Abstract
Two cyclin-dependent kinase inhibitors, p18Ink4c and p27Kip1, are required for proper cerebellar development. Loss of either of these proteins conferred a proliferative advantage to granule neuron progenitors, although inactivation of Kip1 exerted a greater effect. Mice heterozygous for Patched1 (Ptc1+/−) that are either heterozygous or nullizygous for Kip1 developed medulloblastoma (MB) rapidly and with high penetrance. All tumors from Ptc1+/−;Kip1+/− or Ptc1+/−;Kip1−/− mice failed to express the wild type Ptc1 allele, consistent with its role as a canonical “two-hit” tumor suppressor. In contrast, expression of the wild type p27Kip1 protein was invariably maintained in MBs arising in Ptc1+/−;Kip1+/− mice, indicating that Kip1 is haploinsufficient for tumor suppression. Although MBs occurring in Ptc1+/− mice were histopathologically heterogeneous and contained intermixed regions of both rapidly proliferating and nondividing more differentiated cells, tumors that also lacked Kip1 were uniformly less differentiated, more highly proliferative, and invasive. Molecular analysis showed that the latter MBs exhibited constitutive activation of the Sonic hedgehog signaling pathway without loss of functional p53. Apart from gains or losses of single chromosomes, with gain of chromosome 6 being the most frequent, no other chromosomal anomalies were identified by spectral karyotyping, and half of the MBs so examined retained a normal karyotype. In this respect, this mouse MB model recapitulates the vast majority of human MBs that do not sustain TP53 mutations and are not aneuploid.
Keywords: p27Kip1, p18Ink4c, Patched-1, medulloblastoma, cerebellum
Introduction
Normal cerebellar development requires the correct balance between the early proliferation of granule neuron progenitors (GNPs) and their subsequent post-mitotic differentiation and migration to their final location within the mature organ. In the mouse, GNPs arise in the rhombic lip during embryonic days E9.5 and E11.5 and migrate outward to form the external germinal layer (EGL) of the developing cerebellum (1). At birth (post-natal (P) day 0), the EGL is composed of a single layer of GNPs overlaying Purkinje cells that secrete the mitogen Sonic hedgehog (Shh). GNPs expressing the Shh receptor Patched1 (Ptc1) are stimulated to undergo cell division, resulting in the rapid expansion of the EGL with maximum proliferation occurring at P5. GNPs then exit the cell cycle, differentiate and migrate through the Purkinje cell layer to reside as post-mitotic neurons within the internal granule layer (IGL). As they migrate, granule neurons extend retrograde axons that synapse with Purkinje neuron dendrites in the vacated EGL, thereby generating the outer molecular layer of the adult cerebellum, which is largely devoid of neuronal cell bodies. The cerebellum is completely formed within the first 3–4 weeks of life in the mouse and within 12–16 months in humans (1, 2).
Cell cycle progression of GNPs is regulated by the cyclin-dependent kinases, Cdk4 and Cdk6 that, in complexes with their allosteric regulators, cyclins D1 and D2, are required for proper development of the cerebellum (3). Opposing the action of cyclin-dependent kinases are two Cdk inhibitors, p18Ink4c and p27Kip1, that enforce cell cycle exit and help to maintain the neuronal post-mitotic state (4). Whereas p18Ink4c is a specific inhibitor of Cdk4 and Cdk6, p27Kip1 preferentially targets cyclin E- and A-dependent Cdk2, which acts later in G1 phase to help guide the G1 to S-phase transition (5). Ink4c mRNA is transiently expressed just as proliferating GNPs in the EGL exit the cell division cycle and begin to migrate to the IGL (6). Although Ink4c mRNA expression is extinguished by P14, p18Ink4c is a stable protein that remains detectable throughout the migratory phase (Antoine Forget, OA, CJS, and MFR, unpublished observations). Unlike the transient expression of p18Ink4c, p27Kip1 expression is restricted to post-mitotic neurons in the inner EGL and is maintained in granule neurons within the IGL throughout the life of the animal (6–8).
Medulloblastomas (MBs), the most common malignant pediatric brain tumors, are thought to arise, at least in part, from GNPs that fail to exit the cell cycle, migrate and properly differentiate (9). Genetic anomalies in the SHH signaling pathway including mutations in PATCHED and SUFU (a negative regulator of the SHH-stimulated transcription factor GLI1) have been detected in ~25% of human MBs (1). Methylation of the CDKN2C/INK4C promoter was demonstrated in ~10% of MBs from 43 patients admitted to St. Jude Children’s Research Hospital, and a somewhat higher percentage (22% of 73 MBs) expressed no detectable p18INK4C protein (6). While mice heterozygous for Ptc1 develop MB at relatively low frequency (10–12), the time of onset and incidence of tumor development increases dramatically in an Ink4c nullizygous or heterozygous background (6). Low levels of p27KIP1 have been associated with poor outcome in many forms of human cancer, including brain tumors such as astrocytoma and glioblastoma (13). However, the role of Kip1 in MB has not previously been evaluated. We now provide evidence that genetic disruption of either one or two alleles of Kip1 in Ptc1+/− mice greatly increases MB incidence. All such MBs lose expression of the wild type Ptc1 allele but retain functional p53, thereby providing a highly penetrant mouse MB model that closely mimics a subset of the human disease (1).
Results
Expression of Cell Cycle Regulatory Proteins during Cerebellar Development
To compare the expression of key cell cycle regulators with that of p27Kip1 and p18Ink4c during postnatal cerebellar development, we analyzed their protein levels in normal cerebellar tissues between P1 and P30 (Fig. 1A). Cyclin D1, Cyclin D2, Cdk4 and Cdk2 protein levels peaked at P5, corresponding to the time of maximum GNP proliferation, and slowly decreased thereafter. Cdk2 levels were undetectable by P17, whereas Cyclin D1, Cyclin D2 and Cdk4 levels were maintained at low levels until P30. Cdk6 levels remained relatively constant between P1 and P20. P27Kip1 was detected from P1 through P30; its level was relatively constant between P1 and P7 but increased at P10, as more cells exited the cell division cycle (6) and remained elevated thereafter. In contrast, p18Ink4c was barely detectable at P1. Its expression reached a peak at P10 after which it progressively declined and was no longer detectable by P20. Two other Cip/Kip proteins, p21Cip1 and p57Kip2, were not expressed at significant levels (data not shown), consistent with earlier reports (7, 8). Together, these data suggest that p18Ink4c and p27Kip1 coordinate cell cycle exit, whereas the continuing expression of p27Kip1 presumably helps to maintain neurons in their post-mitotic state.
FIGURE 1.
Expression of cell cycle regulators during cerebellar development. A. Expression of proteins (indicated at the left of the panel) was detected by immunoblotting of protein lysates generated from the cerebella of postnatal (P1 to P30) wild type mice. Actin was used as a loading control. B. The bar graphs indicate the percentage of primary GNPs that incorporated BrdU under different culture conditions (panels a, b, and c). GNPs were purified from the cerebella of P10 mice of different genotypes [wild type (wt) (black bar), Ink4c-null (unfilled bar), and Kip1-null (shaded bar)]. GNPs were cultured for one day (panel a) or 3 days (panel b) without Shh, or cultured one day without Shh followed by 2 days with Shh (panel c). BrdU was added 24 hours before the fixation of the cells.
We next evaluated the effect of Kip1 inactivation on the proliferation of cultured GNPs and compared the results with those obtained with GNPs lacking Ink4c, whose loss was previously revealed to endow cells with an enhanced proliferative capacity (6). GNPs purified from the cerebella of wild type, Ink4c-null, and Kip1-null mice were cultured in the absence of Shh for one day (Fig. 1B, panel a) or for three days (Fig. 1B, panel b), or in the complete absence of Shh for 1 day followed by 2 day restimulation with Shh (Fig. 1B, panel c). Cultures were exposed to BrdU for 24 hours either throughout the duration of the 24 hour experiment (panel a) or during the final 24 hour intervals (panels b and c) before fixing the cells and determining the percentage that had incorporated the precursor. When deprived of Shh for one day, GNPs from Kip1-null, Ink4c-null, and wild type mice displayed comparable levels of DNA synthesis with at least 30% of the cells incorporating BrdU during the labeling period (Fig. 1B, panel a). However, after two days of Shh starvation, virtually no GNPs from wild type mice incorporated BrdU in the ensuing 24 hours; in contrast, a significant fraction of GNPs explanted from Ink4c-null and, to a greater extent, Kip1-null mice could still incorporate BrdU (Fig. 1B, panel b). When GNPs were grown in the absence of Shh for one day followed by a two day restimulation with Shh, an even more significant proliferative advantage was documented for cells lacking either Ink4c or Kip1 (Fig. 1B, panel c). These data are consistent with previous reports demonstrating enhanced in vitro proliferation of GNPs from Kip1-null mice compared to those from wild type animals (7, 8). Therefore, loss of Kip1 can delay cell cycle exit and maintain GNP proliferation even in the absence of Shh.
Increased MB Incidence in Ptc1+/−;Kip1+/− and Ptc1+/−;Kip1−/− Mice
To assess whether loss of Kip1 might accelerate MB formation in tumor-prone Ptc1 heterozygous mice, Kip1-null mice were interbred with Ptc1+/− animals to derive Ptc1+/−;Kip1+/− offspring and later generation Ptc1+/−;Kip1−/− cohorts that were prospectively followed for tumor formation. The overall survival of Ptc1+/− mice of the three possible Kip1 genotypes revealed detrimental effects of Kip1 loss of function on lifespan, with bi-allelic Kip1 deletion exerting the most drastic effect (Fig. 2A, panel a). Although the majority of morbidity in these cohorts was due to MB development, a fraction of the mice in each group developed other tumor types (hemangiosarcomas, intestinal tumors, rhabdomyosarcomas, and lymphomas) as well as hydrocephalus unrelated to tumor development (Fig. 2A, panel b). The latter abnormalities collectively accounted for MB-independent deaths as the animals were further aged (10, 12, 14).
FIGURE 2.
Kip1 loss increases the incidence of MBs in Ptc1 heterozygous mice. A. Panel a illustrates survival curves for Ptc1+/− mice retaining two wild type Kip1 alleles (+/+: black line, n= 70), or lacking one (+/−: grey dotted line, n= 144) or two (−/− grey line, n= 30) Kip1 alleles. The incidence of MBs and other tumors arising in these cohorts is indicated in the Table in panel b. Mice showing signs of disease without any obvious brain tumors developed hydrocephaly, hemangiosarcomas, intestinal tumors, rhabdomyosarcomas or lymphomas. Not all mice succumbed to tumors. B. Survival curves of Ptc1+/− mice of different Kip1 genotypes with confirmed MB development. Line designations are the same as for panel a; the number of mice in each cohort was 30 for Kip1+/+, 86 for Kip1+/−, and 20 for Kip1−/− genotypes. C. Detection by immunoblotting of the p27Kip1 protein in GNPs purified from a P7 wild type cerebellum and in purified tumor cells from MBs arising in Ptc1+/−;Kip1+/+ and Ptc1+/−;Kip1+/− mice as designated at the top of the panels. Actin was used a loading control. D. Expression of p27Kip1 determined by immunohistochemistry (brown) in cerebellar sections from Ptc1+/− mice of different Kip1 genotypes. All sections were counterstained with hematoxylin (blue). Panels a, b, and c illustrate sections taken from normal cerebellar tissues from Ptc1+/−;Kip1+/+, Ptc1+/−;Kip1−/−, and Ptc1+/−;Kip1+/− mice, respectively. Panels d, e, and f illustrate p27Kip1 expression in MBs arising in Kip1+/+, Kip1−/−, and Kip1+/− mice, respectively. Panel b was photographed at 10X magnification; the unstained region represents the external molecular layer of the adult cerebellum which is practically devoid of cell bodies. All other panels were photographed at 40X magnification. Panels a and c denote cells within the p27Kip1-positive IGL.
Ptc1+/− mice were previously reported to develop MB with a relatively low incidence (12–22%) and a mean time of tumor occurrence of 5–6 months (10, 12). A greater incidence of MB (42%) in Ptc1+/−;Kip1+/+ mice from our colony (Fig. 2A, panel b) was most likely due to strain-specific genetic differences (14, 15). Loss of one or two alleles of Kip1 further increased the overall incidence of MB in Ptc1+/− mice to ~60% and ~67%, respectively (Fig. 2A, panel b). Although the inactivation of one Kip1 allele did not increase the incidence of other tumor types observed in Ptc1+/− mice (12–14% overall), 30% of Ptc1+/−;Kip1−/− mice developed tumors other than MBs (Fig. 2A, panel b). No grossly overt pituitary tumors, a hallmark of Kip1-null mice (16–18), were observed in Ptc1+/−;Kip1−/− mice with MB, reflecting the more rapid deaths of the compound-deficient animals. Indeed, when we limited our focus to those mice that developed MB only (Fig. 2B), their median times of survival were shorter than those of their overall cohorts, documenting that MBs arose earlier than other tumor types (compare Fig. 2B to 2A, panel a). Among the animals with MB only, the Ptc1+/−;Kip1−/− mice died somewhat more rapidly (median survival 114 days) than the Ptc1+/−;Kip1+/− (127 days) or Ptc1+/−;Kip1+/+ mice (158 days). Differences in survival between Ptc1+/−;Kip1+/+ versus Ptc1+/−;Kip1+/− mice were not significant (p = 0.13), but inactivation of both Kip1 alleles significantly accelerated MB formation (Ptc1+/−;Kip1+/+ versus Ptc1+/−;Kip1−/− p = 0.016; Ptc1+/−;Kip1+/− versus Ptc1+/−;Kip1−/− p = 0.034). Therefore, Kip1 collaborates with Ptc1 to prevent development of MB and other tumor types.
We confirmed by immunoblotting that p27Kip1 expression was maintained in tumor cells purified from MBs resected from six of six Ptc1+/−;Kip1+/− mice (Fig. 2C, lanes 4 to 9). The levels of p27Kip1 expressed in these tumors were approximately half those detected in MBs arising in Ptc1+/−;Kip1+/+ mice (Fig. 2C, lanes 2 and 3), consistent with the concept that Kip1 is haploinsufficient for tumor suppression (19). Immunohistochemical staining (IHC) of sections of normal cerebellar tissue from one month old Ptc1+/− animals confirmed that p27Kip1 was localized within the nuclei of mature post-mitotic neurons residing in the IGL (Fig. 2D, panel a). As expected, no staining was detected in mature granule neurons in the IGL of Ptc1+/−;Kip1−/− mice (Fig. 2D, panel b), whereas p27Kip1 was present in their Kip1+/− counterparts (Fig. 2D, panel c). P27Kip1 was also found in the nuclei of MBs that arose spontaneously in Ptc1+/−;Kip1+/+ mice (Fig. 2D, panel d) while, as expected, it was not detected in MBs from Ptc1+/−;Kip1−/− mice (Fig. 2D, panel e). Notably, p27Kip1 expression was retained in the nuclei of four of four Ptc1+/−;Kip1+/− MBs (Fig. 2D, panel f shows representative data). Because nucleotide sequencing of PCR-amplified Kip1 cDNAs from the latter MBs revealed no mutations, the collective findings confirmed that Kip1 is haploinsufficient for MB suppression, as it is in other tumor types (19).
MBs from Ptc1+/−;Kip1−/− Mice Are Less Differentiated and More Invasive Than MBs from Ptc1+/−;Kip1+/+ Animals
Histological examination of MBs revealed morphological patterns that displayed distinctive immunohistochemical features, consistent with their degrees of cell differentiation (Fig. 3). MBs from Ptc1+/−;Kip1+/+ mice, although capable of expanding into large masses (Fig. 3A), were uniformly noninvasive (0/11). In 90% of cases, there was a biphasic architectural pattern (Fig. 3B) in which cells either expressed Ki-67, a marker of proliferation (Fig. 3C), or instead, the neuronal nuclear antigen (NeuN), a marker of differentiated neurons (Fig. 3D). However, focal regions of these tumors exhibited more anaplastic features in which virtually all cells were Ki-67-positive and NeuN-negative (not shown). The histological patterns in these murine tumors mimicked different phenotypes encountered in the human disease, including the classic biphasic phenotype and anaplastic variants seen in tumors with MYC amplification (20, 21).
FIGURE 3.
Pathological characteristics of MBs. Tumors from Ptc1+/−;Kip1+/+ mice (panels A–D) were non invasive and confined to the periphery of the cerebellum (A), whereas those arising in Ptc1+/−;Kip1−/− mice (panels E–H) were more invasive and extended into the white matter (E). Interrupted black dashes in E designate the contours of the invaded lobes. Sections were stained with hematoxylin and eosin (A, B, E, F) and by immunohistochemistry with antibodies to Ki-67 (C and G) or to NeuN (D and H) with hematoxylin counterstain. Tumors that retained both Kip1 alleles commonly exhibited a biphasic pattern (B) in which differentiated Ki-67-negative and Neu-N-positive neurons were surrounded by proliferating Ki-67-positive and NeuN-negative cells (serial sections shown in C and D). Kip1−/− MBs were comprised of more uniform sheets of tumor cells (F) that were Ki-67-positive (G) and NeuN-negative (H). Magnifications were 2X (panel A), 40X (panels B, C, D and F) and 4X (panels E, G and H).
In contrast, MBs from Ptc1+/−;Kip1−/− and Ptc1+/−;Kip1+/− animals were more invasive than MBs arising in Ptc1+/−;Kip1+/+ animals and penetrated the white matter, in some cases completely obliterating the normal contours of the cerebellar lobes (Fig. 3E). Uniform sheets of tumor cells (Fig. 3F) were mostly Ki-67-positive (Fig. 3G) and NeuN-negative (Fig. 3H). A similar frequency of invasion into the white matter was observed in 3 of 10 Ptc1+/−;Kip1+/− and 4 of 12 Ptc1+/−;Kip1−/− MBs, implying that invasiveness per se did not account for the accelerated demise of animals that were completely Kip1-deficient. Indeed, invasion was seen even more frequently in MBs arising in Ptc1+/−;Ink4c−/− mice (7 of 11, data not shown), although these animals have a better overall survival than Ptc1+/− mice lacking one or both Kip1 alleles (6). Analysis of cerebella from one month old Ptc+/−;Kip1+/+, Ptc1+/−;Kip1+/−, Ptc1+/−;Kip1−/− and Ptc1+/−;Ink4c1−/− MB-prone mice revealed preneoplastic lesions in the outer molecular layer where neuronal progenitors within the EGL had previously resided (data not shown); this suggests that MBs arose from GNPs within the EGL that failed to migrate. Thus, the increased morbidity of mice lacking Kip1 alleles correlated most closely with the increased proliferative index of the tumor cells and their failure to differentiate.
Molecular Characterization of MBs from Ptc1+/−;Kip1−/− Mice
MBs from Ptc1+/− mice lose the expression of the wild type Ptc1 allele (22). We therefore assessed expression of Ptc1 in purified tumor cells from MBs occurring in Ptc1+/−;Kip1+/− and Ptc1+/−;Kip1−/− mice. By quantitative real time PCR, we found that Ptc1 mRNA could not be detected in all such tumors (data not shown) implying that the Shh signaling pathway was constitutively activated. To confirm this, tumor cells purified from the cerebella of Ptc1+/−;Kip1−/− animals were cultured in the presence of cyclopamine, a specific inhibitor of Shh signaling (23). These cells completely ceased proliferating after 3 days of treatment (data not shown), underscoring their dependence on the Shh pathway.
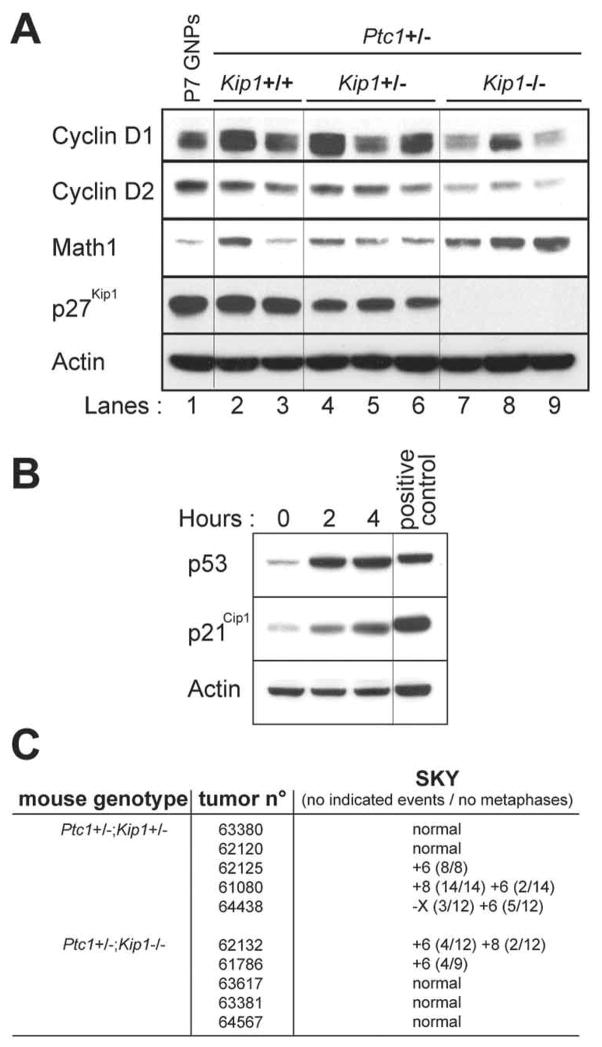
The transcription factor, Math1/Atoh1, is expressed in proliferating GNPs, but its expression is extinguished once GNPs in the EGL exit the cell cycle and begin to differentiate and start migrating toward the IGL (24). Downregulation of Math1/Atoh1 in response to signaling by bone morphogenic proteins (BMPs) or transcriptional repression mediated by Hic1 induces the neuronal differentiation of GNPs and of Ptc1−/− MB tumor cells derived from them (25, 26), suggesting that Math1/Atoh1 expression is required to prevent the differentiation of GNPs. Math1/Atoh1 expression is also a hallmark of human MBs harboring a SHH signaling pathway expression signature (25). Immunoblotting of protein lysates revealed that Math1/Atoh1 was expressed in Ptc1−/− tumor cells purified from mice of all three Kip1 genotypes (Fig. 4A) confirming that the Shh signaling pathway was constitutively activated in these MBs. The levels of Math1/Atoh1 were elevated in MBs from Ptc1+/−;Kip1−/− mice, consistent with the enhanced proliferative capacity and more aggressive nature of these tumors.
FIGURE 4.
Molecular analysis of GNPs and MBs. A. Immunoblotting of the indicated proteins (left) expressed in primary GNPs purified from a cerebellum of a P7 wild type mouse and from GNP-like tumor cells purified from MBs occurring in Ptc1+/− mice lacking no (+/+), one (+/−) or two (−/−) Kip1 alleles. Actin was used as loading control. B. Accumulation of p53 and p21Cip1 2 and 4 hours after exposure to 15 Gy ionizing radiation in tumor cells purified from MBs from Ptc1+/−;Kip1−/− mice. Zn2+-treated MT-Arf cells were used a positive control. C. Spectral karyotyping (SKY) of MBs from representative tumors arising in Ptc1+/−;Kip1+/− and Ptc1+/−;Kip1−/− animals was used to determine the nature and frequency of chromosomal anomalies.
BMPs inhibit the proliferation of tumor cells from Ptc1+/−;Ink4c−/− mice and induce their differentiation by rapid post-transcriptional downregulation of Math1/Atoh1 (25). Despite their relatively elevated levels of Math1/Atoh1 expression, tumor cells from Ptc1+/−;Kip1−/− mice remained sensitive to BMP treatment (S phase: 18% without treatment; 5% after BMP treatment, n = 2) suggesting that BMP-dependent cell cycle arrest and differentiation is independent of both Kip1 and Ink4c.
P21Cip1 and p27Kip1 are necessary for the assembly and stability of D-type cyclins into complexes with Cdk4 and Cdk6 (5, 27). In turn, mouse embryo fibroblasts, hepatocytes, and thymic lymphoid cells lacking p27Kip1 and p21Cip1 each exhibited reduced levels of D-type cyclins (27). Although the levels of cyclin D1 in purified tumor cells from MBs from Ptc1+/−;Kip1+/+ and Ptc1+/−;Kip1+/− mice differed between individual tumors, they were higher or equivalent to those observed in P7 wild type GNPs (Fig. 4A). In the same tumors, cyclin D2 levels were somewhat less variable and were similar to those observed in P7 wild type GNPs. However, reduced overall levels of cyclins D1 and D2 were observed in purified tumor cells from MBs arising in Ptc1+/−;Kip1−/− animals, consistent with previous observations that D-type cyclins are more rapidly degraded when p27Kip1 is absent (27, 28). The fact that MBs lacking Kip1 arise more rapidly implies that, in the absence of p27Kip1, unfettered “downstream” Cdks drive the cell cycle even when cyclin D levels are reduced (28).
To assess their p53 status, tumor cells were purified from MBs of Ptc1+/−;Kip1−/− mice, cultured for one day, and then subjected to 15 Gγ of ionizing radiation. P53 and p21Cip1 protein levels, detected by immmunoblotting 2 and 4 hours after irradiation, accumulated in response to ionizing radiation (Fig. 4B) confirming that p53 and a canonical p53-responsive gene product could be activated by DNA damage in these tumors. Spectral karyotyping (SKY) analysis of MBs from Ptc1+/−;Kip1+/− and Ptc1+/−;Kip1−/− mice revealed that they had not sustained chromosomal translocations but exhibited recurrent gains of chromosomes 6 and 8 (Fig. 4C). The most frequent event was trisomy 6 in 50% of cases (5/10), as previously reported in several mouse MB models (29–31). The overall lack of aneuploidy and presence of a normal karyotype in half of the analyzed tumors is consistent with the fact that retention of p53 counters the widespread genomic instability generally associated with its loss of function.
Discussion
Several lines of evidence suggest that Ink4 and Cip/Kip1 proteins collaborate to induce G1-phase arrest (5). In cycling cells, Ink4 gene expression is limited, and most Cip/Kip proteins are assembled into complexes with cyclin D-Cdks. Induction of p18Ink4c and its binding to Cdk4 and Cdk6 not only disrupts cyclin D-Cdk complexes and accelerates cyclin D turnover but mobilizes p27Kip1 into complexes with cyclin E/A-Cdk2, thereby inhibiting all G1 cyclin-dependent kinase activity and inducing exit from the cell cycle. These interrelationships are consistent with observations that inactivation of either Ink4c or Kip1 in mice leads to similar phenotypes, including organomegally and predisposition to certain tumor types (16–18, 32, 33). Moreover, both Ink4c and Kip1 can act as haploinsufficient tumor suppressors (6, 19, 34). However, the fact that the simultaneous disruption of both genes widens the tumor spectrum and further accelerates tumor formation argues that the two Cdk inhibitors not only functionally collaborate but act synergistically (35).
The patterns of expression of p27Kip1 and p18Ink4c during postnatal development of the cerebellum are consistent with their overlapping but differential functions in regulating cell cycle exit in GNPs. During the early postnatal period, the EGL rapidly expands, and maximal overall GNP proliferative rates are manifested at P5–P7, after which the cells exit the division cycle and migrate into the IGL. P27Kip1, which accumulated to maximal levels by P10, is detected exclusively in post-mitotic GNPs, whether present in the inner part of the EGL during its expansion or later in the IGL; its expression in mature granule neurons then persists throughout the lifetime of the mice (6, 7). However, Ink4c mRNA expression in GNPs is transient, peaking at P7 but becoming undetectable by P14 (6). Because the p18Ink4c protein is relatively stable (half-life ~12 hrs; A. Forget, OA, CJS, and MFR, unpublished observations), its expression lags behind that of its mRNA. Peak levels of p18Ink4c were detected at P10 and declined slowly thereafter, vanishing by P17–P20. Thus, p18Ink4c expression persists even in the absence of new synthesis throughout the period when most postmitotic cells migrate into the IGL.
Constitutive activation of the Shh signaling pathway induced MBs whose formation was accelerated when either one or two alleles of Kip1 were co-inactivated. Although co-inactivation of Ptc1 and Kip1 alleles in the mouse germline predisposed to several tumor types, MBs were by far the most frequent in the younger animals. MBs from Ptc1+/−;Kip1−/− mice were less differentiated and more invasive than MBs from their Ptc1+/−;Kip1+/+ counterparts, correlating with the greater incidence and more rapid course of MBs in animals lacking p27Kip1 function. MBs lacking Kip1 expressed high levels of Math1/Atoh1, a basic helix-loop-helix (bHLH) transcription factor expressed exclusively in that subset of GNPs that are actively proliferating. This underscores the idea that p27Kip1 helps to trigger exit of GNPs from the cell cycle and enforces their differentiation. In tumors arising in Ptc1+/−;Kip1+/− mice, the wild type p27Kip1 protein was persistently expressed in purified tumor cells, consistent with previously documented Kip1 haploinsufficiency in other tumor types (19). In contrast, the wild type Ptc1 allele was not expressed in tumor cells and functioned as a canonical tumor suppressor gene.
Although many mouse MB models rely on the loss of functional p53 for high tumor penetrance, MBs from Ptc1+/−;Kip1+/− or Ptc1+/−;Kip1−/− mice, like those arising in Ptc1+/−;Ink4c+/− or Ptc1+/−;Ink4c−/− mice, retained functional wild type p53 and were not aneuploid, similar to the vast majority of human MBs (1). This raises the possibility that inactivation of either Kip1 or Ink4c might limit the p53 response to oncogenic signaling through the altered Ptc1 pathway, thereby bypassing any need to mutate p53 in this setting. Indeed, Carneiro and cowokers suggested that the absence of p27Kip1 might curb the p19Arf-induced p53 response during pituitary tumorigenesis (36); however, the inactivation of Arf does not accelerate MB formation in Ptc1+/− mice (12), effectively ruling out such a mechanism. Neurons lacking Ptc1 exhibit an increased sensitivity to DNA damaging agents (37), so the retention of a functional p53 checkpoint in this subset of MBs implies that they would be more sensitive to genotoxic therapy than surrounding normal tissue.
Low levels of p27KIP1 are associated with poor prognoses in many different human cancers, including brain tumors such as astrocytoma and glioblastoma (13, 38). Although the role of p27Kip1 as a biomarker in human MB remains unclear, determining its level of expression may have prognostic value, and its further evaluation in this disease is warranted.
Materials and Methods
Animal Husbandry
Kip1-null male mice (18) were bred to Ptc1+/− female mice (10) to obtain Ptc1+/−;Kip1+/− F1 animals. By intercrossing these double heterozygotes, we obtained Ptc1+/+;Kip1+/− females and Ptc1+/−;Kip1+/− males which were bred to one another to generate other genotypes. Mice were observed twice weekly for a period of 10 months. All mice were maintained on a mixed C57BL/6 X 129Sv background. The generation of Ptc1+/−;Ink4c−/− mice was previously described (6). Mice were housed in an American Association of Laboratory Animal Care-accredited facility and maintained in accordance with NIH guidelines. The Animal Care and Use Committee at St. Jude Children’s Research Hospital approved all procedures. Survival curves and median survival were plotted and calculated using GraphPad Prism 4.
Purification and Culture of Primary GNPs and GNP-like Tumor Cells
Purification of GNPs from cerebella of mice with different genotypes and of GNP-like tumor cells from MBs were performed as described (6, 25). Proliferation was determined by a BrdU incorporation assay performed with 6 × 105 GNPs per ml plated in poly-D lysine and Matrigel-pre-coated eight-well Lab-Tek glass dishes (6). We counted >500 cells per well and recorded the number of BrdU-positive cells detected by fluorescence within the total population enumerated by counterstaining with DAPI (Sigma, St. Louis MO). Each experiment was repeated at least 3 times. Purified tumor cells were grown in the absence of Shh and treated with cyclopamine or BMP, as previously described (25). Zn2+-inducible MT-Arf cells were used as positive control for p53 and p21Cip1 induction as previously described (39).
Protein Analysis by Immunoblotting and Quantitative Real-time PCR
Proteins extracted from cells were electrophoretically separated on polyacrylamide denaturing gels, transferred onto nitrocellulose membranes, and immunoblotted with the designated antibodies as previously described (30). Antibodies to mouse proteins used at 1:500 dilution were directed to cyclin D1 (72-13G), cyclin D2 (34B1-3), Cdk4 (C-22), Cdk6 (C-21), Cdk2 (M2), p21Cip1 (F-5), and β-Actin (C-11) (all from Santa Cruz Biotechnology, Santa Cruz CA); Math1/Atoh1 (Developmental Studies Hybridoma Bank, NIH); p27Kip1 (clone 57; BD Transduction Laboratories, San Jose CA); and p53 (1C12; Cell Signaling, Danvers MA). Quantitative real-time PCR with RNA extracted from purified GNP-like tumor cells was performed as described (30).
Histopathology, Iimmunohistochemistry and Spectral Karyotyping
Histopathology and immunohistochemistry were performed as previously described (30) using the following antibodies: anti-NeuN (clone A60, 1:50, Chemicon, Temecula CA); anti-Ki67 (1:1000, Vector Laboratories, Burlingame CA), and anti-p27Kip1 (1:100, C19, Santa Cruz Biotechnology). SKY was performed as previously described (30)
Acknowledgments
We thank all members of the Roussel/Sherr laboratory for helpful discussions. We also thank Robert Jenson, Shelly Wilkerson and Deborah Yons for mouse husbandry, Suqing Xie for Q-RT-PCR, Robert Jenson, Suqing Xie and Shelly Wilkerson for mouse genotyping, Shelly Wilkerson and Dorothy Bush for immunohistochemistry staining, Brenda McGowan and Shelly Wilkerson for tissue sectioning, Virginia Valentine for SKY analysis, Rose Mathew for cultures of GNPs, and David Ellison for review of histopathology.
This work was funded in part by NCI grant P01-CA-096832 (MFR & CJS) an NCI Cancer Center Core Grant CA-21765 to St. Jude Children’s Research Hospital, La Fondation pour la Recherche Medicale and a Gephardt Endowed Fellowship (OA), and the American Lebanese-Syrian Associated Charities of St. Jude Children’s Research Hospital. CJS is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Marino S. Medulloblastoma: developmental mechanisms out of control. Trends Mol Med. 2005;11:17–22. doi: 10.1016/j.molmed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;27:484–91. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- 3.Ciemerych MA, Kenney AM, Sicinska E, Kalaszczynska I, Bronson RT, Rowitch DH, et al. Development of mice expressing a single D-type cyclin. Genes Dev. 2002;16:3277–89. doi: 10.1101/gad.1023602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zindy F, Knoepfler PS, Xie S, Sherr CJ, Eisenman RN, Roussel MF. N-Myc and the cyclin-dependent kinase inhibitors p18Ink4c and p27Kip1 coordinately regulate cerebellar development. Proc Natl Acad Sci USA. 2006;103:11579–83. doi: 10.1073/pnas.0604727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherr C, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Uziel T, Zindy F, Xie S, Lee Y, Forget A, Magdaleno S, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev. 2005;19:2656–67. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazawa K, Himi T, Garcia V, Yamagishi H, Sato S, Ishizaki Y. A role for p27/Kip1 in the control of cerebellar granule cell precursor proliferation. J Neurosci. 2000;20:5756–63. doi: 10.1523/JNEUROSCI.20-15-05756.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizaki Y. Control of proliferation and differentiation of neural precursor cells: focusing on the developing cerebellum. J Pharmacol Sci. 2006;101:183–8. doi: 10.1254/jphs.cpj06011x. [DOI] [PubMed] [Google Scholar]
- 9.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol Mech Dis. 2008;3:341–65. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 10.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 11.Goodrich LV, Scott MP. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–57. doi: 10.1016/s0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- 12.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–6. [PubMed] [Google Scholar]
- 13.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 14.Pazzaglia S. Ptc1 heterozygous knockout mice as a model of multi-organ tumorigenesis. Cancer Let. 2006;234:124–34. doi: 10.1016/j.canlet.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Pazzaglia S, Mancuso M, Tanori M, Atkinson MJ, Merola P, Rebessi S, et al. Modulation of patched-associated susceptibility to radiation induced tumorigenesis by genetic background. Cancer Res. 2004;64:3798–806. doi: 10.1158/0008-5472.CAN-03-3716. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 17.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–32. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 18.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–44. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 19.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–80. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellison D. Classifying the medulloblastomas: insights from morphology and molecular genetics. Neuropathol Appl Neurobiol. 2002;28:257–82. doi: 10.1046/j.1365-2990.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 21.McManamy CS, Pears J, Weston CL, Hanzely Z, Ironside JW, Taylor RE, et al. Nodule formation and desmoplasia in medulloblastomas -- defining the nodular/desmoplastic variant and its biological behavior. Brain Pathol. 2007;17:151–64. doi: 10.1111/j.1750-3639.2007.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver TG, Read TA, Kessler JD, Mehmeti A, Wells JF, Huynh TT, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132:2425–39. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 23.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–61. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Arie N, Bellen HJ, Armstrong DLMAE, Gordadze PR, Guo Q, Matzuk MM, et al. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–72. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Ayrault O, Zindy F, Kim J-H, Roussel MF. Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev. 2008;22:722–7. doi: 10.1101/gad.1636408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briggs KJ, Corcoran-Schwartz IM, Zhang W, Harcke T, Devereux WL, Baylin SB, et al. Cooperation between the Hic and Ptch1 tumor suppressors in medulloblastoma. Genes Dev. 2008;22:770–85. doi: 10.1101/gad.1640908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, et al. The p21Cip1 and p27Kip1 CDK “inhibitors” are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–83. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng Y, Yu Q, Sicinska E, Das M, Bronson RT, Sicinski P. Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc Nat Acad Sci USA. 2001;98:194–9. doi: 10.1073/pnas.011522998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan CT, Kaushal D, Murphy M, Zhang Y, Datta A, Chen C, et al. XRCC4 suppresses medulloblastomas with recurrent translocations in p53-deficient mice. Proc Natl Acad Sci U S A. 2006;103:7378–83. doi: 10.1073/pnas.0601938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zindy F, Uziel T, Ayrault O, Calabrese C, Valentine M, Rehg JE, et al. Genetic alterations in mouse medulloblastomas and generation of tumors de novo from primary cerebellar granule neuron precursors. Cancer Res. 2007;67:2676–84. doi: 10.1158/0008-5472.CAN-06-3418. [DOI] [PubMed] [Google Scholar]
- 31.Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzer S, Pullar B, et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomenigeal spread. Cancer Res. 2008;68:1768–76. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- 32.Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, et al. CDK inhibitors p18INK4c and p27Kip1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latres E, Malumbres M, Sotillo R, Martin J, Ortega S, Martin-Caballero J, et al. Limited overlapping roles of P15INK4b and P18INK4c cell cycle inhibitors in proliferation and tumorigenesis. EMBO J. 2000;19:3496–506. doi: 10.1093/emboj/19.13.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai F, Pei XH, Godfrey VL, Xiong Y. Haploinsufficiency of p18INK4c sensitizes mice to carcinogen-induced tumorigenesis. Mol Cell Biol. 2003;23:1269–77. doi: 10.1128/MCB.23.4.1269-1277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franklin DS, Godfrey VL, O’Brien DA, Deng C, Xiong Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol. 2000;20:6147–58. doi: 10.1128/mcb.20.16.6147-6158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carneiro C, Jiao MS, Hu M, Shaffer D, Park M, Pandolfi PP, et al. p27 deficiency desensitized Rb−/− cells to signals that trigger apoptosis during pituitary development. Oncogene. 2003;22:261–9. doi: 10.1038/sj.onc.1206163. [DOI] [PubMed] [Google Scholar]
- 37.Pazzaglia S, Mancuso M, Atkinson MJ, Tanori M, Rebessi S, Majo VD, et al. High incidence of medulloblastoma following X-ray-irradiation of newborn Ptc1 heterozygous mice. Oncogene. 2002;21:7580–4. doi: 10.1038/sj.onc.1205973. [DOI] [PubMed] [Google Scholar]
- 38.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2002;183:10–7. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 39.Kuo M-L, Duncavage EJ, Mathew R, den Besten W, Pie D, Naeve D, et al. Arf induces p53-dependent and independent anti-proliferative genes. Cancer Res. 2003;63:1046–53. [PubMed] [Google Scholar]