Abstract
Tetrahydrobiopterin (BH4) is an essential co-factor required for the activity of endothelial nitric oxide synthase (eNOS). Suboptimal concentrations of BH4 in the endothelium reduce the biosynthesis of nitric oxide (NO), thus contributing to the pathogenesis of vascular endothelial dysfunction. Supplementation with exogenous BH4 or therapeutic approaches that increase endogenous amounts of BH4 can reduce or reverse endothelial dysfunction by restoring production of NO. Improvements in formulations of BH4 for oral delivery have stimulated clinical trials which test the efficacy of BH4 in the treatment of systemic hypertension, peripheral arterial disease, coronary artery disease, pulmonary arterial hypertension, and sickle cell disease. This review discusses ongoing progress in the translation of knowledge, accumulated in pre-clinical studies, into the clinical application of BH4 in the treatment of vascular diseases. This review also addresses the emerging roles of BH4 in the regulation of endothelial function and their therapeutic implications.
Introduction
The role of tetrahydrobiopterin (BH4) in the control of nitric oxide synthase (NOS) activity was recognized almost twenty years ago [1–4]. It is now well established that BH4 is an essential co-factor required for enzymatic activity of all three NOS isoforms, endothelial (eNOS), neuronal (nNOS) and inducible (iNOS). Availability of BH4 is regulated by several mechanisms including de novo synthesis, biopterin recycling, and so called “salvage pathway” (Figure 1). In the early 1990s biochemical studies demonstrated that the loss of BH4 may cause uncoupling of nNOS thereby increasing nNOS-derived production of superoxide anion and hydrogen peroxide [5,6] (Figure 2). In 1995, our group reported that in coronary arteries, reduced availability of BH4 in the endothelium causes increase in eNOS-derived production of reactive oxygen species (ROS) including superoxide anion and hydrogen peroxide [7]. Given the ability of superoxide anion to chemically inactivate nitric oxide (NO) we hypothesized that uncoupled eNOS may represent an important mechanism underlying pathogenesis of endothelial dysfunction.
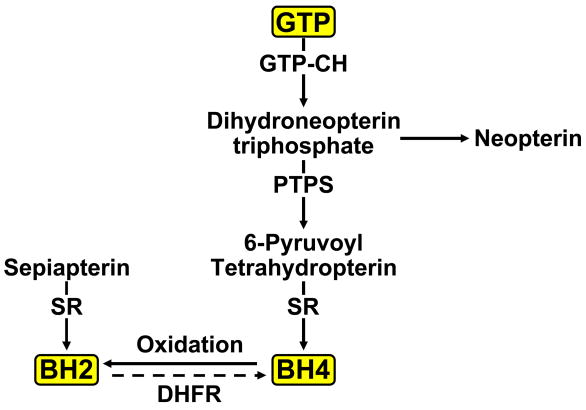
Figure 1.
Biosynthesis of BH4. GTP cyclohydrolase I (GTP-CH) is the rate-limiting enzyme in the de novo synthetic pathway of BH4. Pyruvoyl tetrahydroptrein synthase (PTPS) and sepiapterin reductase (SR) are two additional enzymes required for the final production of BH4. BH4 can also be synthesized from sepiapterin by the so-called “salvage pathway”. BH4 oxidized by peroxynitrite to BH2 can be recycled back to BH4 by activity of dihydrofolate reductase (DHFR). Neopterin is a stable side-product of BH4 that may serve as an indicator of elevated GTPCH activity.
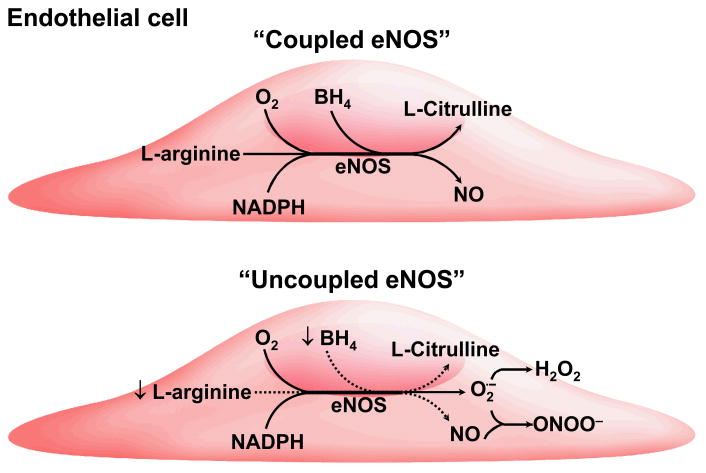
Figure 2.
Schematic representation of endothelial nitric oxide synthase (eNOS) uncoupling. During uncoupling, biosynthesis of nitric oxide (NO) is uncoupled from consumption of NADPH and electron flow is directed towards formation of superoxide anion (O2.−) and hydrogen peroxide (H2O2). Subsequent reaction between superoxide anion and NO generates peroxynitrite anion (ONOO−). Suboptimal concentrations of BH4 (↓).
In attempt to determine the mechanisms responsible for the loss of BH4 in diseased arteries, the attention of many groups, including ours, was initially directed towards interaction between BH4 and ROS. First, these studies confirmed the previously reported propensity of BH4 to autooxidize when exposed to oxygen [8]. Second and more importantly, in 1999 it was discovered that BH4 can be oxidized by peroxynitrite, a potent oxidant, generated by the reaction between superoxide anion and NO [9]. This observation established a chemical antagonism between BH4 and peroxynitrite, thereby suggesting that conditions associated with elevated production of peroxynitrite may induce the uncoupling of eNOS. As a corollary, the ability of BH4 to inactivate peroxynitrite suggested that BH4 may be an important molecule protecting endothelium form oxidative and nitrosative stress induced by peroxynitrite. In this regard, it is important to note that peroxynitrite may alter protein structure and function by reacting with various amino acids in the peptide chain. Cysteine oxidation and tyrosine nitration affect more than 70 different proteins [10]. Theoretically, all of these interactions may be modified by BH4 thereby expanding the influence of BH4 far beyond its role in the regulation of NO production. However, there is no experimental evidence to support the notion that oxidation of BH4 by endogenously generated peroxynitrite reduces intracellular concentration of BH4.
With further progress in the understanding of biochemistry and pharmacology of BH4, it became apparent that BH4 is major vasoprotective molecule. In vivo, this concept was strongly supported by series of studies demonstrating the beneficial effect of genetic supplementation of BH4 on endothelial dysfunction [11–14]. The more recent development of novel pharmaceutical formulations of BH4 enabled investigators to administer BH4 orally, leading to ongoing clinical trials designed to determine the therapeutic efficacy of BH4 in patents with hypertension, peripheral arterial disease, coronary artery disease, and sickle cell disease. In this review we will focus our discussion on the most recent developments in the vascular pharmacology of BH4.
BH4 and Endothelial Dysfunction
Historically, the discovery of endothelium-derived relaxing factor (EDFR) and the subsequent identification of the chemical nature of EDRF as NO were among the most important advances in vascular pharmacology in 1980s. The ground- breaking impact of these discoveries was recognized by the Nobel Prize awarded to Drs. Furchgott, Ignarro and Murad in 1998. The recognition of the endothelium as a major source of vasodilator substances, including NO, had a major impact on the understanding of the pathogenesis of vascular diseases. The concept of endothelial dysfunction evolved as a result of studies on diseased arteries both in experimental animals and in patients with vascular disease [15]. These studies established the loss of NO as a central mechanism of endothelial dysfunction, thereby stimulating the development of novel therapeutic approaches that sought to remedy impaired endothelial production of NO. In addition, drugs currently employed in the treatment of cardiovascular diseases were reevaluated so as to determine the extent to which improved endothelial biosynthesis of NO accounted for their therapeutic effects. Indeed, an increasing focus in the field of cardiovascular drug development is the search for agents that can preserve endothelial function.
Over the last decade numerous studies demonstrated that the chemical inactivation of BH4 by oxidative stress causes uncoupling of eNOS and endothelial dysfunction; the role of BH4 in eNOS uncoupling and the pathogenesis of endothelial dysfunction has been emphasized in recent reviews [16,17]. During the last couple of years, studies utilizing genetically modified mice provided convincing evidence that eNOS uncoupling alone (in the absence of vascular disease) was sufficient to initiate and propagate BH4 oxidation in vivo [18]. In mice over-expressing eNOS, a mismatch between eNOS protein levels and intracellular concentration of BH4 resulted in uncoupling of over-expressed eNOS. This, in turn, led to increased formation of eNOS-derived superoxide anion. Indeed, further pharmacological and genetic analysis demonstrated that uncoupled eNOS was a major source of superoxide anion. This study underscored the fact that even in the absence of vascular disease, up-regulation of eNOS may adversely affect endothelial function if the concentrations of BH4 do not commensurately increase so as to attain levels required for optimal activity of eNOS. Consistent with this concept, long-term transgenic over-expression of eNOS in ApoE-KO (apolipoprotein E knockout) mouse model of atherosclerosis had a detrimental rather than a beneficial effect on plaque formation [19]; and, most importantly, supplementation with BH4 abolished the accelerated atherosclerosis detected in ApoE-KO mice over-expressing eNOS. These observations were explained, and plausibly so, by BH4-induced eNOS re-coupling. They also demonstrated that therapeutic approaches designed to elevate expression of eNOS protein may not improve production of NO if the local environment in the vascular endothelium becomes relatively deficient in BH4. In fact, activity of uncoupled eNOS may contribute to oxidative stress and exacerbate endothelial dysfunction. In accordance with this concept, numerous in vivo studies in rodent models as well as in humans have consistently demonstrated the beneficial effect of BH4 on endothelial dysfunction, thereby attesting to the vasoprotective properties of BH4 (Table).
Table.
Effect of Administration of BH4 in vivo on Human Vascular Function
| Condition | Effect | Reference |
|---|---|---|
| Hypercholesterolemia | prevents endothelial dysfunction | 60 |
| Coronary artery disease | prevents endothelial dysfunction | 61 |
| Chronic smokers | prevents endothelial dysfunction | 20 |
| Long-term smokers | prevents endothelial dysfunction | 62 |
| Coronary risk factors | prevents endothelial dysfunction | 63 |
| Normo- or hypertension | augments endothelium-dependent vasodilatation | 64 |
| Vasospastic angina | prevents endothelial dysfunction | 65 |
| Chronic heart failure | prevents endothelial dysfunction | 66 |
| Hypercholesterolemia | prevents endothelial dysfunction | 67 |
| Glucose challenge | prevents endothelial dysfunction | 68 |
| Type 2 diabetes | increases insulin sensitivity but does not improve endothelial function | 69 |
| Endotoxin-induced endothelial dysfunction | prevents endothelial dysfunction | 70 |
| Aging | improves flow-mediated dilatation | 71 |
| Aging | prevents endothelial dysfunction | 72 |
| Erectile dysfunction | prevents erectile dysfunction | 73 |
| Ischemia reperfusion | prevents endothelial dysfunction | 21 |
| Atherosclerosis | does not improve endothelial function | 74 |
| Hypercholesterolemia | prevents endothelial dysfunction and decreases oxidative stress | 34 |
| Hypertension | prevents endothelial dysfunction and decreases arterial blood pressure | 35 |
| Hypercholesterolemia | improves dysfunction of coronary microcirculation | 75 |
| Type 2 diabetes | prevents endothelial dysfunction | 33 |
It is however also important to keep in mind that BH4 is a potent reducing agent, and that BH4 may act as an anti-oxidant. Analogues of BH4 that are antioxidant but are incapable of supporting eNOS activity have been successfully employed to dissect the contribution of the anti-oxidant capacity of BH4 to its overall beneficial effect on vascular function [20]. Although the existing evidence suggests that re-coupling of eNOS and stimulation of NO production are more important protective mechanisms, anti-oxidant properties of BH4 may also contribute to preservation of endothelial function [21].
BH4 and Oxidative Stress
Oxidative stress is imposed when the generation of oxidants outstrips the rate at which they can be metabolized or degraded, thereby leading to an increase in ambient concentrations of these oxidizing species. As mentioned previously, BH4 is a potent reducing agent and therefore a vulnerable molecular target for oxidation. More recent evidence suggests that besides peroxynitrite, superoxide anions generated by can also oxidize Oxidative degradation of BH4 is complex and depends on a number of factors including pH [9]. At neutral pH, 7,8-dihydropterin is the most abundant product of BH4 oxidation by peroxynitrite. Thus, a major portion of BH4 is oxidized irreversibly to cofactor inactive molecule (7,8-dihydropterin), thereby disabling intracellular regeneration of BH4 [9]. Only about one third of the BH4 that is oxidized to 7,8-dihydrobiopterin (BH2) may be regenerated into BH4 by dihydrofolate reductase. It should be emphasized that BH2 can compete and displace BH4 from eNOS, thereby fostering the uncoupling of eNOS initially incurred by the oxidation of BH4 [22,23]. The potential importance of peroxynitrite as an oxidant responsible for the loss of BH4 is underscored by the fact that the rate constants for the reactions of peroxynitrite with major cellular antioxidants (specifically, ascorbate, cysteine and glutathione) are about 6–10 times lower as compared to the rate constant for the reaction of peroxynitrite with BH4 [24]. This makes BH4 highly susceptible to oxidative attack by peroxynitrite. However, it is important to note that direct experimental evidence supporting the ability of endogenous peroxynitrite to oxidize intracellular BH4 is still lacking. More recent finings suggest that BH4 may be oxidized in the presence of superoxide anion generated by xanthine plus xanthine oxidase [25]. The importance of this observation for the beneficial effects of BH4 is unclear and remains to be determined [25].
We also wish to point out that the chemical antagonism between BH4 and peroxynitrite raises another important question: namely, does the availability of BH4 reduce the risk for local injury by peroxynitrite? Pro-inflammatory cytokines including, tumor necrosis factor-α, interferon-γ, and interleukin-1β, are powerful stimulators of BH4 biosynthesis. In human endothelium, these cytokines increase the expression and activity of GTP cyclohydrolase I, the rate-limiting enzyme in production of BH4 [26,27]. Given the fact that pro-inflammatory cytokines decrease expression of eNOS (but do not increase endothelial expression of iNOS [26]) the role of high endothelial concentration of BH4 during inflammation remains poorly understood. One possibility is that the elevation of BH4 may enhance antioxidant potential of the endothelium in conditions associated with increased production of peroxynitrite [28]. Indeed, BH4 (together with ascorbate) prevents peroxynitrite-induced tyrosine nitration of numerous proteins in cells stimulated with pro-inflammatory cytokines [23]. It is therefore conceivable that under pro-inflammatory conditions, BH4 may be an important anti-oxidant in general, and one that safeguards against peroxynitrite-induced nitration of tyrosine residues, in particular. Since peroxynitrite may affect the function of proteins involved in oxidative stress, energy production, apoptosis, fatty acid metabolism, as well as structural proteins [29], it is likely that the ambient levels of BH4 may thereby influence a wide range of biologic processes. This hypothesis remains to be tested.
Several observations from our group, as yet unpublished, further support an important role for BH4 as an anti-oxidant molecule in the endothelium. For example, we have obtained immunohistochemical evidence that GTP cyclohydrolase I localizes in the caveolar membrane microdomain along with caveolin-1 and eNOS. A strategic, cytoprotective benefit is likely conferred by such localization as the high local concentrations of BH4 within caveolae may safeguard the structural and functional integrity of caveolae under conditions of oxidative stress [30]. Moreover, our prior studies demonstrated that endothelial progenitor cells (EPCs) have a high antioxidant capacity [31], and our recent studies revealed that EPCs also have an intrinsically high intracellular concentration of BH4 (He and Katusic, unpublished observation), despite the fact that expression of eNOS is relatively low [32]. We suggest that these generous amounts of BH4 in EPCs may serve to maintain eNOS in its coupled state in EPCs, to guard against the oxidizing effect of peroxynitrite on eNOS and other targets, and to sustain, in general, the high antioxidant profile of EPCs. An enriched antioxidant capability of EPCs likely confers a survival advantage to EPCs since the regenerative function of EPCs is most needed in injured tissue, the latter commonly exhibiting a milieu of increased oxidative stress. In this regard, the exact contribution of BH4 to the antioxidant capability and regenerative function of EPCs merits further investigation.
BH4 and Therapy of Vascular Disease
Exogenous BH4
Over the last decade, numerous studies have examined the effect of BH4 supplementation on endothelial dysfunction caused by a variety of vascular diseases. Initially, these studies involved a limited number of patients in whom BH4 was administered acutely or on a short-term basis. Quite remarkably, supplementation with BH4 improved endothelial dysfunction in various conditions including hypercholesterolemia, diabetes, hypertension, coronary artery disease, and heart failure; such administration of BH4 also improved endothelial function in smokers and aged subjects (Table). The beneficial effect of BH4 appeared to be endothelium-specific because the effects of endothelium-independent vasodilators (e.g. nitroglycerin) were not affected by BH4 supplementation. The beneficial effects of BH4 arose from the activation of eNOS or antioxidant effect of BH4 [21,33].
More recently, the effects of long-term oral administration of BH4 have been studied in patients with hypercholesterolemia and hypertension. Twenty two hypercholesterolemic patients were randomized to 4 weeks of oral BH4 treatment (400 mg twice daily) or placebo; age-matched healthy volunteers served as controls [34]. BH4 levels in plasma were significantly increased by oral supplementation. BH4 normalized the impairment in endothelium-dependent relaxations to acetylcholine in hypercholesterolemic patients whereas it did not have any effect on endothelial function in control subjects. Interestingly, the authors also demonstrated that BH4 significantly reduced plasma levels of 8-F2 isoprostane, a marker of oxidative stress, and that BH4 prevented uncoupling of eNOS induced by low-density lipoprotein in cultured endothelial cells. Notably, no adverse effects were observed in subjects administered BH4, findings that indicate the safety of BH4, at least as utilized in this study. The authors concluded that oxidative stress and endothelial dysfunction induced by hypercholesterolemia can be normalized by chronic administration of BH4.
To determine whether chronic administration of BH4 may have an anti-hypertensive effect, the effects of 5, 10, mg/kg of BH4 per day (administered orally for 8 weeks) or 200 mg or 400 mg of BH4 (administered orally for 4 weeks) on arterial blood pressure and endothelial dysfunction were studied in patients with poorly controlled hypertension [35]. These investigators observed a beneficial effect of BH4 on both arterial blood pressure and endothelial dysfunction in patients treated with BH4 at doses of 5 or 10 mg/kg for 8 weeks, and at doses of 400 mg for 4 weeks. These findings support the view that administration of BH4 may provide a salutary effect on vascular function. However, as pointed out by the authors, the study lacked a placebo control group, and BH4 was formulated such that vitamin C was present during administration of BH4. Thus, it is possible that the beneficial effects may reflect a synergistic effect of BH4 and vitamin C, or a vitamin C-mediated effect.
In contrast to these two studies demonstrating a beneficial effect of BH4, a phase II clinical trial sponsored by pharmaceutical company BioMarin (United States) failed to observe an ameliorative effect of oral administration of BH4 (5mg/kg, twice daily for 2 months) in patients with poorly controlled hypertension. It is possible that the lack of efficacy observed with such administration of BH4 may reflect aspects of the pharmacokinetic and pharmacodynamic profiles of BH4 that are, currently, incompletely appreciated. For example, from a pathophysiologic standpoint, the ultimate intent when BH4 is administered is the elevation of intracellular levels of BH4 in the endothelium. However, only plasma levels of BH4 are measured in patients treated with BH4. Whether elevation of BH4 in plasma faithfully reflects increased intracellular concentration in the endothelium is unknown. In fact, in patients with coronary artery atherosclerosis, high plasma levels of BH4 are associated with low BH4 levels in endothelium [36]. This dissociation between plasma and vascular content of BH4 suggests that, in humans, BH4 does not passively diffuse from circulating blood into the endothelium. Indeed, it has been demonstrated that demonstrated that in many cell types, including endothelial cells, BH4 is not freely diffusible [37]. In order for BH4 to be imported into the endothelium, it has to undergo oxidation to BH2; imported BH2 is then regenerated back to BH4 by dihydrofolate reductase (DHFR) [37]. Intact DHFR activity is thus an essential requirement in enabling an increase in intracellular concentrations of BH4 following oral supplementation with BH4. However, the extent to which DHFR activity is preserved in the endothelium of diseased blood vessels is poorly understood, and the limited number of studies that address this issue offer divergent findings. For example, under conditions of oxidative stress, as induced by angiotensin II, DHFR activity is decreased in endothelium, an alteration that favors the elevation of BH2 and the uncoupling of eNOS [38]. At variance with this observation, it has been demonstrated that oxidative stress induced by diabetes may increase vascular expression of DHFR, thus raising the possibility that elevation of DHFR may be an adaptive response to oxidative stress [39]. Further investigation of the involvement of DHFR in influencing BH4 metabolism and maintaining the “coupling” of eNOS is clearly needed not only in the healthy vasculature but also in the diseased blood vessels. It is also increasingly apparent that the intracellular concentration of BH4 is determined by a complex network of mechanisms that influence the anabolism and catabolism of BH4, the recycling of BH4, and mechanisms responsible for the transport of BH4 across the endothelial cell membrane. More complete understanding of these mechanisms will facilitate the realization of the therapeutic potential of BH4.
As previously discussed, BH4 is easily oxidized, is thermo- and photo-labile, and has relatively poor cell membrane permeability. Some of these limitations have been addressed by development of sapropterin (BioMarin), a synthetic BH4; the FDA has recently approved the oral administration of sapropterin for the treatment of phenylketonuria. Sapropterin is a synthetic preparation of the dihydrochloride salt of the biologically active (6R)-5,6,7,8-tetrahydro-L-biopterin (BH4). Of note, this compound is currently being tested in clinical trials in patients with peripheral arterial disease, hypertension, coronary artery disease, pulmonary arterial hypertension, and sickle cell disease. However, development of novel BH4 analogues with oral bioavailability, better access into intracellular space, longer duration of action, and greater potency may improve chances for successful therapeutic application. Now available is a novel BH4 analogue (6-acetyl-7,7-dimethyl-5,6,7,8-tetrahydropterin; ADDP) [40] that is a stable compound, soluble in water and in polar, hydrogen binding organic solvents; it is also capable of crossing cell membranes and it supports NOS activity. Interestingly, studies on purified nNOS demonstrated that ADDP requires conversion into 5,6,7,8-tetrahydropterin to support enzymatic activity of nNOS. It was suggested that in vivo, this chemical conversion of ADDP may occur in the presence of DHFR. Based on studies utilizing isolated rat aorta and observations obtained in an experimental model of pulmonary hypertension, it was suggested that ADDP causes vasodilatation by stimulating eNOS activity. These studies illustrate how the discovery of novel BH4 analogues may significantly broaden and diversify the therapeutic options available for patients with cardiovascular diseases.
The approval of BH4 by the FDA as a drug for the treatment of patients with phenylketonuria is based on several clinical trials that demonstrate the safety of BH4. However, it is important to keep in mind that BH4 is also co-factor for iNOS and nNOS. Activation of both of these isoforms may be associated with detrimental effects resulting from markedly increased, local concentration of NO. Marked induction of, and attendant cytotoxicity incurred by iNOS may more likely occur in patients with severe infections, autoimmune disorders, and pathological angiogenesis [41]. In addition, in some patients, the stimulatory effect of BH4 on biosynthesis of catecholamines [42] may adversely affect cardiovascular function. Careful monitoring of patients chronically treated with BH4 will provide important additional information regarding possible unrecognized adverse effects.
Endogenous BH4
The deficiency of BH4 in vascular diseases appears to result mainly from its oxidation by peroxynitrite [9]. Therefore, strategies that protect endogenous BH4 from the oxidative attack by peroxynitrite may result in preservation of optimal endothelial concentration of the co-factor required for activity of eNOS and biosynthesis of NO. In human studies, administration of folic acid or 5-methyltetrahydrofolate (5-MTHF; the active and circulating form of folic acid) received the most attention as agents with a potentially favorable effect on BH4 metabolism. Although the mechanism of this effect remains incompletely understood [43], more recent findings suggest that 5-MTHF is a potent scavenger of peroxynitrite [44]. Moreover, 5-MTHF and folic acid [45] reduce superoxide anion and improves endothelium-dependent relaxations in human arteries both ex vivo and in vivo. Most importantly, the administration of 5-MTHF to patients with atherosclerosis and coronary artery disease leads to increased tissue content of BH4 in arteries and veins, the reversal of the uncoupling of eNOS, and normalization of production of NO [44,46]. Thus, the administration of folates provides an effective strategy to prevent BH4 oxidation and subsequent uncoupling of eNOS. Although the beneficial effects of folates are traditionally explained by their ability to reduce levels of homocysteine, these recent findings indicate that preservation of vascular content of BH4 and effective coupling of eNOS contribute to the vasoprotective effects of folates.
In attempt to define the optimal dose of folates required for vascular protection, the effects of folic acid administered as a low-dose (400 μg/d) or high-dose regimen (5 mg/d) were studied in patients with coronary artery disease [46]. They demonstrated that the low dose folic acid regimen (equivalent to dose that is present in fortified grain) is sufficient to improve vascular function by increasing levels of BH4 in the vascular wall, whereas the high dose folic regimen did not confer any additional benefit. Thus, it appears that folate supplementation is an efficient strategy in the prevention of BH4 oxidation and eNOS uncoupling in the vasculature. Further studies are needed to determine if genetic variants in methyltetrahydrofolate reductase may detect those patients with impaired synthesis of 5-MTHF, and therefore may benefit from 5-MTHF supplementation.
In addition to folates, studies on cultured human endothelial cells or experimental animals suggest that other drugs may increase the availability of BH4: specifically, ascorbic acid [28,47,48], statins [49–52], enalapril [53], captopril [54], telmisartan [39], eplerenone [53], cilostazol [55], insulin [56], and erythropoietin [57] have all been shown to stimulate biosynthesis of BH4 and/or protect BH4 from oxidation. That structurally diverse therapeutic agents employed in cardiovascular diseases all converge on the common biochemical effect of sustaining levels of BH4 bespeaks to a fundamentally important role of BH4 in maintaining the health and function of the vasculature.
Summary
Currently, the endothelium is recognized as a major therapeutic target in the prevention and treatment of vascular disease. From a pathophysiologic standpoint, an important focus in the prevention and treatment of vascular disease is the restoration of normal biosynthesis of NO and the reduction of excessive generation of superoxide anion and ROS. In this regard, supplementation with BH4 and/or strategies that augment endogenous levels of BH4 have been recently identified as novel approaches which exert salutary effects on endothelial dysfunction induced by a variety of vascular diseases. This concept and its therapeutic implications are the focus of considerable investigation, from which will likely emerge an increased spectrum of therapeutic agents available for cardiovascular diseases. Emerging evidence derived from the study of genetic polymorphisms in key enzymes involved in metabolism of BH4 suggests that constitutive deficiency of BH4 may increase the risk for cardiovascular disease [58,59]. Studies of gene polymorphisms may thus assist in identifying those patients who are at risk for cardiovascular disease, and who would most likely benefit from supplementation with BH4 or its analogues.
Box 1. Major steps in development of BH4 as therapeutic agent for cardiovascular diseases
Identification of enzymatic activity of endothelial nitric oxide synthase (eNOS) as a source of nitric oxide (NO), a major vasodilator substance produced and released from vascular endothelium.
Recognition of tetrahydrobiopterin (BH4) as an essential co-factor required for activity of eNOS.
Discovery of eNOS uncoupling caused by suboptimal intracellular concentration of BH4 resulting in increased formation of eNOS-derived superoxide anion and endothelial dysfunction.
Pre-clinical studies demonstrating ability of pharmacological and genetic supplementation of BH4 to prevent eNOS-derived production of superoxide anion and endothelial dysfunction in experimental models of cardiovascular diseases.
Establishment of the beneficial effect of intravenous BH4 supplementation on endothelial dysfunction in humans.
Synthesis of orally active dihydrochloride salt of BH4.
Commencement of randomized placebo controlled clinical trials designed to determine therapeutic efficacy of oral BH4 supplementation in treatment of hypertension, peripheral arterial disease, coronary artery disease, pulmonary hypertension, and sickle cell disease.
Acknowledgments
This work was supported by the National Institutes of Health grant HL-53524, HL-58080, and HL-66958 (Z.S.K.), HL-55552 (K.A.N.), DK-70124 (K.A.N.), and by the Mayo Foundation. L.V.D. is the recipient of a Scientist Development Grant from the American Heart Association (07-301333N).
References
- 1.Tayeh MA, Marletta MA. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate: tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–19658. [PubMed] [Google Scholar]
- 2.Kwon NS, et al. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989;264:20496–20501. [PubMed] [Google Scholar]
- 3.Mayer B, et al. Purification of a Ca2+/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor-role of tetrahydrobiopterin. FEBS Lett. 1990;2771:215–219. doi: 10.1016/0014-5793(90)80848-d. [DOI] [PubMed] [Google Scholar]
- 4.Pollock JS, et al. Purification and characterization of particulate endothelium-derved relaxing factor synthase from cultured and native bovine aoric endothelial cells. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinzel B, et al. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pou S, et al. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 7.Cosentino F, Katusic ZS. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsui M, et al. Effect of tetrahydrobiopterin on endothelial function in canine middle cerebral arteries. Circ Res. 1996;79:336–342. doi: 10.1161/01.res.79.2.336. [DOI] [PubMed] [Google Scholar]
- 9.Milstien S, Katusic ZS. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 10.Pacher P, et al. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alp NJ, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alp NJ, et al. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 13.Khoo JP, et al. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2022–2024. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 14.Du YH, et al. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation. 2008;117:1045–1054. doi: 10.1161/CIRCULATIONAHA.107.748236. [DOI] [PubMed] [Google Scholar]
- 15.Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 17.Schulz E, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 18.Bendall JK, et al. Stochiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo. Insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki M, et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest. 2002;110:331–340. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitzer T, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 21.Mayahi L, et al. (6R)-5,6,7,8-tetrahydro-L-biopterin and its stereoisomer prevent ischemia reperfusion injury in human forearm. Arterioscler Thromb Vasc Biol. 2007;27:1334–1339. doi: 10.1161/ATVBAHA.107.142257. [DOI] [PubMed] [Google Scholar]
- 22.Vásquez-Vivar J, et al. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabtree MJ, et al. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production be eNOS. Am J Physiol Heart Circ Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzkaya N, et al. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 25.Moens AL, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin. Efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenkranz-Weiss P, et al. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells. J Clin Invest. 1994;93:2236–2243. doi: 10.1172/JCI117221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katusic ZS, et al. Cytokines stimulate GTP cyclohydrolase I gene expression in cultured human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:27–32. doi: 10.1161/01.atv.18.1.27. [DOI] [PubMed] [Google Scholar]
- 28.d’Uscio LV, et al. Long-term vitamin C tretament increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 29.Aulak KS, et al. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Nat Acad Sci. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson TE, et al. GTP cyclohydrolase I expression and enzymatic activity are present in caveolae of endothelial cells. Hypertension. 2008 doi: 10.1161/HYPERTENSIONAHA.108.115709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He T, et al. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 32.He T, et al. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008;102:80–88. doi: 10.1161/CIRCRESAHA.108.176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heitzer T, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 34.Cosentino F, et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart. 2008;94:487–492. doi: 10.1136/hrt.2007.122184. [DOI] [PubMed] [Google Scholar]
- 35.Porkert M, et al. Tetrahydrobiopterin: a novel antihypertensive therapy. J Human Hyperten. 2008;22:401–407. doi: 10.1038/sj.jhh.1002329. [DOI] [PubMed] [Google Scholar]
- 36.Antoniades C, et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation. 2007;116:2851–2859. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa H, et al. Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4. Mol Genet Metab. 2005;86:S2–S10. doi: 10.1016/j.ymgme.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenzel P, et al. AT(1)-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic Biol Med. 2008;45:619–626. doi: 10.1016/j.freeradbiomed.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Suckling CJ, et al. 6-Acetyl-7,7-dimethyl-5,6,7,8-tetrahydropterin is an activator of nitric oxide synthases. Bioorg Med Chem Lett. 2008;18:1552–1555. doi: 10.1016/j.bmcl.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 41.Cooke JP, et al. Nitric Oxide Biology and Pathobiology. San Diego, CA: Academic; 2000. Nitric oxide and vascular disease; pp. 759–785. [Google Scholar]
- 42.Baker H, et al. Interleukin-2 enhances biopterins and catecholamine production during adoptive immunotherapy for various cancers. Cancer. 1989;64:1226–1231. doi: 10.1002/1097-0142(19890915)64:6<1226::aid-cncr2820640611>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 43.Verhaar MC, et al. Effects of oral folic acid supplementation on endothelial function in familial hypercholesterolemia. A randomized placebo-controlled trial. Circulation. 1999;100:335–338. doi: 10.1161/01.cir.100.4.335. [DOI] [PubMed] [Google Scholar]
- 44.Antoniades C, et al. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 45.Moens AL, et al. High-dose folic acid pretreatment blunts cardiac dysfunction during schemia coupled by maintenance of high-energy phosphates and reduced postreperfusion injury. Circulation. 2008;117:1810–1819. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirodaria C, et al. Global improvement of vascular function and redox state with low-dose folic acid. Implications for folate therapy inpatients with coronary artery disease. Circulation. 2007;115:2262–2270. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 47.Huang A, et al. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275:17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 48.Heller R, et al. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 49.Hattori Y, et al. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:176–182. doi: 10.1161/01.atv.0000054659.72231.a1. [DOI] [PubMed] [Google Scholar]
- 50.Ding QF, et al. The effect of high glucose on NO and O2- through endothelial GTPCH1 and NADPH oxidase. Life Sci. 2004;75:3185–3194. doi: 10.1016/j.lfs.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Shinozaki K, et al. Pitavastatin restores vascular dysfunction in insulin-resistant state by inhibiting NAD(P)H oxidase activity and uncoupled endothelial nitric oxide synthase-dependent superoxide production. J Cardiovasc Pharmacol. 2007;49:122–130. doi: 10.1097/FJC.0b013e31802f5895. [DOI] [PubMed] [Google Scholar]
- 52.Wenzel P, et al. Mechanisms underlying recoupling of eNOS by HG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imanishi T, et al. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension. 2008;51:734–741. doi: 10.1161/HYPERTENSIONAHA.107.104299. [DOI] [PubMed] [Google Scholar]
- 54.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56:118–126. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki K, et al. Cilostazol activates AMP-activated protein kinase and restores endothelial function in diabetes. Am J Hypertens. 2008;21:451–457. doi: 10.1038/ajh.2008.6. [DOI] [PubMed] [Google Scholar]
- 56.Ishii M, et al. Stimulation of tetrahydrobiopterin synthesis induced by insulin: possible involvement of phosphatidylinositol 3-kinase. Int J Biochem Cell Biol. 2001;33:65–73. doi: 10.1016/s1357-2725(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 57.d’Usco LV, Katusic ZS. Erythropoietin increases endothelial biosynthesis of tetrahydrobiopterin by activation of protein kinase B alpha/Akt1. Hypertension. 2008;52:93–99. doi: 10.1161/HYPERTENSIONAHA.108.114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, et al. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117:2658–2671. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antoniades C, et al. GCH1 haplotype determines vascular and plasma biopterin availability in coronary artery disease. J Am Coll Cardiol. 2008;52:158–165. doi: 10.1016/j.jacc.2007.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stroes ES, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maier W, et al. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Ueda S, et al. Tetrahydrobiopterin restores endothelial function in long-term smokers. J Am Coll Cardiol. 2000;35:71–75. doi: 10.1016/s0735-1097(99)00523-9. [DOI] [PubMed] [Google Scholar]
- 63.Setoguchi S, et al. Tetrahydrobiopterin improves endothelial dysfunction in coronary microcirculation in patients without epicardial coronary artery disease. J Am Coll Cardiol. 2001;38:493–498. doi: 10.1016/s0735-1097(01)01382-1. [DOI] [PubMed] [Google Scholar]
- 64.Higashi Y, et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–332. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 65.Fukuda Y, et al. Tetrahydrobiopterin improves coronary endothelial function, but does not prevent coronary spasm in patients with vasospastic angina. Circ J. 2002;66:58–62. doi: 10.1253/circj.66.58. [DOI] [PubMed] [Google Scholar]
- 66.Setoguchi S, et al. Tetrahydrobiopterin improves impaired endothelium-dependent forearm vasodilation in patients with heart failure. J Cardiovasc Pharmacol. 2002;39:363–368. doi: 10.1097/00005344-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Fukuda Y, et al. Tetrahydrobiopterin restores endothelial function of coronary arteries in patients with hypercholesterolaemia. Heart. 2002;87:264–269. doi: 10.1136/heart.87.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ihlemann N, et al. Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol. 2003;285:H875–H882. doi: 10.1152/ajpheart.00008.2003. [DOI] [PubMed] [Google Scholar]
- 69.Nyström T, et al. Tetrahydrobiopterin increases insulin sensitivity inpatients with type 2 diabetes and coronary heart disease. Am J Physiol Endocrinol Metab. 2004;287:E919–E925. doi: 10.1152/ajpendo.00046.2004. [DOI] [PubMed] [Google Scholar]
- 70.Mittermayer F, et al. Tetrahydrobiopterin corrects Escherichia coli endotoxin-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1752–H1757. doi: 10.1152/ajpheart.00057.2005. [DOI] [PubMed] [Google Scholar]
- 71.Eskurza I, et al. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higashi Y, et al. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 73.Sommer F, et al. Evaluation of tetrahydrobiopterin (BH4) as a potential therapeutic agent to treat erectile dysfunction. Asian J Androl. 2006;8:159–167. doi: 10.1111/j.1745-7262.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 74.Worthley MI, et al. Effects of tetrahydrobiopterin on coronary vascular reactivity in atherosclerotic human coronary arteries. Cardiovasc Res. 76:539–546. doi: 10.1016/j.cardiores.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Wyss CA, et al. Tetrahydrobiopterin restores impaired coronary microvascular dysfunction in hypercholesterolaemia. Er J Nucl Med Mol Imaging. 2005;32:84–91. doi: 10.1007/s00259-004-1621-y. [DOI] [PubMed] [Google Scholar]