and secretion of apoB-containing lipoproteins – the availability of lipids and microsomal triglyceride transfer protein (MTP). Mounting evidence has demonstrated the correlation between triglyceride synthesis and lipoprotein secretion. With low lipid availability, apoB is targeted for degradation, which can occur cotranslationally 5; 6; 7. A key factor during apoB maturation is MTP, a protein when in deficiency causes abetalipoproteinemia 8; 9. MTP is an ER resident protein, which in vitro can transfer lipids, mostly triglyceride, between vesicles 10. In most studies, a positive association between MTP and the efficiency of lipoprotein secretion has been observed, despite the fact that the level of contribution seems to vary in different cell lines 11; 12. MTP physically interacts within the N-terminal 13% of apoB (B13) 13; 14; 15. Both the physical interaction as well as the lipid shuttling activities are required for the secretion of apoB-containing lipoproteins 16. Besides MTP, a number of eukaryotic chaperones interact with apoB during its maturation, including BiP, GRP94, ERp72, calreticulin and cyclophilin B 17. The nature of these interactions has not been studied as thoroughly.
Based on the primary sequence, apoB can be divided into five superdomains βα1-β1-α2-β2-α3, with α and β representing domains containing predominant α-helical or β-sheet structures respectively 18. Studies have shown that apoB sequences as short as 19.5% or 22% can be secreted with significant lipids 19; 20. Thus, essential elements for the initiation of lipoprotein formation reside in the very N-terminal βα1 and part of the early β1 superdomain. Further evidence demonstrates that correct folding of the N-terminal βα1 domain determines the fate of the entire protein. Problems in the disulfide bond formation, N-linked glycosylation and even single mutations can prevent the secretion of apoB-containing lipoprotein 21; 22; 23; 24; 25; 26. The sensitivity to structural perturbations in the N-terminal domains indicates that the initiation of apoB-containing lipoprotein is highly dependent on the folding of this region.
The N-terminal region of apoB is structurally homologous to lipovitellin and MTP 15. Despite the weak sequence identity (21%) between B17 and lipovitellin, the presence of structural similarity to lipovitellin has been confirmed by circular dichroism, limited proteolysis, and chemical cross-linking 27. Based on the crystal structure of lipovitellin, the N-terminal of apoB is composed of a β-barrel domain (B5.9), an α-helical domain (B6.4–13), a C-sheet domain (B13–17) and a potential A-sheet domain (B17–22). In vitro binding assays have mapped the phospholipid interacting surfaces to regions in B17 that are predicted to constitute part of the lipid binding pocket 28. Although predicted to be amphipathic β-sheets, the structural and functional annotation of B17–22 has not been tested experimentally, largely because of the insoluble nature of this domain. However, negative staining electron microscopic imaging showed lipovitellin-like particles produced by B22 in McARH777 cells, which supports this structural annotation 19. The dimension and protein-lipid stoichiometry of a B22 model based on lipovitellin is consistent with the electron microscopic observation and biochemical data 29.
Despite our current knowledge of the structure and function of the N-terminal region of apoB, the initiation of lipoprotein assembly is still poorly understood. In particular, the roles of apoB, lipids and MTP in this process are elusive. Intuitively, a successful particle assembly requires a concerted interplay between protein folding and lipid binding. If the formation of an initiation complex is simply a passive adsorption of protein to a lipid surface, what structural features define the lipid nucleus? Alternatively, if the initiation is an active lipid recruitment process by the protein, how is this recruitment achieved and what role does MTP or other chaperones play? If the lipids come from the ER membrane, how does the protein see the difference between sufficient and insufficient lipid availability? Such questions are difficult to answer by in vivo methods, because lipids and ER resident chaperones are integral cellular components, which cannot be easily dissected. On the other hand, by simply mixing protein with lipids in vitro, it is difficult to reconstitute the events during lipoprotein assembly, because it ignores a key characteristic during particle assembly, which is the sequential availability of apoB domains. In this study, we characterize the early events during apoB maturation using a prokaryotic in vitro translational system, in which protein translation occurs in an environment where apoB, lipids and MTP can be controlled to determine their temporal and spatial relationships.
Results
Choosing an in vitro translational system
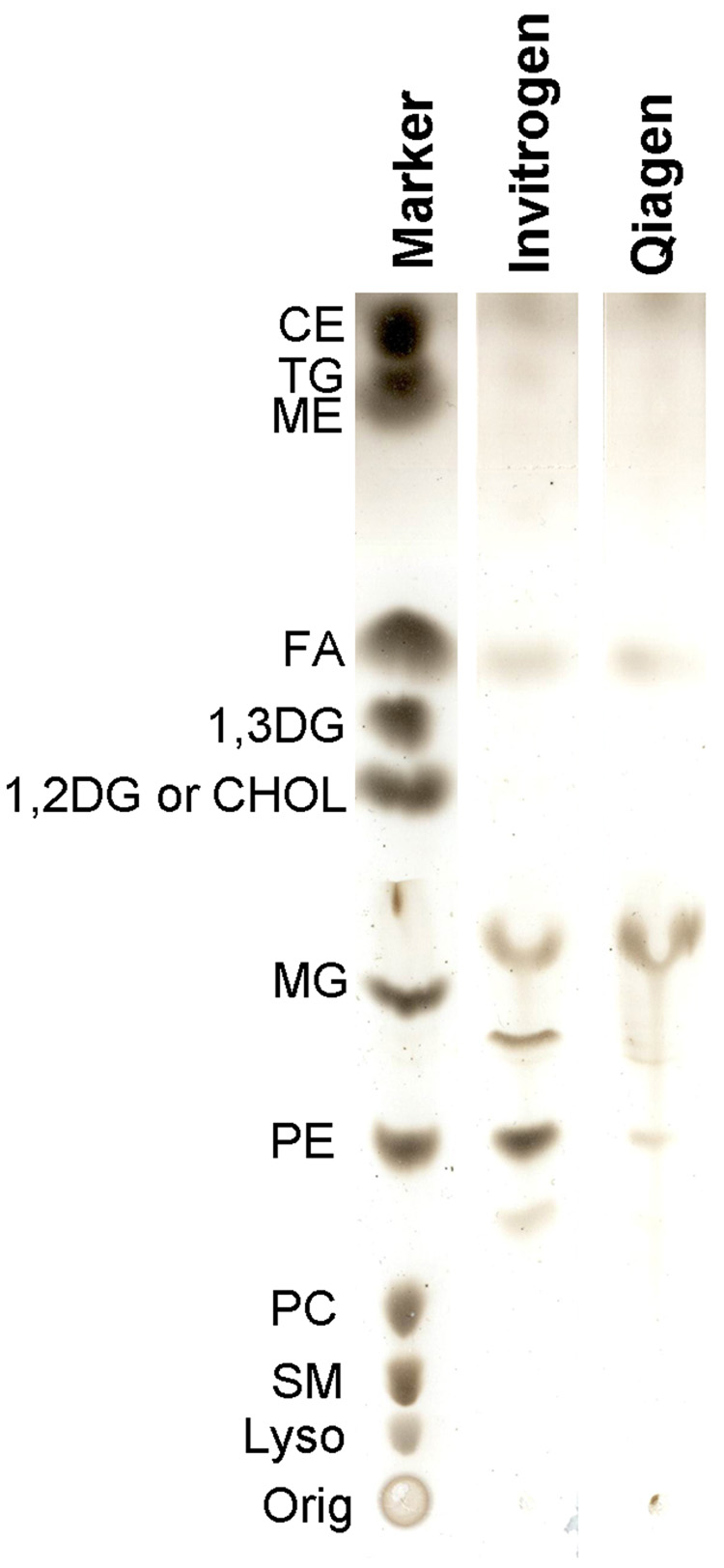
To access lipids and chaperones during apoB translation, we need an open translational system that has minimal endogenous lipids and chaperones. In this regard, prokaryotic cell-free systems are close to these requirements. Two commercially available systems were initially tested: the Expressway system from Invitrogen and the EasyXpress system from Qiagen. In order to control the lipid environment, undesirable contaminants such as endogenous lipids and detergents must be avoided or at least minimized. For proprietary reasons, we couldn’t obtain the preparation scheme for either system from the manufacturer. However, we were assured that neither system used detergent in its preparation. We assayed for the presence of endogenous lipids in both systems by chloroform extraction and thin layer chromatography. Expressway from Invitrogen had a much higher level of bacterial lipids (mostly phosphatidylethanolamine) than the Qiagen EasyXpress system (Fig 1). Therefore, the EasyXpress system was used for all experiments described in this study.
Fig. 1. Lipid analysis of two cell-free expression systems.
Lipids extracted from the cell lysate for one reaction of the Expressway (Invitrogen) and EasyXpress systems (Qiagen) were compared with thin layer chromatography, as described in the experimental procedures section. A lipid marker containing common lipid species is shown on the left.
Domains of apoB required for early lipid recruitment
It has been suggested that apoB constructs as short as B19.5 or B22 can support lipid accumulation and be secreted in a lipid-bound form 19; 20. Three constructs – B6.4–17, B6.4–20.5 and B6.4–22 were used to compare lipid binding in the in vitro expression system (Fig 2). The N-terminal 6.4% of apoB (B6.4) was excluded in our expression constructs, because of the complication in its folding, and its lack of lipid interacting capability 28.
Fig. 2. Domain diagram of apoB.

The proposed domain structure of the N-terminal region of apoB is compared with that of lipovitellin. Sequences missing in the lipovitellin crystal structure are shown in white boxes. In the diagram of apoB, disulfide bond linkages are shown with orange bridges, and N-linked glycosylation sites are shown with green hexagons. A scale with both percentage of B100 and amino acid number is shown below the diagram of apoB. Also listed are the three constructs used in this study – B6.4–17, B6.4–20.5 and B6.4–22.
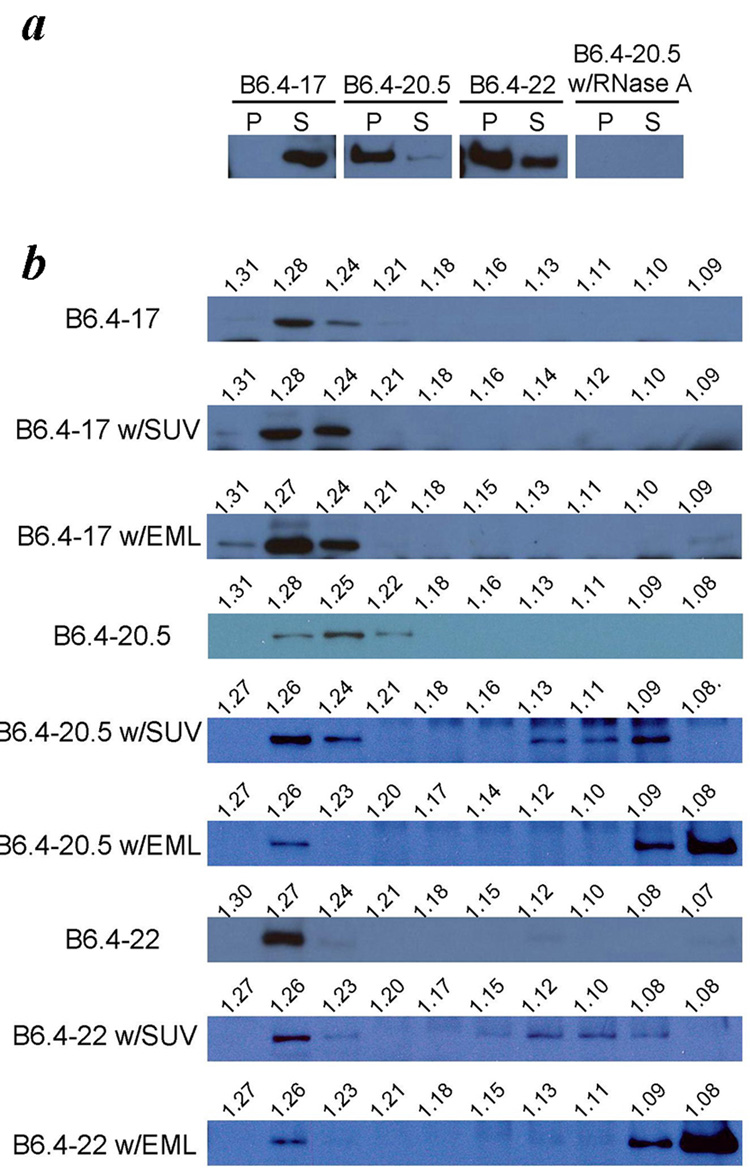
All three proteins, B6.4–17, B6.4–20.5 and B6.4–22 were cloned in pET24a vectors with a C-terminal His-tag and translated under a T7 promoter. Although all proteins expressed well in the EasyXpress system, they exhibited different levels of solubility in the absence of additional lipids. After expression, insoluble aggregates were precipitated by centrifugation and analyzed along with the supernatants by western blot using the penta-His antibody (Fig 3a). B6.4–17 was completely soluble, which is consistent with its defined folding observed previously 27; 30. B6.4–20.5 had the highest tendency toward precipitation, suggesting that it is unstable in a lipid-free state. Interestingly, a higher proportion of B6.4–22 remained soluble after translation compared to B6.4–20.5. It should be emphasized that the protein length is not an absolute predictor for solubility, but rather, the completeness of the domain structure may determine its behavior.
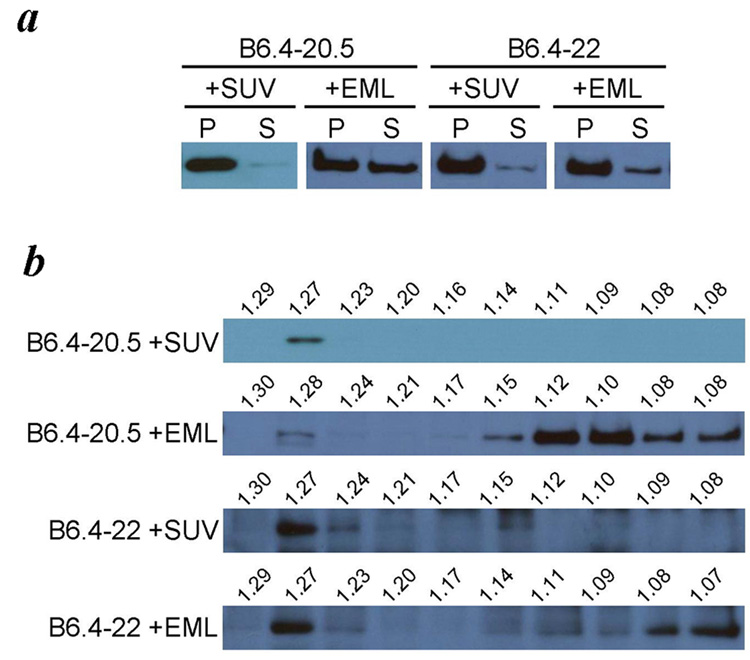
Fig. 3. Cotranslational cell-free apoB expression.
a. Comparison of the solubility of B6.4–17, B6.4–20.5 and B6.4–22. Each construct was expressed in the cell-free system in the absence of additional lipids. The cell pellet (P) and supernatant (S) were separated by centrifugation and analyzed by western blot using anti-His antibody. The expression of B6.4–20.5 in the presence of RNase A was included as a negative control. b. Density of apoB expressed with lipids. Supernatant of the ApoB expressed without lipids, with SUV or with emulsion (EML) were further separated by potassium bromide density gradient centrifugation and analyzed by western blot using anti-His antibody. The density of each fraction was determined by measuring its refractory index, and the calculated density is labeled above each lane.
All three constructs were then expressed in the presence of either egg PC small unilamellar vesicles (SUVs) (Fig 3b, Fig 6a), or egg PC / triolein emulsions (EML, 1:2 weight ratio, Fig 3b, Fig 6b). The supernatant fractions were further separated by KBr density gradient centrifugation, and analyzed by SDS-PAGE and western blot (Fig 3b). In the absence of lipids, all constructs migrated to high density (1.24–1.28 g/ml). In the presence of SUVs or emulsions, the formation of lipoprotein particles resulted in the observation of significant amount of proteins shifted to a lower density. B6.4–17 failed to migrate to lower densities either in the presence of SUV or emulsions, suggesting that B6.4–17 cannot efficiently bind to these lipids in the cell free system. In comparison, B6.4–20.5 and B6.4–22 bound to both types of lipid, and migrated to densities around 1.08–1.13 g/ml in the presence of SUVs and densities less than 1.1 g/ml in the presence of emulsions. Therefore, the apoB sequence between B17–20.5 enables the formation of a protein-lipid complex in this in vitro assembly system.
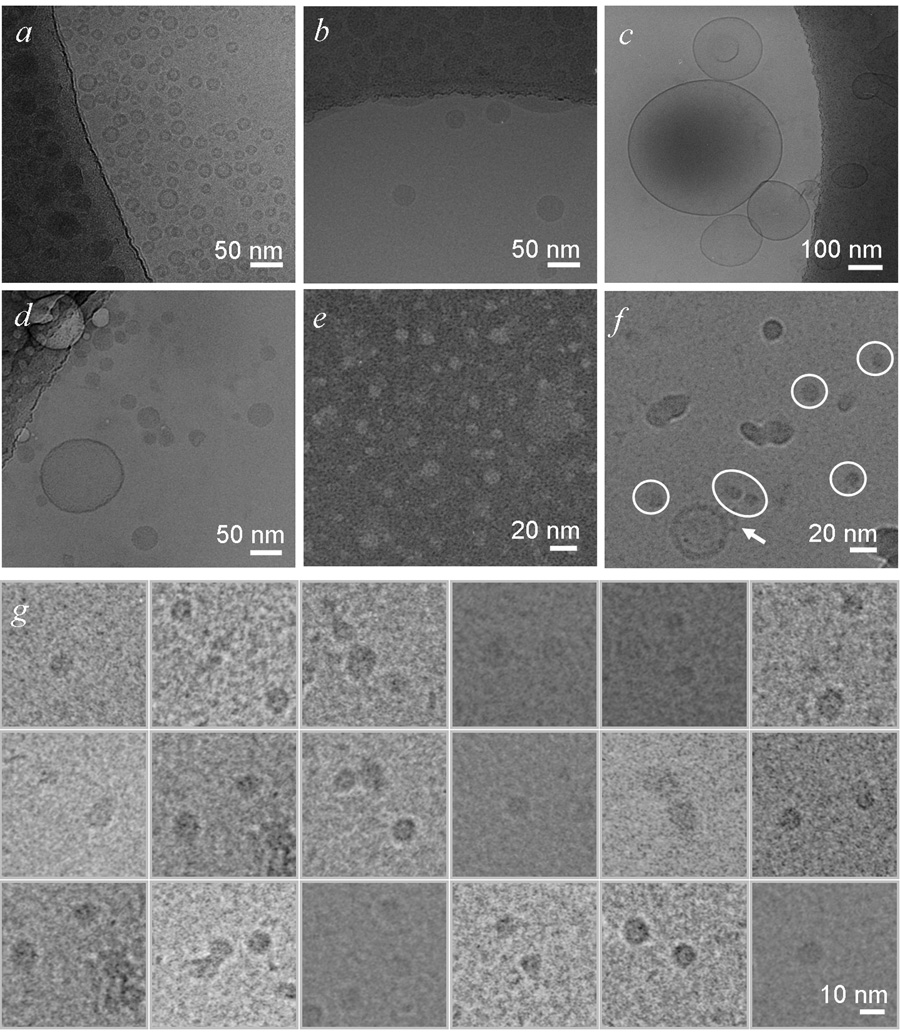
Fig. 6. Electron microscopy imaging of lipids and protein lipid complexes.
a. Egg PC SUVs. A high density ring with a thickness of 5 nm is characteristic for unilamellar vesicles. b. Egg PC / Triolein emulsions. Emulsion particles have a homogenous density. In this view, many emulsion particles are adsorbed on the grid (top third of the image), and only a few were trapped in the vitreous ice. Egg PC vesicles are sometimes found in the emulsion sample. c. B6.4–20.5 refolded with SUV. Large unilamellar vesicles are observed in this sample, likely due to the fusion of unstable small unilamellar vesicles overtime. d. B6.4–20.5 refolded with emulsions. The appearance of most particles is similar to pure emulsion (b). It is noteworthy that samples in c and d had been purified by Ni-NTA resin, thus proteins were present on all lipid particles. e. and f. B6.4–20.5 expressed with SUV in the cell-free system. The sample was analyzed by negative staining electron microscopy in e. Samples imaged by cryo-electron microscopy in f. is less concentrated on the grid and often contains impurities from liquid ethane. This contamination can be differentiated by a characteristic white ring on the edge, suggesting their presence on the surface of the vitreous ice. Circled particles are the identified complex containing B6.4–20.5 and egg PC. The arrow points to a vesicle with additional densities on its surface, probably representing B6.4–20.5 particle. g. A collage of 18 cropped images containing B6.4–20.5 translated in the presence of egg PC particles from 8 negative films. These particles adopt relatively uniform shape and dimension. Preparation of samples for electron microscopy is described in the experimental procedures section. All grids were prepared with cryo method, except for the sample in e, which was stained by 1% phosphotunstate. Images were acquired at different magnifications, and scale bars are shown in each image.
Cotranslational and posttranslational lipid recruitment
To examine the role of the translational process, we compared the efficiency of cotranslational and posttranslational lipid binding of B6.4–20.5 and B6.4–22 in the in vitro expression system. Neither B6.4–20.5 nor B6.4–22 was capable of binding to egg PC SUV if lipids were added after the translation, since both proteins remained at densities greater than 1.25 g/ml (Fig 4). Most proteins form aggregates after 2 hours translation, and incubation with SUV does not change the ratio between soluble and insoluble fractions. It should be noted that even proteins in the soluble fraction can indeed form aggregates, which are not big enough to precipitate during a brief centrifugation. Therefore, posttranslational addition of SUV may have missed the window of lipid recruitment during apoB folding.
Fig. 4. Posttranslational incubation with lipids.
B6.4–20.5 and B6.4–22 were incubated with SUV (0.6 mg/ml) or emulsion (EML) (1.8 mg/ml) after their cell free expression. The supernatants were separated by potassium bromide density gradient centrifugation and analyzed by western blot using anti-His antibody. The density of each fraction was determined by measuring its refractory index, and the calculated density is labeled above each lane.
The binding with emulsions however was not abolished if emulsions were added after the translation (Fig 4). In addition, emulsions added posttranslationally seem to increase the solubility of B6.4–20.5 and B6.4-22. Since apoB misfolding and aggregation are likely to be irreversible in the absence of eukaryotic chaperones, the increased proportion of soluble apoB in the presence of emulsions is probably a nonspecific process due to the high affinity of the exposed hydrophobic apoB segments for emulsions. In fact, posttranslationally formed apoB / emulsion particles have a higher density than cotranslationally formed lipoprotein particles (Fig 4 and Fig 3b). This is probably due to the association of multiple proteins on the same emulsion particle through locally exposed lipid binding patches from apoB, thus increasing the density of the overall complex.
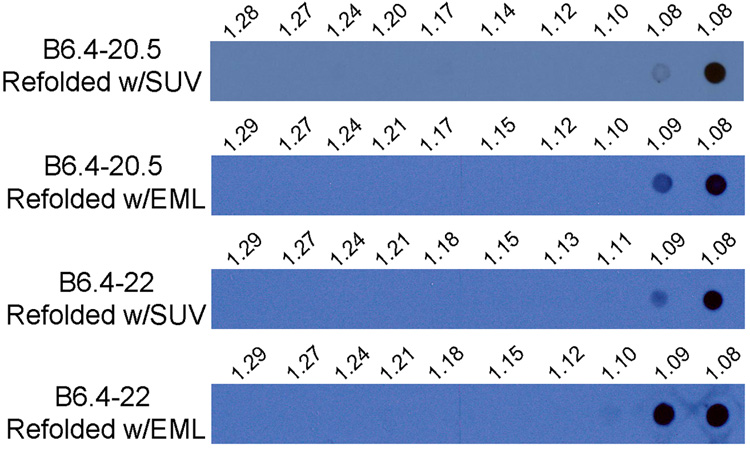
The failed posttranslational lipid recruitment can be attributed to two causes, the absence of lipids during protein folding and the lack of sequential exposure of the domains within B6.4–20.5. Therefore, we further examined lipid recruitment during protein folding, but after protein translation. B6.4–20.5 and B6.4–22 from BL21 E. coli cells was purified under denaturing condition and refolded in the presence of 1:8 (protein to egg PC SUV weight ratio) or 1:8:16 (protein to egg PC to triolein emulsion weight ratio). The denatured proteins were slowly added from 6 M GuHCl stocks to the lipid-containing refolding solution to allow instantaneous dilution of the proteins by over 1000 fold. Refolded B6.4–20.5 and B6.4–22 appear to form the correct disulfide bonds as they migrate as a single species in non-reducing gels with a trace amount (less than ~5%) of higher molecular weight species that correspond to disulfide bonded dimers or higher order species (data not shown). When the reconstituted products were analyzed on the potassium bromide density gradient, B6.4–20.5 migrated to the top of the gradient in both SUV and emulsion conditions (Fig 5). Compared to the cotranslational condition, products with similar density were observed for emulsion binding, but particles with lower density were produced with SUV binding (Fig 3b, 5). It should be noted that fractions at the top of the density contained particles with densities equal to or less than 1.08 g/ml, which may represent particles with a broad range of compositions. Without further structural characterization, we cannot ascertain whether the same particles were formed with emulsion in both cotranslational and posttranslational assembly approaches.
Fig. 5. Refolding of B6.4–20.5 and B6.4–22 in the presence of lipids.
B6.4–20.5 and B6.4–22 were refolded in the presence of SUV or emulsion at 8 or 24 times the protein weight concentration, respectively. Arginine and glutathione that had assisted in the protein refolding were removed by dialysis, and the products were separated on a potassium bromide gradient. Density separated fractions were analyzed on a dot blot using anti-His antibody. The density of each fraction was determined by measuring its refractory index, and the calculated density is labeled above each lane.
The difference in protein density indicates a compositional difference of the lipoprotein particles formed with SUVs. The particles produced in the refolding method appear to have a lower density, suggesting higher lipid to protein ratios. Since pure SUVs have a density less than 1.08 g/ml, the cotranslational formation of particles at densities greater than 1.08/ml was a result of drastic modification of the lipid particle. In contrast, such modification was not observed in the refolded protein. Therefore, the presence of phospholipids during refolding is not sufficient, but rather the translational process and the sequential availability of domains in apoB are required for efficient lipid remodeling.
Structure of B6.4–20.5 /SUV complexes
Cotranslationally assembled B6.4–20.5 / SUV complexes fall into the density range between 1.10–1.15 g/ml. This density range suggests a lipid to protein weight ratio between 1.6–2.7 by a rough estimate based on the density of pure protein and phosphatidylcholine. However, the density information cannot differentiate between two scenarios: 1) multiple protein molecules attach to a SUV, or 2) the protein breaks the SUV into smaller fragments and forms more discrete protein-lipid complexes.
The density of the B6.4–20.5 / SUV complex provides a convenient approach to purify them from both excess lipids and contaminating proteins. The amount of endogenous bacterial proteins from the cell-free system is insignificant in this density range between 1.10–1.20 g/ml (data not shown). The fractions containing B6.4–20.5 / SUV were desalted and further analyzed by electron microscopy using both negative stain and cryo sample preparation methods. Particles of similar dimensions were observed in both negative stain and cryo samples, reassuring our identification of the assembled complex (Fig 6e, f). Technically, the negative stained samples can achieve a higher concentration, but cannot be used to differentiate SUVs from large particles (Fig 6e). In comparison, samples made by the cryo method allow clear identification of unbroken SUVs, but were much less concentrated, and were often contaminated by impurities in the ethane, which could be differentiated by the presence of a prominent white edge, suggesting a different focal plane (Fig 6f). Compared to the protein-free pure SUVs (Fig 6a), the B6.4–20.5-containing SUVs show coarse and discontinuous rings, and were sometimes decorated with protrusions possibly representing budding lipoproteins (Fig 6f, arrow). Such morphology was indicative of protein insertion into the bilayer.
B6.4–20.5 refolded with SUVs or emulsions were purified by Ni-NTA resin to remove the extra lipid and were also examined by cryo-EM. Interestingly, many very large vesicles were observed in samples containing B6.4–20.5 and SUVs (Fig 6c). SUVs are metastable, and tend to fuse into large vesicles over time. It is likely that the refolded protein fails to stabilize the protein-SUV complex and these lipids fuse into big vesicles over the 4 day preparation period. In contrast, the refolded protein-emulsion complex appeared similar to pure emulsion particles in terms of morphology and size (Fig 6b, d).
The morphology of the particles formed during protein refolding therefore differs from those formed cotranslationally. During cotranslational lipid binding, the observation of a rather discrete population of B6.4–20.5 / egg PC complexes provides interesting information on the nature of this lipid recruitment process (Fig 6g). The protein / SUV interaction is not merely a nonspecific protein-lipid interaction; rather the protein is capable of breaking up the SUV and forming smaller complexes. By a direct measurement of 165 particles from cryo microscopy images, the average diameter for the B6.4–20.5 / egg PC complexes is 95.0 ± 13.1 Å.
Roles of MTP in the in vitro particle assembly
The lipid shuttling activity of MTP has long been recognized as a pivotal mechanism for the lipoprotein maturation. However, during the initiation stage of lipoprotein assembly, the proposed phospholipid shuttling activity has never been directly demonstrated. In the cell-free system, we found that MTP can significantly increase the solubility of B6.4–20.5 in a concentration-dependent manner (Fig 7a). The increase in solubility is an indication for a decrease in protein misfolding. Since this was achieved in a lipid-free environment, MTP is capable of performing a classic chaperone role for B6.4–20.5 by directly assisting in its folding and maintaining its solubility. However, the chaperone activity of MTP cannot reverse B6.4–20.5 aggregation when it is added after the translation (Fig 7a).
Fig. 7. Role of MTP for protein folding and lipid binding.
a. The effect of MTP on B6.4–20.5 expression. MTP was added either cotranslationally or posttranslationally during B6.4–20.5 expression. RNase inhibitor increases the expression yield when MTP was present cotranslationally. b. The effect of MTP on the lipid binding of B6.4–20.5. Four combinations of MTP and SUV during B6.4–20.5 translation were tested, and their respective outcome on protein density were examined with potassium bromide density gradient and western blot using anti-His antibody: cotranslational presence of MTP (B6.4–20.5 w/MTP), posttranslational incubation of SUV (B6.4–20.5 + SUV), posttranslational incubation of both MTP and SUV (B6.4–20.5 + MTP & SUV) and cotranslational presence of MTP and posttranslational incubation of SUV (B6.4–20.5 w/MTP + SUV). The density of each fraction was determined by measuring its refractory index, and the calculated density is labeled above each lane.
We then studied the role of MTP in cotranslational lipidation of nascent B6.4–20.5. Translation of B6.4–20.5 in the presence of SUVs results the formation of particles with a density of 1.09–1.13 g/ml (Fig. 3b). However, posttranslational addition of SUVs does not lead to particle formation (Fig. 7b). Translation of B6.4–20.5 with MTP in the absence of SUVs resulted in the generation of lipid-poor protein that was present at a density of 1.28 g/ml (Fig. 7b). Addition of MTP and SUVs to translated B6.4–20.5 did not affect its flotation properties, indicating that a protein synthesized in the absence of MTP is not a good acceptor of lipids. In contrast, translation of B6.4–20.5 in the presence of MTP and posttranslational supplementation of SUVs resulted in the formation of particles with a density of 1.08–1.14 g/ml. These data indicate that B6.4–20.5 synthesized in the presence of MTP acquires a conformation that can accept lipids from SUVs.
Discussion
The assembly of an apoB-containing lipoprotein produces two important lipid transporting particles in the body –chylomicrons and VLDLs. The signal sequence of apoB targets the protein translation and translocation into the ER lumen. Lipids are then added to the nascent protein either in the ER or in the downstream cellular organelles such as the golgi apparatus. Many questions regarding this assembly process remain elusive. For instance, at what stages of apoB translation are lipids added, where are the lipids from, in what form are the lipids added to apoB, and how do MTP and other ER chaperones affect this assembly process?
The goal of this study is to establish an in vitro system, where key components for lipoprotein assembly can be controlled. In this regard, cell-free systems provide a simplified platform to reconstitute biological processes using limited components in a physiologically relevant environment. It has been successfully used in the study of apoB secretion and vesicular trafficking 31; 32. We choose the prokaryotic cell-free expression system as a platform because it is free from eukaryotic lipids and chaperones, thus the endogenous factors favoring the assembly process are minimized. This system is suitable to address the protein-lipid interaction in a cotranslational manner, which is often overlooked in in vitro studies that simply mix proteins with lipids. Due to the hydrophobic nature and the large size of apoB, the sequential availability of various apoB domains is likely to be essential for successful assembly, especially during the initiation process, when the globular N-terminal region is involved.
On the other hand, the prokaryotic expression precludes posttranslational modifications and probably the formation of disulfide bonds due a relatively reducing environment (Fig. 2). It has been reported that glycosylation is largely involved in protein sorting through the secretory pathway, which is not the focus of this study 22. However, the lack of oxidizing environment and appropriate chaperones may pose difficulty in the folding of the Cys-rich very N-terminus of apoB. Based on its homology to lipovitellin, the N-terminal 6.4% of apoB presumably forms a β-barrel structure 15; 27; 29; 33. In the current study, this domain has been excluded based on several reasons. Since the purpose of this study is to reconstitute a translational event in vitro with minimized eukaryotic components, constructs containing the β-barrel domain may not be compatible with the current system setup. Most available evidence suggest that the β-barrel domain is not involved directly in lipoprotein particle formation; rather it may facilitate apoB secretion 22, and assist in apoB maturation through interaction with MTP 15. Therefore, studies in the absence of the β-barrel domain may still provide mechanistic insights for in vivo apoB lipidation, if constructs without the β-barrel domain exhibit independent protein folding and lipid recruitment activities. Indeed, both electron microscopic reconstructions and homologous modeling suggest that the β-barrel domain protrudes away from the rest of the protein, forming only a small interface with the following domains 15; 27; 28; 34; 35. Indeed, limited proteolysis of B17 and chemical crosslinking between N-terminal domains suggest weak interactions between the N-terminal β-barrel domain and the rest of the protein 27. Moreover, unlike the immediately-following α-helical and β-sheet domains, the β-barrel domain exhibits no phospholipid remodeling activity, which is consistent with the lipovitellin-based model that the β-barrel domain has a hydrophilic surface, and cannot be involved in direct binding to hydrophobic surfaces 27; 28. In the current study, the observation that constructs lacking the β-barrel domain exhibit cotranslational SUV remodeling capability and MTP-dependent posttranslational SUV remodeling capability indicates that the truncated constructs represent biologically active and independent functional domains. Therefore, we believe that constructs lacking the β-barrel domain can be folded in the cell-free system and likely maintain their intrinsic lipid interacting properties. Prokaryotic-based in vitro translation using truncated constructs shed light on apoB lipidation, the essential subject of the current study, but cannot yet provide information for certain physiological events that require vesicular transportation and N-terminus specific protein recognition, such as apoB secretion. However, it should be noted that the cell-free system is an open system, and in theory does allow future optimization to accommodate the expression of full-length constructs and more sophisticated cellular organelles.
As a model system, two lipid forms are used in our study – bilayer egg PC in the form of SUVs and small emulsion particles, which have a monolayer of egg PC and a hydrophobic triolein core. Protein constructs longer than B6.4–20.5 are capable of binding both SUV and emulsions cotranslationally, suggesting that apoB sequences between 17–20.5% are responsible for this gain of function. Notably, this region corresponds to the A-sheet (also termed βB-sheet) in the lipovitellin-based structural model 15; 27; 29. Interestingly, we have previously shown that B6.4–17 can remodel dimyristoylphosphatidylcholine (DMPC) multilamellar vesicles and egg PC unilamellar vesicles 28. The differences in the physical properties between DMPC and egg PC vesicles may underlie this apparent inconsistency. When DMPC undergoes its phase transition at 24 °C, defects are generated in the bilayer, facilitating the insertion of lipid binding motifs such as amphipathic α-helices or β-sheets 36. It should be noted that the in vitro expression system is a closer mimic to the physiological situation than simply mixing the protein with lipids. It is also consistent with the observation that B17 is secreted mostly in a lipid-free state, while constructs between B19.5 to B22 are able to accumulate significant amount of lipids 19; 20; 37.
This study shows that B6.4–20.5 or B6.4–22 responded differently to SUVs and emulsions. Both sonicated SUV and emulsion particles have densities lower than 1.08 g/ml. Since the size of the protein is much smaller than the lipid particle, if one protein simply binds to the lipid particle without remodeling it, the density of the protein-lipid complex will not change much. This is indeed the case when proteins are expressed in the presence of emulsions. However, when B6.4–20.5 and B6.4–22 are expressed with SUVs, the protein-lipid complex migrates to a higher density (1.10–1.15 g/ml). Therefore, a lower lipid to protein ratio is expected in the final product, suggesting that either SUVs are bound with multiple proteins, or the SUVs are remodeled by the protein to form new particles. Electron microscopic imaging of the egg PC / B6.4–20.5 complex suggest that the latter scenario occurs. Indeed, a rather homogenous product, with a diameter of 95.0 ± 13.1 Å is observed, suggesting a defined structure of the nascent particle formed by B6.4–20.5. The diameter of these particles is close to the molecular dimension of lipovitellin without the β-barrel domain, which is about 80–100 Å. Manchekar and coworkers reported negative stain images of B22 expressed in McA-RH7777 cells with dimensions of 100 × 150 Å 19. Considering the absence of the β-barrel domain of our construct, the particles that we observed are of a similar size. However, they also reported the failure of B20.5 to assemble into a lipid-containing complex, while B22 can. Such difference between B6.4–20.5 and B6.4–22 are not observed in our in vitro expression system. Nonetheless, B6.4–20.5 does seem to be less soluble than B6.4–22. It is possible that the truncation ending at 20.5% of apoB is not very compatible with the protein folding, thus leading to more folding defects, which could be treated differently by the eukaryotic cellular machinery than the cell-free system.
A surprising observation in our study is the intrinsic lipid recruitment property of B6.4–20.5 or B6.4–22 during translation, which is independent of eukaryotic chaperones, including MTP. The property relies on the sequential availability of the N-terminal domains within apoB, and is lost when synthesized protein is denatured and refolded. Technically, we found that the success of this lipid recruitment relies on the freshness of the SUV sample. The SUV is not a stable structure. It fuses over time and forms larger vesicles or multilamellar liposomes. Indeed, the lipid recruitment is not efficient for egg PC large unilamellar vesicles (data not shown). This difference indicates that structural defects in the phospholipid bilayer induced by the tight curvature may facilitate this spontaneous lipid recruitment event. The thickness of rough ER sheets or the diameter of smooth ER tubule is 60–100 nm, sufficient to accommodate small vesicles 38. Furthermore, structural defects in the ER membrane are also possible, which can be caused by physical bending of the membrane, protein insertion, or heterogeneous lipid composition. Regardless of lipid availability, all nascent apoB polypeptides have to cross the bilayer ER membrane and the N-terminal domain of apoB likely stays in close proximity to the phospholipid rich ER membrane for minutes during the synthesis of the rest of this large protein. Since the secretion of apoB-containing particles depends on lipid availability, an ER membrane without active lipid synthesis is insufficient to support particle maturation. Therefore, certain structural features in the membrane, being a result of lipid synthesis, may serve as a nucleus for the assembly of apoB-containing particles.
The in vitro expression experiments demonstrate that spontaneous phospholipid recruitment by apoB occurs cotranslationally. The presence of lipids after or even during protein folding is insufficient to effectively form a protein / lipid complex. Rather, the sequential availability of apoB domains through translation appears important, which may affect protein folding and lipid recruitment, the two tightly associated events. Cotranslational availability of phospholipids in the appropriate form may facilitate protein folding. It is conceivable that the extensive lipid binding sequences in apoB can lead to protein misfolding and aggregation in the absence of lipids or appropriate chaperones. Since cotranslational apoB degradation occurs in lack of lipid availability, the lifespan of a nascent apoB polypeptide can be shorter than the times it takes for apoB translation. This rather short life of the nascent apoB peptide in the ER lumen indicates a small window for initial lipid recruitment that determines the fate of the protein early on.
The efficient production of apoB-containing lipoproteins requires MTP, an ER resident chaperone that carries lipid shuttling activity. In the cell-free expression system, cotranslational presence of MTP increases the solubility of B6.4–20.5 and maintains B6.4–20.5 in a conformation that is acceptable to phospholipids from SUVs. In contrast, adding MTP and SUVs postranslationally cannot promote lipid recruitment. Since the increase in protein solubility is achieved in the absence of lipids, this observations suggests a chaperone role of MTP in assisting apoB folding. Notably, the β-barrel domain is not required to recruit this chaperone activity, although the β-barrel is a reported MTP binding site 15. It has been reported that the phospholipid transfer activity of MTP can support lipoprotein assembly and secretion 39. Our study does not disprove the presence of this activity for the N-terminal domain of apoB. However, our data does indicate that the presence of MTP is not required for spontaneous lipid recruitment by the N-terminal domains of apoB, but MTP can significantly facilitate lipid recruitment through assisting apoB folding. A recent study showed that the inhibition of MTP lipid transfer activity does not prohibit the initiation stage of lipoprotein formation 40. Most literature indicates that apoB lipidation and secretion require a cell line that contains MTP. The level of MTP requirement varies from high to extremely low, such as C127 cells 41; 42. A few reports suggest that the secretion of apoB-containing lipoprotein is still possible in the absence of MTP activity, albeit at extremely low yield 43; 44. Dashti and coworkers also showed that a 60–70% decrease in MTP level by iRNA had no effect in the initiation of apoB assembly 40. This observation raises the question of whether the proposed chaperone role of MTP is indispensable for the initiation of apoB assembly. It is possible that the remaining 30–40% of MTP activity is sufficient to support this chaperone role during initiation, or other chaperones may compensate the role of MTP.
Many seemingly inconsistent observations have been made regarding the lipoprotein assembly and the secretion of the N-terminal domains of apoB. The capacity of lipidation seems to vary significantly among different apoB constructs and cell lines, which had led to different hypotheses 19; 20; 45; 46. Our observation provides an alternative hypothesis for the initiation of apoB-containing lipoprotein assembly, which in a sense is compatible with many apparent inconsistencies in various expression conditions. Our data suggest that phospholipid recruitment ability is intrinsic in the N-terminal domain of apoB during the translational process. However, this activity is highly dependent on the correct protein folding and lipid availability. Considering that the ER membrane is ubiquitous in all eukaryotic cells, it is not just the presence of lipids, but rather the appropriate structure that the lipids adopt that affects successful apoB-directed lipid recruitment. During the initiation stage, MTP may play a pivotal role in assisting apoB folding, and assuring the lipid recruitment activity of apoB (Fig 8c). The significant difference among various cell lines probably reflects the different specific lipid environment in the ER, which has direct implications in apoB maturation. The specific structural features of the lipids that favor particle assembly are yet to be determined. Factors that lead to the formation of such features can be involved in lipid synthesis as well as mobilization. It is possible that MTP plays an active role in forming these structural features through its lipid transfer activity.
Fig. 8. Model of B20.5.
a. A homology model of B20.5. The homology model of B20.5 was built upon the crystal structure of lipovitellin as described in the experimental procedures section. The β-barrel domain is shown in green, α-helical in cyan, C-sheet in red, and A-sheet in blue. b. A simplified cartoon for the folding topology of B20.5, with a proposed lipid binding site shown as an orange sphere. c. A proposed mechanism during the initiation of lipid binding. The nascent apoB polypeptide tested in our system beginning from the α-helical domain has an intrinsic lipid binding and remodeling property, which can break the appropriate lipid bilayer substrates, such as egg PC SUVs into discrete protein/lipid complexes. However, in the absence of such lipid substrates, the protein misfolds and irreversibly aggregates. MTP during apoB expression facilitates apoB folding even in the absence of appropriate lipid substrate, and retains the lipid binding capability of apoB.
Materials and Methods
Cloning
Constructs of B6.4–17, B6.4–20.5 and B6.4–22 were cloned from B37 initially described by Carraway et al. 45. The PCR products containing a 6-His tag at the C-terminus were digested with Nde I and EcoR I, and then inserted into pET24a vector. The DNA sequence was confirmed at the Molecular Genetics Core Facility at Boston University School of Medicine.
Small unilamellar vesicle and emulsion preparation
Egg PC (Avanti Polar Lipids) in chloroform was first dried in a round bottom flask on a rotary evaporator (Buchi) to form a uniform thin layer, and then lyophilized for 1 hour to remove residual solvent. The dried lipids were suspended in 20 mM tris-hydroxymethylaminomethane (Tris), 150 mM sodium chloride pH 7.5 (TS buffer), and sonicated with a Branson probe sonicator for 1 hour on ice, or till the solution clears. The lipid preparation was centrifuged at 14,000 g to remove titanium debris. Each small unilamellar vesicle (SUV) sample was freshly prepared before use. Egg PC and triolein emulsions were prepared at 1:2 weight ratio using the same drying and sonicating protocol.
Thin layer chromatography
Cell extracts from the Expressway (Invitrogen) and EasyXpress (Qiagen) prokaryotic cell-free expression kits were diluted in 100 µl TS buffer and vigorously mixed with 2 ml chloroform and 1 ml methanol. After centrifugation, the organic supernatant was separated, and then vigorously mixed with 0.6 ml TS buffer. After waiting for 30 mins, the organic phase was separated from the aqueous phase with a separatory funnel. The organic solvent was evaporated under a gentle flow of nitrogen, and dried on a lyophilizer. The organic extract was hydrated in 100 µl TS buffer again, and the same extraction procedure was repeated two more times. Finally, the sample was dissolved in chloroform and spotted 1.5 cm from the bottom of a washed Analtech Silica Gel G plate (ThermoFisher). The plate was first run in a polar system composed of chloroform, methanol, water and acetic acid at a volume ratio of 65:25:4:1 to one quarter of the plate’s height. The plate was air dried and then run in a neutral system composed of hexane, diethyl ether, acetic acid at a volume ratio of 70:30:1 to the full height of the plate. After air-drying, the plate was developed by spraying with 50% sulfuric acid and then charred on a hot plate.
Cell-free protein expression
Slight modifications have been adopted based on the EasyXpress protocol from Qiagen. Generally, plasmid DNA encoding the gene of interest at 20 nM was mixed with the cell-free extract, and the reaction buffer either with or without lipids. Egg PC SUVs were added to the mixture to 600 µg/ml final concentration, and emulsions were added to 1.8 mg/ml. Purified MTP (Chylos) was first treated with 2 U/µl RNase inhibitor (Ambion), and 5 µl of MTP was used for each reaction. The expression reaction was incubated on a vertical rotational mixer at 30 °C for 2 hours. Expression was terminated by the addition of 15 µM Rnase A before the addition of any posttranslational proteins or lipids.
Protein purification and refolding
B6.4–20.5 and B6.4–22 proteins were expressed in BL21 DE3 cells using standard protocols. Protein expression was performed at 37 °C and induced by the addition of IPTG to 1 mM after the cell concentration reached an optical density of 0.6–0.8 at 600 nm. Cells were allowed to grow for another three hours, and were then harvested by centrifugation. Cell pellets were lysed with 1 mg/ml lysozyme at room temperature for 30 min and then sonicated with a probe sonicator (Branson). The protein-containing inclusion bodies were dissolved in 8 M urea after washing with 1% Triton X-100 and 1 M Urea. Soluble proteins were loaded on a Ni-NTA sepharose column (Qiagen) and eluted with 250 mM imidazole and 6 M guanidine hydrochloride (GuHCl). Protein refolding was achieved by slowly adding concentrated protein stocks in 6 M GuHCl into a refolding buffer containing 50 mM Tris, 800 mM arginine, 10 mM reduced glutathione, 2 mM oxidized glutathione, 0.02% sodium azide at pH 8.0. The refolding solutions were supplemented with either SUVs at 8 times the weight ratio of the protein or emulsions at 24 times the weight ratio of the protein. Protein at 1 µM in the refolding buffer was incubated at 4 °C overnight and then dialyzed extensively against the TS buffer.
Density gradient centrifugation
Protein samples were placed in a potassium bromide density gradient composed of three layers: the bottom layer, 3.3 ml at 1.25 g/ml; the top layer, 1.3 ml at 1.06 g/ml; and the middle layer, 0.4 ml at 1.20 g/ml, which also contained the protein sample. Each sample was centrifuged in a SW55 rotor at 55,000 rpm for 40 hours at 15 °C. After centrifugation, the gradient was manually fractionated from the bottom of the centrifuge tube into 10 fractions. The density of each fraction was determined by measuring the refractory index of the solution on a refractometer (American Optical Corp.).
Dot blot and western blot
For dot blot analysis, samples without desalting were directly filtered onto a PVDF membrane (Millipore) by gravity using a dot blot microfiltration system (Bio-rad). The membrane was washed with TS buffer before antibody incubation. For western blot, samples after gradient centrifugation were first desalted by methanol and chloroform extraction to precipitate the protein. A purified B6.4–15 with His-tag was added prior to desalting as an internal standard to ensure consistent sample recovery. Proteins were then dissolved in 8 M urea and gel loading buffer, separated on 15% Sodium Dodecyl Sulfate – polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto an Immobilon membrane (Millipore). For both dot blot and western blot, membranes were blocked by 3% bovine serum albumin, labeled with penta-His antibody (Qiagen) and anti-mouse antibody linked to horse radish peroxidase. The protein signal was captured on CL-Xposure films (Pierce) after the incubation of the membrane in the chemiluminescent substrate (Perkin Elmer).
Electron microscopic imaging
B6.4–20.5 expressed with SUVs in the cell-free system was first separated from the gradient centrifugation. Fractions containing B6.4–20.5 particles were combined, dialyzed against TS buffer by more than 1000 fold and concentrated 50 – 100 fold with Microcon YM-30 (Millipore). Purified B6.4–20.5 refolded in the presence of SUVs or emulsions was concentrated 20 – 50 fold by Ni-NTA resin (Qiagen). The elution fractions were dialyzed against TS buffer. SUVs and emulsions were diluted to 2 mg/ml and 6 mg/ml with the TS buffer.
For negative stain electron microscopy, B6.4–20.5 samples (4 µl) were loaded on a carbon-coated and glow-discharged copper grid (SPI Supplies) for 1 min, washed with 10 drops of distilled water and stained with 1% sodium phosphotungstate, pH 7.5 for 30 s. The grid was imaged on a Philips CM12 transmission electron microscope operated at 120 kV with a LaB6 filament and recorded on SO-163 EM (Kodak) film at 45,000 × magnification under minimal electron dose conditions.
The cryo-preservation of the sample was performed in the Vitrobot (FEI company) system. The glow discharged Quantifoil grid (Quantifoil Micro Tools GmbH) was held in a closed chamber which has 100% humidity at 22 °C. A sample of 4 µl was applied onto the grid then blotted with filter paper, to allow the formation of a thin layer of liquid film containing individual particles over the grid holes. The grid was immediately plunged into liquid ethane, which lowered the temperature of the grid to −175 °C in 10−4 second, and was preserved in liquid nitrogen until use. The grid was examined on the same electron microscope and images were recorded at 60,000 × magnification under minimal electron dose conditions on SO-163 EM film.
Films from both imaging methods were processed with undiluted Kodak D-19 developer for 12 min and Kodak rapid fixer for 5 min. Electron micrographs were digitized on a Creo IQ Smart2 Scanner (Global Imaging) at 1270 dpi. Photoshop (Adobe) was used for measurements on the digitalized images.
Molecular modeling
The sequence of B20.5 was aligned with lipovitellin by Blast-2-Sequence to generate the input file for comparative modeling 47. The homology model of B20.5 was generated with Modeller 8.0 using standard parameters 48. Molecular images were generated in MOLMOL 49.
Acknowledgements
We would like to thank Michael Gigliotti and Cheryl England for help with lipid analysis, and Drs. Donald M. Small, Haya Herscovitz, Margaretha Carraway and Libo Wang for insightful discussions. This work was supported by National Institutes of Health grant HL26335 (to CJM), DK46900 (to MMH) and American Heart Association postdoctoral fellowship grant 0625943T (to ZGJ)
Abbreviations
- apoB
apolipoprotein B
- ER
endoplasmic reticulum
- GuHCl
guanidine hydrochloride
- MTP
microsomal triglyceride transfer protein
- PC
phosphatidylcholine
- SDS-PAGE
Sodium dodecyl sulfate – polyacrylamide gel electrophoresis
- SUV
small unilamellar vesicle
- Tris
tris-hydroxymethylaminomethane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knott TJ, Rall SC, Jr, Innerarity TL, Jacobson SF, Urdea MS, Levy-Wilson B, Powell LM, Pease RJ, Eddy R, Nakai H, et al. Human apolipoprotein B: structure of carboxyl-terminal domains, sites of gene expression, and chromosomal localization. Science. 1985;230:37–43. doi: 10.1126/science.2994225. [DOI] [PubMed] [Google Scholar]
- 2.Chen SH, Habib G, Yang CY, Gu ZW, Lee BR, Weng SA, Silberman SR, Cai SJ, Deslypere JP, Rosseneu M, et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238:363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 3.Powell LM, Wallis SC, Pease RJ, Edwards YH, Knott TJ, Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987;50:831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- 4.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 5.Yao Z, Tran K, McLeod RS. Intracellular degradation of newly synthesized apolipoprotein B. J Lipid Res. 1997;38:1937–1953. [PubMed] [Google Scholar]
- 6.Oyadomari S, Yun C, Fisher EA, Kreglinger N, Kreibich G, Oyadomari M, Harding HP, Goodman AG, Harant H, Garrison JL, Taunton J, Katze MG, Ron D. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell. 2006;126:727–739. doi: 10.1016/j.cell.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 7.Davidson NO, Shelness GS. APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu Rev Nutr. 2000;20:169–193. doi: 10.1146/annurev.nutr.20.1.169. [DOI] [PubMed] [Google Scholar]
- 8.Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- 9.Sharp D, Blinderman L, Combs KA, Kienzle B, Ricci B, Wager-Smith K, Gil CM, Turck CW, Bouma ME, Rader DJ, et al. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature. 1993;365:65–69. doi: 10.1038/365065a0. [DOI] [PubMed] [Google Scholar]
- 10.Atzel A, Wetterau JR. Mechanism of microsomal triglyceride transfer protein catalyzed lipid transport. Biochemistry. 1993;32:10444–10450. doi: 10.1021/bi00090a021. [DOI] [PubMed] [Google Scholar]
- 11.Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136–150. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 12.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 13.Hussain MM, Bakillah A, Nayak N, Shelness GS. Amino acids 430–570 in apolipoprotein B are critical for its binding to microsomal triglyceride transfer protein. J Biol Chem. 1998;273:25612–25615. doi: 10.1074/jbc.273.40.25612. [DOI] [PubMed] [Google Scholar]
- 14.Bakillah A, Hussain MM. Binding of microsomal triglyceride transfer protein to lipids results in increased affinity for apolipoprotein B: evidence for stable microsomal MTP-lipid complexes. J Biol Chem. 2001;276:31466–31473. doi: 10.1074/jbc.M100390200. [DOI] [PubMed] [Google Scholar]
- 15.Mann CJ, Anderson TA, Read J, Chester SA, Harrison GB, Kochl S, Ritchie PJ, Bradbury P, Hussain FS, Amey J, Vanloo B, Rosseneu M, Infante R, Hancock JM, Levitt DG, Banaszak LJ, Scott J, Shoulders CC. The structure of vitellogenin provides a molecular model for the assembly and secretion of atherogenic lipoproteins. J Mol Biol. 1999;285:391–408. doi: 10.1006/jmbi.1998.2298. [DOI] [PubMed] [Google Scholar]
- 16.Bakillah A, Nayak N, Saxena U, Medford RM, Hussain MM. Decreased secretion of ApoB follows inhibition of ApoB-MTP binding by a novel antagonist. Biochemistry. 2000;39:4892–4899. doi: 10.1021/bi9924009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Herscovitz H. Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B. J Biol Chem. 2003;278:7459–7468. doi: 10.1074/jbc.M207976200. [DOI] [PubMed] [Google Scholar]
- 18.Segrest JP, Jones MK, Mishra VK, Anantharamaiah GM, Garber DW. apoB-100 has a pentapartite structure composed of three amphipathic alpha-helical domains alternating with two amphipathic beta-strand domains. Detection by the computer program LOCATE. Arterioscler Thromb. 1994;14:1674–1685. doi: 10.1161/01.atv.14.10.1674. [DOI] [PubMed] [Google Scholar]
- 19.Manchekarc M, Richardson PE, Forte TM, Datta G, Segrest JP, Dashti N. Apolipoprotein B-containing lipoprotein particle assembly: lipid capacity of the nascent lipoprotein particle. J Biol Chem. 2004;279:39757–39766. doi: 10.1074/jbc.M406302200. [DOI] [PubMed] [Google Scholar]
- 20.Shelness GS, Hou L, Ledford AS, Parks JS, Weinberg RB. Identification of the lipoprotein initiating domain of apolipoprotein B. J Biol Chem. 2003;278:44702–44707. doi: 10.1074/jbc.M307562200. [DOI] [PubMed] [Google Scholar]
- 21.Tran K, Boren J, Macri J, Wang Y, McLeod R, Avramoglu RK, Adeli K, Yao Z. Functional analysis of disulfide linkages clustered within the amino terminus of human apolipoprotein B. J Biol Chem. 1998;273:7244–7251. doi: 10.1074/jbc.273.13.7244. [DOI] [PubMed] [Google Scholar]
- 22.Vukmirica J, Nishimaki-Mogami T, Tran K, Shan J, McLeod RS, Yuan J, Yao Z. The N-linked oligosaccharides at the amino terminus of human apoB are important for the assembly and secretion of VLDL. J Lipid Res. 2002;43:1496–1507. doi: 10.1194/jlr.m200077-jlr200. [DOI] [PubMed] [Google Scholar]
- 23.Burnett JR, Shan J, Miskie BA, Whitfield AJ, Yuan J, Tran K, McKnight CJ, Hegele RA, Yao Z. A novel nontruncating APOB gene mutation, R463W, causes familial hypobetalipoproteinemia. J Biol Chem. 2003;278:13442–13452. doi: 10.1074/jbc.M300235200. [DOI] [PubMed] [Google Scholar]
- 24.Burnett JR, Zhong S, Jiang ZG, Hooper AJ, Fisher EA, McLeod RS, Zhao Y, Barrett PH, Hegele RA, van Bockxmeer FM, Zhang H, Vance DE, McKnight CJ, Yao Z. Missense mutations in apoB within the beta alpha 1 domain of human APOB-100 result in impaired secretion of apob and APOB-containing lipoproteins in familial hypobetalipoproteinemia. J Biol Chem. 2007 doi: 10.1074/jbc.M702442200. [DOI] [PubMed] [Google Scholar]
- 25.Huang XF, Shelness GS. Identification of cysteine pairs within the amino-terminal 5% of apolipoprotein B essential for hepatic lipoprotein assembly and secretion. J Biol Chem. 1997;272:31872–31876. doi: 10.1074/jbc.272.50.31872. [DOI] [PubMed] [Google Scholar]
- 26.Burch WL, Herscovitz H. Disulfide bonds are required for folding and secretion of apolipoprotein B regardless of its lipidation state. J Biol Chem. 2000;275:16267–16274. doi: 10.1074/jbc.M000446200. [DOI] [PubMed] [Google Scholar]
- 27.Jiang ZG, Carraway M, McKnight CJ. Limited proteolysis and biophysical characterization of the lipovitellin homology region in apolipoprotein B. Biochemistry. 2005;44:1163–1173. doi: 10.1021/bi048286y. [DOI] [PubMed] [Google Scholar]
- 28.Jiang ZG, Gantz D, Bullitt E, McKnight CJ. Defining lipid-interacting domains in the N-terminal region of apolipoprotein B. Biochemistry. 2006;45:11799–11808. doi: 10.1021/bi060600w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson PE, Manchekar M, Dashti N, Jones MK, Beigneux A, Young SG, Harvey SC, Segrest JP. Assembly of lipoprotein particles containing apolipoprotein-B: structural model for the nascent lipoprotein particle. Biophys J. 2005;88:2789–2800. doi: 10.1529/biophysj.104.046235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang ZG, Simon MN, Wall JS, McKnight CJ. Structural analysis of reconstituted lipoproteins containing the N-terminal domain of apolipoprotein B. Biophys J. 2007;92:4097–4108. doi: 10.1529/biophysj.106.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodsky JL, Gusarova V, Fisher EA. Vesicular trafficking of hepatic apolipoprotein B100 and its maturation to very low-density lipoprotein particles; studies from cells and cell-free systems. Trends Cardiovasc Med. 2004;14:127–132. doi: 10.1016/j.tcm.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Gusarova V, Brodsky JL, Fisher EA. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. J Biol Chem. 2003;278:48051–48058. doi: 10.1074/jbc.M306898200. [DOI] [PubMed] [Google Scholar]
- 33.Anderson TA, Levitt DG, Banaszak LJ. The structural basis of lipid interactions in lipovitellin, a soluble lipoprotein. Structure. 1998;6:895–909. doi: 10.1016/s0969-2126(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 34.Spin JM, Atkinson D. Cryoelectron microscopy of low density lipoprotein in vitreous ice. Biophys J. 1995;68:2115–2123. doi: 10.1016/S0006-3495(95)80392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlova EV, Sherman MB, Chiu W, Mowri H, Smith LC, Gotto AM., Jr Three-dimensional structure of low density lipoproteins by electron cryomicroscopy. Proc Natl Acad Sci U S A. 1999;96:8420–8425. doi: 10.1073/pnas.96.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson D, Small DM. Recombinant lipoproteins: implications for structure and assembly of native lipoproteins. Annu Rev Biophys Biophys Chem. 1986;15:403–456. doi: 10.1146/annurev.bb.15.060186.002155. [DOI] [PubMed] [Google Scholar]
- 37.Herscovitz H, Hadzopoulou-Cladaras M, Walsh MT, Cladaras C, Zannis VI, Small DM. Expression, secretion, and lipid-binding characterization of the N- terminal 17% of apolipoprotein B. Proc Natl Acad Sci U S A. 1991;88:7313–7317. doi: 10.1073/pnas.88.16.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126:435–439. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Rava P, Ojakian GK, Shelness GS, Hussain MM. Phospholipid transfer activity of microsomal triglyceride transfer protein is sufficient for the assembly and secretion of apoB-lipoproteins. J Biol Chem. 2006 doi: 10.1074/jbc.M512823200. [DOI] [PubMed] [Google Scholar]
- 40.Dashti N, Manchekar M, Liu Y, Sun Z, Segrest JP. Microsomal triglyceride transfer protein activity is not required for the initiation of apolipoprotein B-containing lipoprotein assembly in McA-RH7777 cells. J Biol Chem. 2007;282:28597–28608. doi: 10.1074/jbc.M700229200. [DOI] [PubMed] [Google Scholar]
- 41.Herscovitz H, Kritis A, Talianidis I, Zanni E, Zannis V, Small DM. Murine mammary-derived cells secrete the N-terminal 41% of human apolipoprotein B on high density lipoprotein-sized lipoproteins containing a triacylglycerol-rich core. Proc Natl Acad Sci U S A. 1995;92:659–663. doi: 10.1073/pnas.92.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellers JA, Shelness GS. Lipoprotein assembly capacity of the mammary tumor-derived cell line C127 is due to the expression of functional microsomal triglyceride transfer protein. J Lipid Res. 2001;42:1897–1904. [PubMed] [Google Scholar]
- 43.Gretch DG, Sturley SL, Wang L, Lipton BA, Dunning A, Grunwald KA, Wetterau JR, Yao Z, Talmud P, Attie AD. The amino terminus of apolipoprotein B is necessary but not sufficient for microsomal triglyceride transfer protein responsiveness. J Biol Chem. 1996;271:8682–8691. doi: 10.1074/jbc.271.15.8682. [DOI] [PubMed] [Google Scholar]
- 44.Aguie GA, Rader DJ, Clavey V, Traber MG, Torpier G, Kayden HJ, Fruchart JC, Brewer HB, Jr, Castro G. Lipoproteins containing apolipoprotein B isolated from patients with abetalipoproteinemia and homozygous hypobetalipoproteinemia: identification and characterization. Atherosclerosis. 1995;118:183–191. doi: 10.1016/0021-9150(95)05605-x. [DOI] [PubMed] [Google Scholar]
- 45.Carraway M, Herscovitz H, Zannis V, Small DM. Specificity of lipid incorporation is determined by sequences in the N- terminal 37 of apoB. Biochemistry. 2000;39:9737–9745. doi: 10.1021/bi000791h. [DOI] [PubMed] [Google Scholar]
- 46.Ledford AS, Weinberg RB, Cook VR, Hantgan RR, Shelness GS. Self-association and lipid binding properties of the lipoprotein initiating domain of apolipoprotein B. J Biol Chem. 2006 doi: 10.1074/jbc.M507657200. [DOI] [PubMed] [Google Scholar]
- 47.Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- 48.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 49.Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. 29–32. [DOI] [PubMed] [Google Scholar]