Abstract
Individuals with a family history of alcoholism (FH+) are at enhanced risk of developing an alcohol or other substance use disorder relative to those without this history (FH-). Recent studies comparing FH+ and FH- individuals have revealed differences in cognition, emotion processing, sociability, and decision-making. These differences suggest possible altered brain functioning in FH+ individuals that may play a crucial role in vulnerability to substance use disorders. In the present study, 15 FH+ and 19 FH- individuals performed the IGT, a simulated card game requiring integration of payoff-to-penalty ratios, while undergoing functional magnetic resonance imaging. All participants performed the task more conservatively as the session progressed, and the FH groups achieved similar payoffs by the end of the game. Imaging revealed a distributed network of brain regions that was engaged when subjects performed this task, including the right inferior frontal and postcentral gyri, left parahippocampal gyrus, insula and precuneous cortices, left inferior and superior parietal lobules, left lentiform nucleus and bilateral culmen, claustrum, lingual gyri and cerebellar tonsils. Despite a lack of behavioral differences between groups, the FH+ participants showed significantly more activation in the left dorsal anterior cingulate cortex and left caudate nucleus. These findings correspond to models of risk in FH+ persons that postulate biases in brain decision-making systems as underlying elevated risk for alcoholism.
Keywords: Iowa Gambling Task, alcoholism, family history, anterior cingulate, caudate nucleus, vulnerability
1. Introduction
Individuals with a family history of alcoholism (FH+) are at increased risk for developing alcohol and other substance use disorders compared with those lacking such a history (FH-) (Finn et al., 1990; Lieb et al., 2002; Merikangas et al., 1998). This risk has a significant genetic basis, as indicated by twin, adoption, cross-fostering, and pedigree analysis studies (Cloninger et al., 1981; Merikangas, 1990; Reich et al., 1998; Slutske et al., 2002), suggesting that the degree of risk is regulated at least in part by underlying biological differences. A better understanding of the biological factors associated with a family history of alcoholism may prove invaluable for identifying the underlying pathology of addiction vulnerability and developing more effective substance abuse treatment and prevention programs.
Psychological comparisons of nonabusing FH+ and FH- young adults has revealed a pattern of “behavioral undercontrol” (Sher et al., 2004; Sher and Trull, 1994) or “neurobehavioral disinhibition” (Tarter et al., 2003) present in FH+, consisting of increased sensation seeking, risk-taking, aggressiveness, and antisocial behaviors. FH+ is also associated with subtle impairments on tests of executive functioning, and these impairments are predictive of later alcohol use disorders (Deckel, 1999; Stevens et al., 2003). The presence of these cognitive differences in FH+ and FH- individuals suggests underlying differences in neural functioning which may mediate or contribute to substance abuse risk.
Despite increased use of noninvasive neuroimaging techniques such as functional magnetic resonance imaging (fMRI) and the mounting evidence that FH+ individuals have cognitive and affective processing deficits that may mediate abuse liability, few neuroimaging studies have been conducted comparing at-risk populations (Glahn et al., 2007; Schweinsburg et al., 2004). Schweinsburg and colleagues (2004) found FH+ youths performing a go/no go task had less blood oxygenation level dependent (BOLD) activation in the left medial frontal gyrus on ‘no go’ vs. ‘go’ trials, suggesting less inhibitory activation, despite a lack of behavioral differences. Glahn and colleagues (2007) found FH+ individuals with high behavioral undercontrol showed decreased amygdala (BOLD) activation in response to faces displaying negative emotions compared to FH- individuals with low behavioral undercontrol. These findings suggest that systematic exploration of cerebral function in relation to appropriate behavioral challenges may yield useful information about at-risk populations.
The present study was designed to compare BOLD activation differences in FH+ and FH-individuals performing the Iowa Gambling Task (IGT) (Bechara et al., 1997; Bechara et al., 2001) to better understand differences in neural functioning and recruitment associated with FH+. The IGT is a simulated card game that requires subjects to track response outcomes in relation to making safe vs. risky choices. Performance on this task involves cognitive processes such as working memory, set shifting, and risk-taking; and these processes appear to be subtly altered in FH+ individuals (Deckel, 1999; Dunn et al., 2006; Lovallo, 2006). Previously, it was found there were no overall differences in the proportion of safe vs. risky choices FH+ and FH-made on this task, although further analysis indicated FH+ males choices were slightly more influenced by gains on previous trials (Lovallo et al., 2006). Given that several FH relevant cognitive processes are involved with the IGT and the expected lack of overall performance differences between FH+ and FH- individuals, we reasoned the this task would be useful assay for exploring differences in neural functioning between FH+ and FH- individuals. We predicted that the FH+ and FH- individuals would show differences in task-induced forebrain cortical and subcortical regions. We suspected that differences would be seen in prefrontal regions associated with decision-making and possibly in limbic areas associated with processing reward value of outcomes.
2. Methods
2.1. Subjects
19 FH- individuals and 15 FH+ were recruited from a larger cohort of 250 volunteers participating in the Oklahoma Family Health Patterns (OFHP) project (Saunders et al., 2008). The sample consisted of young adults in good physical health and free of Axis I and II disorders (clusters A and C) by DSM-IV criteria (APA, 1994), including current depression , current drug or alcohol abuse or any history of drug or alcohol dependence (Table 1). Smokers are included. Participants with FH+ had a biological parent that met DSM-IV criteria for alcohol use disorders by Family History Research Diagnostic Criteria (FH-RDC) (Andreasen et al., 1977). Participants with FH- had an absence of alcohol or substance use disorders in parents and grandparents. Participants were excluded if they or the parent reported possible fetal exposure to alcohol or other drugs. Volunteers traveled from Oklahoma City, OK, to San Antonio, TX, and were scanned at the Research Imaging Center at the University of Texas Health Science Center at San Antonio. Participants and parents signed consent forms approved by the supervising Institutional Review Boards and were paid for participating.
Table 1.
Sample Characteristics
| Family History | FH- | FH+ | p Values |
|---|---|---|---|
| N (M/F) | 19 (10/9) | 15 (6/9) | |
| Age (Years) | 23 (.7) | 24 (.8) | |
| Education (Years) | 15.7 (.4) | 14.5 (.4) | .06 |
| SES | 42 (3.4) | 37 (4.1) | |
| Shipley Vocabulary | 30 (0.9) | 29.7 (1.1) | |
| Ethnicity | |||
| Caucasian | 19 | 12 | |
| African American | 0 | 1 | |
| Other | 0 | 2 | |
| CPI-So | 35.2 (.6) | 26.9 (1.2) | <.0001 |
| BDI | 2 (.4) | 7.1 (1.5) | .006 |
| EPI-Neuroticism | 4.3 (1.3) | 5.6 (1.0) | |
| AUDIT | 3 (.6) | 4 (.9) | |
| Alcohol Intake (oz/mo) | 39 (9) | 64 (9) | .07 |
| Caffeine (mg/day) | 102 (26) | 102 (27) | |
| Tobacco (% Using Weekly) | 5 | 9 |
Entries (mean ± SEM) unless given otherwise. SES scores shown are considered “Middle Class.” Only p's < .10 are shown.
FH-, negative family history; FH+, positive family history; M, male; F, female; SES, Hollingshead & Redlich Socioeconomic Status Score; Shipley Vocabulary, Shipley Institute of Living Vocabulary Score; CPI-So, California Personality Inventory Sociability Scale; BDI, Beck Depression Inventory; EPI, Eysenck Personality Inventory; AUDIT, Alcohol Use Disorders Identification Test.
Behavioral undercontrol was indexed by low scores (< 30) on the Sociability scale of the California Psychological Inventory (CPI-So) (Gough, 1994; Lovallo et al., 2006), a 46-item measure of norm abiding and prosocial behavior. Groups scoring ≥ 30 are highly norm-abiding (research scientists, students in nursing and engineering) whereas scores < 30 are seen in deviant groups (marijuana smokers, shoplifters, alcoholics, pathological gamblers) (Gough 1994). In the total OFHP sample, the FH+ group scores in the antisocial direction on the CPI-So with average scores < 30, whereas the FH- group scores in the prosocial direction, with average scores > 30. This same difference is observed in the present sample (mean ± SEM: FH+ = 26.9 ± 1.2 , FH- = 35.2 ± 0.6; t (31) = 6.66, p < 0. 001; Table 2). The relevance of low CPI-So scores is indicated by the fact that there is a progressive relationship between family density of alcoholism and prevalence of low CPI-So scores in the total OFHP sample (Glahn et al., 2007).
Table 2.
Family Density of Alcoholism in Relation to Behavioral Disinhibition
| Family Density |
CPI-So Score |
|
|---|---|---|
| <30 | ≥30 | |
| 0 | 0% | 100% |
| 1 | 20% | 80% |
| 2 | 75% | 25% |
| 3-6 | 83% | 17% |
Entries show the percentage of persons in the present study (n=34) representing four levels of family density of alcoholism and the percentages of those subjects that scored in the low (< 30) versus high (≥ 30) range on the Sociability scale of the California Personality Inventory as an index of behaviorally disinhibited temperament. χ2 = 21.0, p<.0001.
CPI-So, California Personality Inventory Sociability Scale.
2.2. Procedure
2.2.1. Behavioral Procedure
The Iowa Gambling Task (IGT) is a simulated card game presented on a computer display. The subject sees 4 decks of cards face down on the screen, and on each of the 140 trials he or she turns the top card on 1 of the 4 decks face up. After each trial the subject is informed of the amount of money won on that trial along with the amount of loss. The net difference determines the winnings for that trial. In this version of the game, the decks were stacked to provide a balance of wins to losses that changed as play progressed. Decks A and B yield large gains ($100 per play) but also large or frequent lossses, so that consistent play from these risky decks results in a net loss for the game. In contrast, decks C and D yield smaller gains ($50) but also smaller or less frequent losses so that consistent play from these safe decks results in net winnings for the game. The player cannot predict when a given deck will yield a gain or a penalty and does not know how many plays he or she will have. The only adequate strategy is to respond according to the long-run payoffs from each deck. Thirty-second task blocks were interleaved with a rest condition (15 seconds) and the procedure required 9 minutes to complete. All subjects had performed the identical behavioral task approximately 2-12 months prior to the imaging experiment (Lovallo et al., 2006). The primary dependent measure on this task was the number of cards drawn from the safe decks (C & D) minus the number of cards drawn from the risky decks (A & B).
2.2.2. Imaging Parameters
Imaging was carried out on a research-dedicated Siemens 3T MRI (Siemens, Munich, Germany) housed in the Research Imaging Center at UTHSCSA. Functional imaging used a gradient-echo, echo-planar sequence, acquiring 30 slices (4 mm thick, 1 mm gap) parallel to the anterior commissure-posterior commissure (AC-PC) plane (repetition time/echo time [TR/TE] = 2500/30 msec, 128 × 128 × 5 mm, and field of view [FOV] = 256 mm). For anatomical reference, we acquired a higher resolution coplanar T1-weighted series (TR/TE = 500/20 msec, flip angle = 90°, 128 × 128 × 5 mm, FOV = 256 cm) and a high-quality three-dimensional (3-D) image (TR/TE = 33/12 msec, and flip angle = 60°, 1 mm isotropic). The imaging session was completed in one hour.
2.3. Analysis of fMRI images
Analysis of fMRI data was carried out with FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004). Prior to statistical modeling, data were motion corrected (Woolrich et al., 2001), non-brain tissue was removed (Jenkinson et al., 2002; Jenkinson and Smith, 2001), spatially smoothed with a Gaussian kernel of FWHM 5mm and a high-pass temporal filtering was applied (Gaussian-weighted least-squares straight line fitting, with sigma=50.0s) (Smith et al., 2004). Time-series statistical analysis was carried out using local autocorrelation correction (Beckmann et al., 2003b; Woolrich et al., 2004a). Registration to high resolution and/or standard images was carried out using FLIRT (Worsley et al., 1992). Higher-level analysis was carried out using FLAME (FMRIB's Local Analysis of Mixed Effects) stage 1 only (i.e., without the final MCMC-based stage) and were performed with a mixed-effects model (Beckmann et al., 2003a; Woolrich et al., 2004b) where subject was treated as a random effect and images contrasting the ‘task’ condition versus rest (implicit baseline), respectively, were generated. Spatial mixture modeling was applied to z statistical images of group differences and thresholded conservatively (p < 0.0001, z ≥ 2.3) (Woolrich et al., 2005).
3. Results
3.1. Behavioral Performance
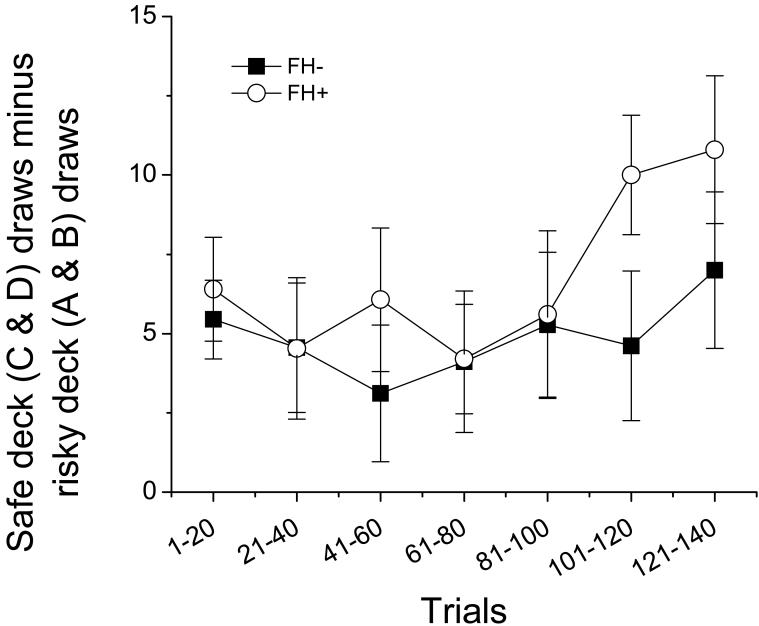
Participants in both groups chose more cards from the safe decks (C& D) as the session progressed [main effect of time; F (6,186) = 2.703, p = 0.015, Figure 1]. There were no significant differences in the ratio of safe to risky cards drawn between FH+ and FH- individuals at any time point.
Figure 1.
Mean (± SEM) safe cards (decks C & D) minus risky cards (decks A & B) drawn across the course of the session in FH- (filled squares) and FH+ (open circles) participants. There a significant increase in the number of safe cards drawn as the session progressed in all participants [F (6,186) = 2.703, p = 0.015] and there were no group differences.
3.2. Imaging Results
3.2.1. All subjects
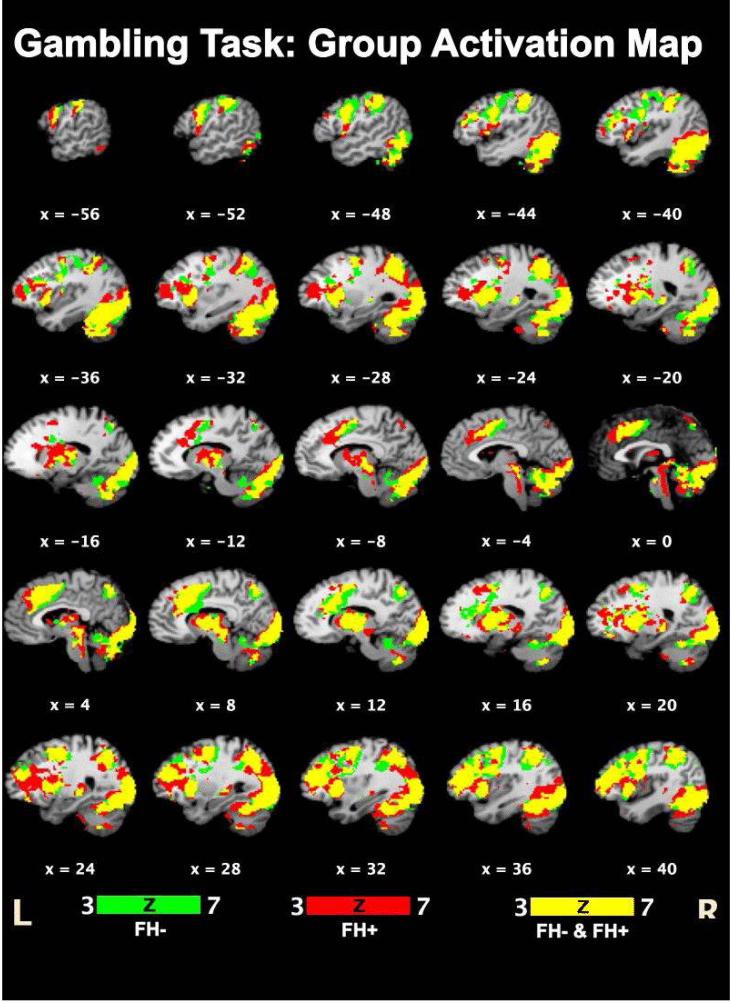
Task versus rest comparison showed similar activation patterns in both FH- and FH+ participants (Figure 2, Table 3). Common activated regions included the right inferior frontal and postcentral gyri, left parahippocampal gyrus, insula and precuneous cortices, left inferior and superior parietal lobules, left lentiform nucleus, and bilateral culmen, claustrum, lingual gyri and cerebellar tonsils.
Figure 2.
Areas activated in FH- (green) and FH+ (red) and all participants (yellow). See Table 3 for details.
Table 3.
Regions activated in all subjects (Z score > 4.0)
| Anatomy (Brodmann area) | Z score | Talairach- Tournoux coordinates (mm) |
||
|---|---|---|---|---|
| X | Y | Z | ||
| Rt. Inferior Frontal Gyrus (BA 44) | 7.69 | 48 | 0 | 16 |
| Lt. Culmen | 7.79 | -34 | -54 | -26 |
| Rt. Culmen | 4.36 | 26 | -28 | -32 |
| Lt. Inferior Parietal Lobule (BA 40) | 6.11 | -52 | -36 | 46 |
| Lt. Inferior Parietal Lobule (BA 40) | 6.42 | -46 | -38 | 38 |
| Lt. Superior Parietal Lobule (BA 7) | 6.29 | -28 | -54 | 40 |
| Lt. Superior Parietal Lobule (BA 7) | 6.01 | -28 | -62 | 46 |
| Lt. Precuneus (BA 7) | 6.53 | -26 | -64 | 32 |
| Lt. Precuneus (BA 7) | 6.48 | -26 | -62 | 38 |
| Rt. Postcentral Gyrus (BA 40) | 5.04 | 58 | -24 | 20 |
| Lt. Parahippocampal Gyrus (BA 27) | 6.04 | -24 | -28 | -4 |
| Lt. Insula(BA 13) | 7.26 | -36 | 12 | -4 |
| Lt. Claustrum | 7.27 | -30 | 10 | 4 |
| Rt. Claustrum | 7.43 | 32 | 10 | 6 |
| Rt. Claustrum | 7.52 | 34 | 10 | 2 |
| Lt. Lentiform Nucleus | 7.27 | -26 | 8 | 4 |
| Lt. Lingual Gyrus (BA 18) | 7.54 | -24 | -94 | -6 |
| Lt. Lingual Gyrus (BA 18) | 7.6 | -12 | -90 | -14 |
| Rt. Lingual Gyrus (BA 17) | 7.48 | 12 | -96 | -10 |
| Rt. Lingual Gyrus (BA 17) | 7.48 | 18 | -96 | -10 |
| Lt. Uvula | 7.8 | -6 | -72 | -30 |
| Rt. Cerebellar Tonsil | 4.85 | 22 | -32 | -38 |
| Rt. Cerebellar Tonsil | 4.44 | 30 | -34 | -34 |
3.2.2. FH- Versus FH+
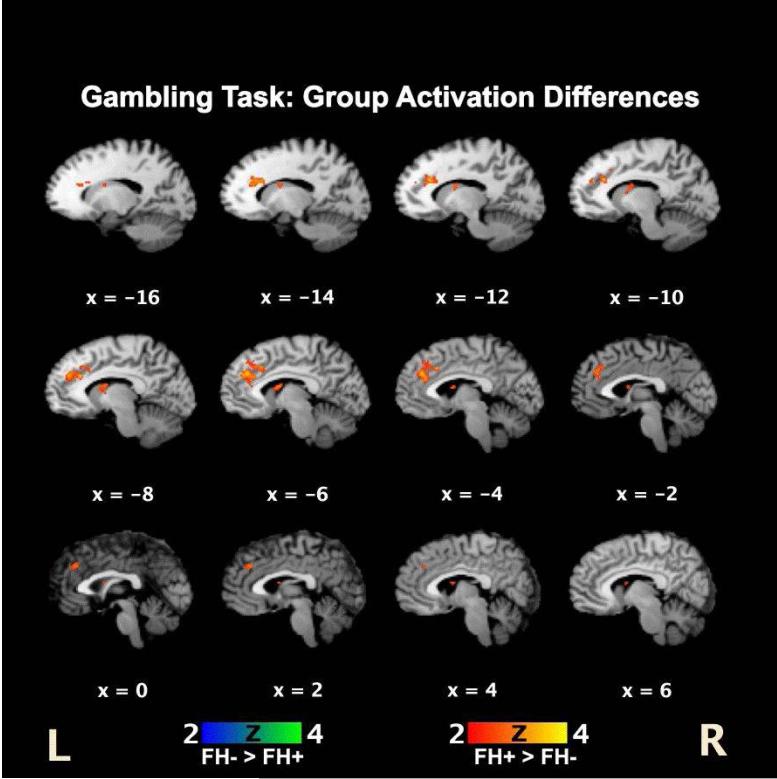
Statistical comparison of FH- vs. FH+ activation maps demonstrated relatively greater activations in the left dorsal anterior cingulate gyrus and left caudate nucleus in the FH+ compared to the FH- group (Figure 3). There were no regions showing increased activations in the FH- group compared to the FH+ group.
Figure 3.
Activation differences in FH- and FH+ individuals. FH+ participants showed greater relative activations localized primarily to the left anterior cingulate cortex and left caudate nucleus.
4. Discussion
In the present study, FH- and FH+ participants performed the IGT while undergoing fMRI. There were no significant differences in task performance between the groups, and both groups showed largely overlapping patterns of task induced BOLD activations. However, FH+ individuals showed greater relative activation in the left dorsal anterior cingulate cortex (dACC) and left caudate nucleus. The dACC region has been implicated in attention, decision-making, emotion processing and initiation of motor behavior (Paus, 2001). The caudate nucleus is involved with linking behaviors to outcomes (Knutson and Cooper, 2005; Yamada et al., 2007). Our findings suggest that neural regulation of these processes mediated by the dACC and caudate nucleus may be altered in FH+ individuals.
There were no significant differences in the number of cards drawn from the safe and risky decks between the FH- and FH+ groups, consistent with analysis from a larger sample (n=175) study from which the present subjects were drawn (Lovallo et al., 2006). The relatively high rates of cards drawn from the safe decks early in the session may be due to subjects having previously performed the task 2-12 months earlier. Indeed participants in both groups drew significantly more cards from the safe deck in the first 20 trials during the second relative to the first administration of the task, but both groups performed the remainder of the task similarly across both administrations (data not shown). Consequently, the activations observed in the present study may relate more to risk-taking aspects of the task and perhaps less so to learning the card contingencies.
Subtle performance differences on the task were found in the much larger sample in the main study cohort (n = 175), with FH+ males' choices being more influenced by past gains relative to past losses, however it was not feasible to detect these differences with the relatively small sample used in the present study. No FH group differences in performance were found in the earlier administration of the task among the participants included in the present study sample either (data not shown). Despite the lack of gross performance differences in FH+ individuals on the IGT, we considered this measure useful for exploring differences in functional brain activity because it likely involves numerous cognitive processes subtly affected in FH+ individuals, including working memory, set-shifting, and risk-taking, and the task has been demonstrated to activate numerous cortical and subcortical structures (Bechara et al., 1997; Bechara et al., 2001; Deckel, 1999; Dunn et al., 2006; Lovallo, 2006).
Although it is presently unclear how altered activity in the dACC and caudate nucleus may relate to the FH+ behavioral phenotype, a number of possibilities merit consideration. The dACC is important for response-conflict monitoring (Critchley et al., 2005; Roelofs et al., 2006; Swick and Jovanovic, 2002), and thus general altered dACC activity in FH+ may relate to impaired Stroop Task performance in FH+ individuals (Lovallo et al., 2006). More related to the task at hand, the ACC region is important for risk prediction, including signaling the extent of risk and the severity of consequences (Brown and Braver, 2007). Similarly, the caudate nucleus is important for linking behaviors to outcomes, both rewarding and aversive (Knutson and Cooper, 2005; Yamada et al., 2007), and increased activation has also been observed in the caudate nucleus in young adult substance abusers on a different risk-taking task, albeit in association with corresponding increases in risky responses (Leland et al., 2006). The ACC and caudate are connected anatomically and may work together during tasks such as the IGT as prior outcomes are weighed and choices are made. Given the altered activity in both the dACC and caudate nucleus, it is possible that FH+ and FH- individuals may show more distinct differences in risk-taking behavior on other, more specific measures of risk-taking.
It is possible that the increased activity in the dACC and caudate nucleus, two key recipients of dopamine projections, may be related to altered dopamine functioning as there is evidence that dopamine systems may be working differently in FH+ individuals (Kohnke, 2008; Volkow et al., 2006). The caudate nucleus receives extensive dopaminergic inputs from both the ventral tegmental area (VTA) and the substantia nigra, and the dACC receives the highest density of VTA dopaminergic projections in the entire primate cortex (Ouimet et al., 1992; Paus, 2001). Volkow et al. (2006) reported increased D2 receptor availability in the caudate nucleus and ventral striatum in FH+ individuals, which may indicate either increased D2 receptors or decreased dopamine levels (but see also Munro et al., 2006). Additionally, there is accumulating evidence that alcoholism may be linked to allelic variations in genes for D2 and D4 dopamine receptors, dopamine transporters, and dopamine metabolizing enzymes (Kohnke, 2008). It is possible that underlying physiological alterations (dopaminergic or nondopaminergic) in FH+ individuals require differential utilization of the anterior cingulate and caudate nucleus to perform the IGT. Alternatively, it is possible that a subtly increased attention to gains in FH+ individuals may be related to the altered activity in these regions even in the absence of gross physiological differences. It is not possible to separate these alternatives with the present data. FH+ is a clearly identified predisposing factor for developing alcohol and other substance use disorders and a better understanding of the neural substrates underlying this vulnerability will provide insight into the pathology of substance abuse.
The present study contributes to an emerging body of literature implicating biases in decision-making and risk-taking in FH+ persons. These models are consistent with recent studies reporting functional activation differences in FH+ individuals (Glahn et al., 2007; Schweinsburg et al., 2004). Future imaging studies in FH+ individuals will be needed to better elucidate differences in neural functioning in this population and how these differences relate to addiction liability. The relevance for the present findings for future abuse potential will require testing a larger sample of FH+ individuals and longitudinal studies of drinking behaviors, which are now underway.
Acknowledgements
Supported by the Medical Research Service of the Department of Veterans Affairs, the National Institutes of Health, NIAAA (AA12207) (W.R.L.), NIRR (RR14467) (W.R.L.) and the UTHSCSA GCRC (M01-RR-01346). We thank Katherine Knight, Ginger D. Haines, and Kristen H. Sorocco for subject screening and assessment.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. (New York, NY. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003a;20:1052. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003b;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: Cross fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Deckel AW. Tests of executive functioning predict scores on the MacAndrew Alcoholism Scale. Progress in neuro-psychopharmacology & biological psychiatry. 1999;23:209–223. doi: 10.1016/s0278-5846(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: a critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kleinman I, Pihl RO. The lifetime prevalence of psychopathology in men with multigenerational family histories of alcoholism. The Journal of Nervous and Mental Disease. 1990;178:500–504. [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biological psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough H. Theory, development, and interpretation of the CPI socialization scale. Psychological Reports. 1994;75:651–700. doi: 10.2466/pr0.1994.75.1.651. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current opinion in neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kohnke MD. Approach to the genetics of alcoholism: a review based on pathophysiology. Biochem Pharmacol. 2008;75:160–177. doi: 10.1016/j.bcp.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Leland DS, Arce E, Feinstein JS, Paulus MP. Young adult stimulant users' increased striatal activation during uncertainty is related to impulsivity. NeuroImage. 2006;33:725–731. doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychological Medicine. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Individual Differences in Response to Stress and Risk for Addiction. In: al'Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Academic Press; New York: 2006. pp. 227–248. [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcoholism, clinical and experimental research. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR. The genetic epidemiology of alcoholism. Psychological Medicine. 1990;20:11–22. doi: 10.1017/s0033291700013192. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Archive of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Oswald LM, Wong DF, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Striatal dopamine release and family history of alcoholism. Alcoholism, clinical and experimental research. 2006;30:1143–1151. doi: 10.1111/j.1530-0277.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, LaMantia AS, Goldman-Rakic P, Rakic P, Greengard P. Immunocytochemical localization of DARPP-32, a dopamine and cyclic-AMP-regulated phosphoprotein, in the primate brain. The Journal of comparative neurology. 1992;323:209–218. doi: 10.1002/cne.903230206. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature reviews. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr., Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. American Journal of Medical Genetics. 1998;81:207–215. [PubMed] [Google Scholar]
- Roelofs A, van Turennout M, Coles MG. Anterior cingulate cortex activity can be independent of response conflict in Stroop-like tasks. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13884–13889. doi: 10.1073/pnas.0606265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Jr., Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcoholism, clinical and experimental research. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Reviews of Clinical Psychology. 2004;22:1–22. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. Journal of Abnormal Psychology. 2002;111:124–133. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kaplan RF, Hesselbrock VM. Executive-cognitive functioning in the development of antisocial personality disorder. Addictive behaviors. 2003;28:285–300. doi: 10.1016/s0306-4603(01)00232-5. [DOI] [PubMed] [Google Scholar]
- Swick D, Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–1253. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004a;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Smith SM. Mixture models with adaptive spatial regularization for segmentation with an application to FMRI data. IEEE Trans Med Imaging. 2005;24:1–11. doi: 10.1109/tmi.2004.836545. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004b;21:1732. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yamada H, Matsumoto N, Kimura M. History- and current instruction-based coding of forthcoming behavioral outcomes in the striatum. Journal of neurophysiology. 2007;98:3557–3567. doi: 10.1152/jn.00779.2007. [DOI] [PubMed] [Google Scholar]