Abstract
This study examined the effect of an on-frequency precursor on growth-of-masking (GOM) functions measured using an off-frequency masker. The signal was a 6-ms, 4-kHz tone. A GOM function was measured using a 40-ms, 2.8-kHz tone (the off-frequency masker). GOM functions were then measured with an on-frequency, fixed level precursor presented before the off-frequency masker. The precursor was 50 or 60 dB SPL, and 160 ms in duration. For the 60-dB SPL precursor, a 40-ms duration was also used. Two-line functions were fit to the GOM data to estimate the basilar membrane input-output function. The precursors reduced the gain of the input-output function, and this decrease was graded with precursor level. Both precursor durations had the same effect on gain. Changes in masking following a precursor were larger than would be predicted by additivity of masking. The observed decrease in gain may be consistent with activation of the medial olivocochlear reflex by the precursor.
INTRODUCTION
Most sounds of interest are time varying in nature, so the response of the auditory system must be examined for dynamic as well as steady signals. This response changes for a short period after the presentation of a sound. This sound will be called a precursor. If the precursor is followed by a short-duration signal, it may increase the threshold for detecting the signal, an effect called forward masking. If the signal is presented with a simultaneous masker, the precursor may make the signal audible at a lower signal-to-masker ratio. This effect has been called overshoot (Zwicker, 1965a) or the temporal effect (Hicks and Bacon, 1992). That is, in simultaneous masking, a precursor may make a subsequent sound easier to hear, but in forward masking, a precursor may have the opposite effect. The point of this paper is to try to reconcile these two effects with one underlying mechanism.
The physiological bases of forward masking are not well understood, and indeed, forward masking probably depends on processes at several levels of the auditory system. One hypothesis is that forward masking is related to neural adaptation (Smith 1977, 1979). Neural firing decreases, or adapts, during the course of the precursor. If another sound, a signal, follows the precursor, the firing to the signal is decreased from what it would be if the signal were preceded by silence (Harris and Dallos, 1979). This decrease in firing might be expected to increase the threshold for the signal, i.e., the signal would be masked. An analysis of firing rates using signal detection theory predicts much less masking at the level of the auditory nerve than is seen in psychophysical tests (Relkin and Turner, 1988). Meddis and O’Mard (2005) were able to adjust an auditory model to predict psychophysical forward masking results from neural adaptation. Oxenham (2001) also showed that neural adaptation, modeled as a decrease in gain, was able to predict many forward masking results, if compression in the cochlea was also included in the model.
A second theory for forward masking is persistence of excitation. That is, the response to the precursor persists and overlaps with the response to the signal, making it harder to hear the signal. This is not seen at the level of the auditory nerve, but has been hypothesized to involve a “temporal window” at some higher level of the auditory system (Moore et al., 1988). Oxenham (2001) found that if compression in the cochlea was included, the temporal window model was able to predict forward masking results.
A third hypothesis is that efferent feedback from the central auditory system is responsible for some forward masking. Shore (1998) observed changes in forward masking in the ventral cochlear nucleus after lesions to efferent pathways.
The present experiment was developed to examine the hypothesis that forward masking is partially due to efferent feedback, but at the level of the cochlea. There would be a known physiological basis in the medial olivocochlear reflex (MOCR). The MOCR is a decrease in the gain of the active process of the cochlea, caused by activation of the medial olivocochlear bundle of efferent fibers (Warr and Guinan, 1979; Warr, 1980; Liberman, 1989). Oxenham (2001) modeled neural adaptation as a decrease in gain, although at a postcochlear level, and the time course of recovery is in the same range as the offset of MOCR effects (Backus and Guinan, 2006). Temporal effects in simultaneous masking have been successfully modeled on the basis of a frequency-specific decrease in gain in the cochlea, which would be consistent with MOCR activation (Strickland 2001, 2004, 2008; Strickland and Krishnan, 2005). Although neural adaptation has also been proposed as a basis for the temporal effect in simultaneous masking, it cannot explain all aspects of the temporal effect (Bacon and Healy, 2000). The temporal window model does not predict the temporal effect in simultaneous masking.
The MOCR hypothesis for forward masking would be consistent with a decrease in gain, but at the level of the cochlea. A decrease in the gain of the active process would be expected to produce effects that would be distinguishable from the effects of a temporal window or of neural adaptation. Specifically, a decrease in the gain of the active process should produce a decrease in the gain of the input-output function of the cochlea.
This hypothesis may be studied using a paradigm called additivity of masking. In this paradigm, the masking produced by two maskers is compared to the masking produced by each masker alone. Previous research on the effects of two maskers has shown that it can be assumed that the effects of the maskers add linearly, if cochlear compression is taken into account (e.g., Penner and Shiffrin, 1980; Oxenham and Moore, 1994). The premise of the present study is that there are probably at least two types of forward masking. Forward masking by short maskers seems unlikely to be due to the efferent feedback to the cochlea. The most rapid MOCR effects fall in the range of 60–80 ms (Backus and Guinan, 2006). Therefore, a short forward masker may be used that should not activate the MOCR. Growth-of-masking (GOM) functions have been used in forward masking to obtain estimates of the cochlear input-output function (Oxenham and Plack, 1997). A masker approximately an octave below the signal frequency is used to mask a short signal, as a function of signal level. If the masker response is linear, the thresholds give an estimate of the input-output function at the signal frequency place. Thus, a GOM function measured with a short masker and signal should give an estimate of gain at the signal frequency place.
In the remainder of the paper, the longer masker will be referred to as the “precursor.” For longer precursor durations, efferent feedback could play a role in forward masking. Because the MOCR is frequency specific, a long-duration precursor will be presented at the signal frequency, and the GOM function measured. If input-output functions estimated from the two GOM functions show a decrease in gain following a precursor, this would be consistent with MOCR activation. The signal threshold will also be measured with the precursor but no short forward masker, so that the results may be analyzed in terms of additivity of masking.
METHODS
Stimuli
The signal was a 6-ms, 4.0-kHz sinusoid, with 3-ms cosine-squared onset and offset ramps (no steady state). This frequency was chosen because large temporal effects in simultaneous masking have been found for this signal frequency. The off-frequency masker was a 2.8-kHz tone with a duration of 40 ms, including 5-ms cosine-squared gating. This masker duration was chosen to minimize activation of the MOCR, yet enable thresholds to be measured within the range of the equipment. There was no delay between masker offset and signal onset. In the precursor conditions, a 4.0-kHz precursor preceded the off-frequency masker with no delay between precursor offset and masker onset. Previous studies using a separate precursor in simultaneous masking have reported a temporal effect at the 40-ms precursor-signal delay used in this study (Bacon and Smith, 1991; Bacon and Healy, 2000).
The effects of precursor level and duration were examined. The precursor level was fixed at 50 or 60 dB SPL, based on pilot data that showed that these levels were effective. The duration was set at 160 ms. This duration was based on previous data in simultaneous masking that showed that the temporal effect plateaued for a signal delay of about 200 ms from masker onset (Zwicker, 1965a). The combined duration of the precursor and masker was 200 ms. The effect of precursor duration was also examined by using a 40-ms, 60-dB SPL precursor.
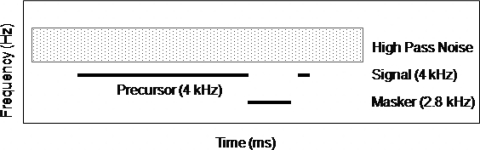
Throughout the experiment, high-pass noise was presented to prevent off-frequency listening. The lower cutoff frequency was 1.2fs, where fs refers to the signal frequency. The spectrum level of this high-pass noise was set at 40 dB below the signal level. This level was used in a similar study (Rosengard et al., 2005). The high-pass noise was turned on 50 ms before precursor onset and turned off 50 ms after the offset of the signal, to avoid confusion with the other stimuli. Conditions are shown schematically in Fig. 1.
Figure 1.
Schematic showing the spectral (y-axis) and temporal (x-axis) characteristics of the (4 kHz,6 ms) signal, (2.8 kHz,40 ms) off-frequency masker, (4 kHz) on-frequency precursor with variable duration and level (bold lines), and high-pass noise (hatched rectangle).
The stimuli were created digitally and were routed through four separate D∕A channels (TDT DA3-4). They were low pass filtered at 10 kHz (TDT FT5 and FT6-2). The levels of the stimuli were controlled by programmable attenuators (TDT PA-4), mixed (TDT-SM3) and routed to a headphone buffer (TDT HB6) prior to presentation through one of two ER-2A insert earphones to a listener seated in a sound-treated booth. These earphones have a flat frequency response from 250 to 8000 Hz.
Procedures
A three-interval forced choice task with a two-down, one-up stepping rule was used to determine thresholds. Subjects were asked to identify the interval containing the signal by pressing a key on a computer keyboard. Visual feedback was provided via a computer monitor. Within each trial, the signal level was fixed and the level of the off-frequency masker varied based on the response. The initial step size was 5 dB, and decreased to 2 dB after the second reversal. Thresholds were taken as the mean of the last even number of reversals at the smaller step size in a set of 50 trials. This adaptive tracking procedure estimated the 70.7% correct point on the psychometric function (Levitt, 1971). The thresholds from at least two runs were averaged to obtain the final threshold. Runs were discarded if the standard deviation exceeded 5 dB or the threshold exceeded the limits of the equipment. Signal thresholds in quiet or following the precursor alone (with no masker) were also measured. When no masker was present, the delay between the precursor and signal was still fixed at 40 ms. Data were collected over several experimental sessions, each lasting 1–1.5 hours.
The listener without previous experience in psychoacoustic tasks was trained for 2–3 hours prior to data collection. In a given session, data were collected for all precursor conditions at one or two signal levels.
Subjects
Three subjects participated in this study. All subjects had air conduction thresholds within normal limits (<20 dB HL) and normal middle ear function bilaterally. There were two females and one male, with a median age of 28 years. Two of these subjects had prior experience in psychoacoustic tasks.
RESULTS
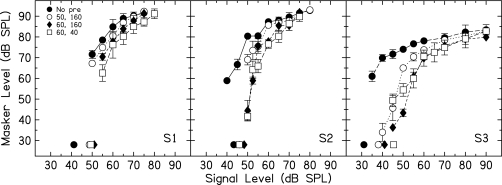
GOM functions for the three listeners are shown in Fig. 2. Filled circles are off-frequency masker thresholds with no precursor. Thresholds are also shown for the off-frequency masker following the 50-(open circles) and 60-dB SPL (filled diamonds) 160-ms precursors, and the 60-dB SPL, 40-ms precursor (open squares). The on-frequency precursor decreased masker thresholds for low signal levels, but had little effect at high signal levels. This effect increased with precursor level. For a 60-dB SPL precursor, there was little to no effect of decreasing the precursor duration from 160 to 40 ms.
Figure 2.
Plots showing the masker level necessary to mask a signal, plotted as a function of signal level without a precursor (filled circle) and with the three different precursors (open circles: 50 dB SPL, 160 ms; filled diamonds: 60 dB SPL, 160 ms; open squares: 60 dB SPL, 40 ms). Symbols at bottom left of individual panels indicate signal thresholds obtained for each of the four conditions without the off-frequency masker.
Signal thresholds were also obtained in the presence of the on-frequency precursor with no off-frequency masker. These are indicated by symbols at the bottom of individual panels. The precursors cause a shift in threshold of approximately 2–15 dB. In a few cases, listeners were able to detect a signal that was slightly below quiet threshold, in the presence of the off-frequency masker. For example, for S2, the absolute threshold of the signal alone was 43 dB SPL (filled circle), but when the off-frequency masker was presented, the signal could be detected at 40 dB SPL. This slight improvement in signal threshold with a forward masker has been seen in several subjects, and has been observed previously (Zwislocki et al., 1959).
Input-output functions were estimated for each of the precursor conditions using the lower two segments of a three-line function described by Plack et al. (2004).
| (1) |
| (2) |
where Lin was the level of the signal, Lout was the estimated output for a given signal level, and G, c, and BP1 were free parameters. The two-line function had a slope of 1 below the lower breakpoint (BP1), and a compressive slope (c) between the two BPs. The correction factor k1, where k1=BP1 (1−c), ensured that the two lines met at the breakpoint. As the GOM data did not show a BP at higher signal levels, BP2 was assumed to be fixed at 100 dB SPL for all subjects. For some listeners, the presence of the precursor produced a slope greater than 1 on the lower leg of the GOM (e.g., S3). This has been seen before with a precursor (Strickland, 2008), and may be due to an effect near threshold which is not predicted by the model (see Plack and Skeels, 2007, for a discussion). These points were excluded from the fit. A least-squares minimization procedure was used to estimate the free parameters. The parameter estimates from the model are shown in Table 1. The model fit the experimental data very well, with rms errors typically less than 3 dB.
Table 1.
Parameters from the two-line fit to the data using a technique derived from Plack et al. (2004). G=gain, c=slope of compressive function, BP1=lower breakpoint, BP2=upper breakpoint. BP2 was fixed at 100 dB for all subjects and conditions.
| Subject | Precursor level (dB) | Precursor duration (ms) | BP1 | c | G | rms error |
|---|---|---|---|---|---|---|
| S1 | No | ⋯ | 65.54 | 0.27 | 23.47 | 0.98 |
| 50 | 160 | 69.84 | 0.51 | 19.87 | 1.40 | |
| 60 | 160 | 82.22 | 0.85 | 17.20 | 1.19 | |
| 60 | 40 | 70.00 | 0.74 | 13.50 | 2.82 | |
| S2 | No | ⋯ | 60.39 | 0.32 | 26.22 | 2.22 |
| 50 | 160 | 63.76 | 0.44 | 22.26 | 1.93 | |
| 60 | 160 | 75.51 | 0.88 | 16.25 | 4.76 | |
| 60 | 40 | 82.84 | 0.87 | 14.80 | 2.04 | |
| S3 | No | ⋯ | 43.67 | 0.39 | 27.93 | 0.98 |
| 50 | 160 | 59.05 | 0.29 | 15.21 | 0.59 | |
| 60 | 160 | 67.61 | 0.40 | 6.39 | 1.90 | |
| 60 | 40 | 67.85 | 0.20 | 7.85 | 1.27 |
In examining Table 1, it can be seen that BP1 increased with the addition of the on-frequency precursor for all subjects. The slope c also increased for S1 and S2. This change increased with precursor level. As a result, the maximum gain G decreased with the addition of an on-frequency precursor. A 10-dB increase in on-frequency precursor level resulted in a decrease in G of approximately 2.5–9 dB. The short duration (40 ms) precursor produced the same decrease in gain as a longer (160 ms) precursor of the same level.
DISCUSSION
Although the results show a decrease in the gain of input-output functions following a precursor, it is possible that they could simply reflect additivity of the masking of the on-frequency precursor and the off-frequency masker. This possibility is explored below.
Additivity of masking
Many previous studies have examined the combined effects of two temporally nonoverlapping maskers on signal threshold, based on their individual effects. This has been called additivity of masking. The results in these studies may be explained if it is assumed that the effects of the two maskers add linearly. For example, suppose there are two maskers, and each is at the level at which it just masks a 70-dB SPL tone. Then when the two maskers are presented sequentially, the threshold for the tone should increase to 73 dB SPL, as if the intensity effects of the two maskers are added linearly. The threshold for the tone may increase more than 3 dB, and this has been attributed to compression.
Estimates for the individual effects for the precursor and masker were obtained by assuming that the GOM measured without a precursor estimated the input-output function at the signal place. The masker effect of the precursor alone was estimated by using the signal threshold in the presence of the precursor alone (symbols at the bottom of Fig. 2) and using the GOM function to estimate the output level. For example, for S3, the threshold for the signal following a 50-dB SPL precursor was 38 dB SPL (open circle). On the input-output function, using the function fitted by the equations, this signal would be masked by an off-frequency masker of 66 dB SPL. Thus, this fixed precursor has an effective level of 66 dB SPL. The precursor level was fixed for a given GOM function, so it was assumed that its effective level was constant. The level of the masker varied according to the signal level. The effective masker level was taken from the input-output function for each signal level. For example, for S3, the masker level needed to mask a 50-dB SPL signal was 74 dB SPL. Now, when the precursor is presented before the masker, what level should the masker be so that the combined effect of the two together is 74 dB SPL? The masker response is assumed to be linear. By subtracting the intensities, it can be determined that a masker level of 73.3 dB SPL would be needed. The actual masker level measured for a 50-dB SPL signal following a 50-dB SPL precursor was 65 dB SPL. This level is much lower than would be predicted by additivity of masking.
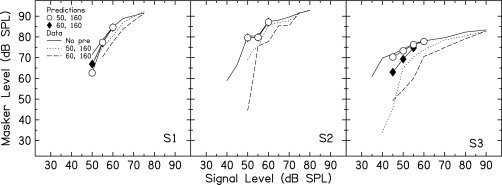
Figure 3 shows the masker levels predicted by additivity of masking for the 50- and 60-dB SPL precursors (symbols), along with the data from Fig. 2 replotted as lines. For S1 and S2, the predicted results for the two precursors overlie each other for most signal levels, while the actual data do not. It can be seen that the precursors cause a larger change in masker level than is predicted by additivity of masking.
Figure 3.
Predictions using an additivity of masking analysis (symbols), along with data from Fig. 2 replotted as lines.
Decrease in gain
Now consider the hypothesis that the gain of the GOM function does decrease following a long-duration precursor at the signal frequency. The interpretation would be that the on-frequency precursor turned down the gain in the cochlea, while the off-frequency masker produced some other type of forward masking. The decrease in gain is graded with precursor level. The decrease in gain observed here is similar to that reported in a simultaneous masking study by Strickland (2008). She reported a decrease in maximum gain of 4–6 dB for every 10 dB increase in precursor level, which is similar to the 2.5–9 dB decrease in the present data. That study also found a decrease in compression with a precursor for some listeners and not others, as in the present study. These data are consistent with Rosengard et al. (2005), who found that compression decreased with gain, but not with Plack et al. (2004), who found no correlation between compression and gain. In the present study, S3, who had the most data points on the compressive part of the GOM function, shows the least change in compression with a precursor. Thus, the apparent change in compression may be due to the limited number of data points to be fitted for the other listeners.
Data from both simultaneous and forward masking are consistent with the hypothesis that preceding stimulation decreases the gain of the basilar membrane input-output function. As noted in the Introduction, this would be consistent with the activation of the MOCR. The MOCR hypothesis fits in the context of adaptation of sensory systems in response to the changing environment. Forward masking would then just be a by-product of a system which is generally beneficial for listening in background noise in simultaneous masking.
Comparison to other aspects of temporal effects in simultaneous masking
In this study, the effect of the on-frequency precursor was characterized by a decrease in masker level, which may seem contrary to some temporal effects in simultaneous masking, where the masker level increases. The present data are consistent with some conditions in Strickland (2001), where the temporal effect was measured in simultaneous masking with a notched-noise masker. Adding a broadband noise precursor before the masker decreased masker levels for wide notch widths. Carlyon (1989) showed that a narrowband noise precursor at the signal frequency increased signal threshold. This is similar to the effect of the on-frequency precursor in our experiment, which decreases the masker level required to mask the signal. Both the previous simultaneous masking and the current forward masking results are consistent with a decrease in gain at the signal frequency following a precursor.
The effectiveness of the short-duration precursor was surprising. In a simultaneous masking study, Bacon and Smith (1991) showed that a short-duration precursor was no different from a longer precursor in its effectiveness, as long as it was long enough to activate the efferent system. In this study, it was expected that the 40-ms on-frequency precursor would be too short to activate the efferent system. However, in addition to precursor duration, it appears that the delay between precursor and signal onsets may play a role. For a 160-ms precursor, this delay is 200 ms, whereas for the shorter duration precursor, it is 80 ms. The time course of onset of the MOCR is on average about 70 ms (Backus and Guinan, 2006; Guinan, 2006). It is, therefore, possible that the 40-ms on-frequency precursor could have activated the MOCR.
Another interesting aspect of this study is the fact that a temporal effect is seen when the preceding stimulation is only at the signal frequency. Past studies have typically shown that in order to produce a temporal effect, the precursor and∕or simultaneous masker must contain energy above the signal frequency (Zwicker, 1965b; McFadden, 1989; Bacon and Smith, 1991). One reason that this might be true is that it would eliminate the possibility of off-frequency listening. If listeners are able to attend to filters above the signal frequency, where the growth of excitation to the signal is more linear, a temporal effect might not be seen. Another reason could be that the temporal effect depends on suppression. The results of the present study are consistent with the off-frequency listening hypothesis. High-pass noise presented with the signal eliminated the possibility of off-frequency listening. This is consistent with temporal effects seen with tonal stimuli at high frequencies, where off-frequency listening would also not be effective due to the sharp increase in thresholds above the signal frequency (Schmidt and Zwicker, 1991; Carlyon and White, 1992). Thus, this shows that a temporal effect can be seen in a condition in which suppression is not playing a role. This is not to suggest that suppression never plays a role in the temporal effect, only that it is not a necessary condition.
ACKNOWLEDGMENTS
We thank Skyler Jennings for his valuable comments on the manuscript. This research was supported in part by funds from the Speech, Language, and Hearing Sciences Department at Purdue University and Grant No. R01-DC008327 from the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH).
Portions of this research were presented at the 30th Midwinter Meeting of the Association for Research in Otolaryngology, Denver, CO, February 2007.
References
- Backus, B. C., and Guinan, Jr., J. J. (2006). “Time-course of the human medial olivocochlear reflex,” J. Acoust. Soc. Am. 10.1121/1.2169918 119, 2889–2904. [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Healy, E. W. (2000). “Effects of ipsilateral and contralateral precursors on the temporal effect in simultaneous masking with pure tones,” J. Acoust. Soc. Am. 10.1121/1.428443 107, 1589–1597. [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Smith, M. A. (1991). “Spectral, intensive, and temporal factors influencing overshoot,” Q. J. Exp. Psychol. A 43, 373–399. [DOI] [PubMed] [Google Scholar]
- Carlyon, R. P. (1989). “Changes in the masked thresholds of brief tones produced by prior bursts of noise,” Hear. Res. 10.1016/0378-5955(89)90014-2 41, 223–235. [DOI] [PubMed] [Google Scholar]
- Carlyon, R. P. and White, L. J. (1992). “Effect of signal frequency and masker level on the frequency regions responsible for the overshoot effect,” J. Acoust. Soc. Am. 10.1121/1.402629 91, 1034–1041. [DOI] [PubMed] [Google Scholar]
- Guinan, Jr., J. J. (2006). “Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans,” Ear Hear. 10.1097/01.aud.0000240507.83072.e7 27, 589–607. [DOI] [PubMed] [Google Scholar]
- Harris, D. M., and Dallos, P. (1979). “Forward masking of auditory nerve fiber responses,” J. Neurophysiol. 42, 1083–1107. [DOI] [PubMed] [Google Scholar]
- Hicks, M. L., and Bacon, S. P. (1992). “Factors influencing temporal effects with notched-noise maskers,” Hear. Res. 10.1016/0378-5955(92)90174-L 64, 123–132. [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 10.1121/1.1912375 49, 467–477. [DOI] [PubMed] [Google Scholar]
- Liberman, M. C. (1989). “Rapid assessment of sound-evoked olivocochlear feedback: Suppression of compound action potentials by contralateral sound,” Hear. Res. 10.1016/0378-5955(89)90127-5 38, 47–56. [DOI] [PubMed] [Google Scholar]
- McFadden, D. (1989). “Spectral differences in the ability of temporal gaps to reset the mechanisms underlying overshoot,” J. Acoust. Soc. Am. 10.1121/1.397732 85, 254–261. [DOI] [PubMed] [Google Scholar]
- Meddis, R., and O’Mard, L. P. (2005). “A computer model of the auditory-nerve response to forward-masking stimuli,” J. Acoust. Soc. Am. 10.1121/1.1893426 117, 3787–3798. [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., Glasberg, B. R., Plack, C. J., and Biswas, A. K. (1988). “The shape of the ear’s temporal window,” J. Acoust. Soc. Am. 10.1121/1.396055 83, 1102–1116. [DOI] [PubMed] [Google Scholar]
- Oxenham, A. J. (2001). “Forward Masking: Adaptation or integration?” J. Acoust. Soc. Am. 10.1121/1.1336501 109, 732–741. [DOI] [PubMed] [Google Scholar]
- Oxenham, A. J., and Moore, B. C. J. (1994). “Modeling the additivity of nonsimultaneous masking,” Hear. Res. 10.1016/0378-5955(94)90014-0 80, 105–118. [DOI] [PubMed] [Google Scholar]
- Oxenham, A. J., and Plack, C. J. (1997). “A behavioral measure of basilar-membrane non-linearity in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. 10.1121/1.418327 101, 3666–3675. [DOI] [PubMed] [Google Scholar]
- Penner, M. J., and Shiffrin, R. M. (1980). “Nonlinearities in the coding of intensity within the context of a temporal summation model,” J. Acoust. Soc. Am. 10.1121/1.383885 67, 617–627. [DOI] [PubMed] [Google Scholar]
- Plack, C. J., Drga, V., and Lopez-Poveda, E. A. (2004). “Inferred basilar-membrane response functions for listeners with mild to moderate sensorineural hearing loss,” J. Acoust. Soc. Am. 10.1121/1.1675812 115, 1684–1695. [DOI] [PubMed] [Google Scholar]
- Plack, C. J., and Skeels, V. (2007). “Temporal integration and compression near absolute threshold in normal and impaired ears,” J. Acoust. Soc. Am. 10.1121/1.2769829 122, 2236–2244. [DOI] [PubMed] [Google Scholar]
- Relkin, E. M., and Turner, C. W. (1988). “A reexamination of forward masking in the auditory nerve,” J. Acoust. Soc. Am. 10.1121/1.396836 84, 584–591. [DOI] [PubMed] [Google Scholar]
- Rosengard, P. S., Oxenham, A. J., and Braida, L. D. (2005). “Comparing different estimates of cochlear compression in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. 10.1121/1.1883367 117, 3028–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, S., and Zwicker, E. (1991). “The effect of masker spectral asymmetry on overshoot in simultaneous masking.” J. Acoust. Soc. Am. 10.1121/1.400656 89, 1324–1330. [DOI] [PubMed] [Google Scholar]
- Shore, S. E. (1998). “Influence of centrifugal pathways on forward masking of ventral cochlear nucleus neurons,” J. Acoust. Soc. Am. 10.1121/1.423294 104, 378–389. [DOI] [PubMed] [Google Scholar]
- Smith, R. L. (1977). “Short-term adaptation in single auditory nerve fibers: Some poststimulatory effects,” J. Neurophysiol. 40, 1098–1112. [DOI] [PubMed] [Google Scholar]
- Smith, R. L. (1979). “Adaptation, saturation, and physiological masking in single auditory-nerve fibers,” J. Acoust. Soc. Am. 10.1121/1.382260 65, 166–178. [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2001). “The relationship between frequency selectivity and overshoot,” J. Acoust. Soc. Am. 10.1121/1.1357811 109, 2063–2073. [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2004). “The temporal effect with notched-noise maskers: Analysis interms of input-output functions,” J. Acoust. Soc. Am. 10.1121/1.1691036 115, 2234–2245. [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2008). “The relationship between precursor level and the temporal effect,” J. Acoust. Soc. Am. 10.1121/1.2821977 123, 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland, E. A., and Krishnan, L. A. (2005). “The temporal effect in listeners with mild to moderate cochlear hearing impairment,” J. Acoust. Soc. Am. 10.1121/1.2074787 118, 3211–3217. [DOI] [PubMed] [Google Scholar]
- Warr, W. B. (1980). “Efferent components of the auditory system,” Ann. Otol. Rhinol. Laryngol. 90, 114–190. [DOI] [PubMed] [Google Scholar]
- Warr, W. B., and Guinan, Jr., J. J. (1979). “Efferent innervation of the organ of Corti: Two separate systems,” Brain Res. 10.1016/0006-8993(79)91104-1 173, 152–155. [DOI] [PubMed] [Google Scholar]
- Zwicker, E. (1965a). “Temporal effects in simultaneous masking by white-noise bursts,” J. Acoust. Soc. Am. 10.1121/1.1909389 37, 653–666. [DOI] [PubMed] [Google Scholar]
- Zwicker, E. (1965b). “Temporal effects in simultaneous masking and loudness,” J. Acoust. Soc. Am. 10.1121/1.1909588 38, 132–141. [DOI] [PubMed] [Google Scholar]
- Zwislocki, J., Pirodda, E., and Rubin, H. (1959). “On some poststimulatory effects at the threshold of audibility,” J. Acoust. Soc. Am. 10.1121/1.1907619 31, 9–14. [DOI] [Google Scholar]