Abstract
Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme for prostaglandin biosynthesis. Its inducible expression is regulated by complex pathways.
To monitor Cox-2 transcriptional activity in vivo, we generated a knock-in mouse expressing a firefly luciferase reporter. In this study we examined, by comparing luciferase activity of Cox-2luc/+ and Cox-2luc/- cells and mice, effects of prostanoid products on Cox-2 promoter transcriptional activation. In peritoneal macrophages, luciferase induction by LPS in Cox-2luc/- cells was less than that of Cox-2luc/+ cells. However, in the presence of PGE2, induction was comparable, suggesting positive Cox-2 feedback regulation by PGE2 occurs for macrophages. In contrast, feedback modulation was not observed in TPA-induced Cox-2luc/+ and Cox-2luc/- mouse embryonic fibroblasts (MEFs). Using non-invasive in vivo imaging, we observed negative feedback regulation of Cox-2 expression during paw inflammation in living mice. Our results suggest Cox-2 expression is regulated by cell type specific feedback mechanisms, both in cultured cells and in living animals.
Keywords: prostaglandin, peritoneal macrophage, embryonic fibroblast, in vivo imaging, inflammation
Introduction
The cyclooxygenases (COXs) catalyze the conversion of arachidonic acid to PGH2, the common intermediate in prostaglandin, prostacyclin and thromboxane synthesis. Expression of the constitutive Cox-1 gene is regulated by a GC-rich “housekeeping” promoter lacking TATA or CAAT box sequences [1, 2]. The Cox-2 gene is an immediate-early gene whose transcription is induced by a variety of ligands, including tumor promoters, growth factors, cytokines, endotoxins and mitogens, in a variety of cells [3, 4].
A number of signal transduction pathways and transcription factors mediate Cox-2 induction; moreover, signal transduction pathways for Cox-2 induction vary depending on stimulus and cell type [5]. The cyclic AMP responsive element (CRE), nuclear factor-interleukin 6 elements (NF-IL6), and an NF-κB element were characterized initially as key transcriptional regulatory sequences in the Cox-2 promoter [6, 7]. A peroxisome proliferator response element (PPRE), sterol response element, NFAT element, PU.1/ets binding site and erb-B2/HER2 binding site were also identified as functional Cox-2 promoter elements [5, 8-10]. In addition to transcriptional regulation, COX-2 expression is also regulated by post-transcriptional message stabilization and by regulation of Cox-2 message translation [11, 12].
Prostanoids are reported to feedback regulate Cox-2 gene expression. However, prostanoid feedback regulation of Cox-2 gene expression is dependent both on cell type and prostanoid product [13-17]. Moreover, COX-2 is upregulated in a tissue-specific fashion in Cox-1 knockout mice, indicating that compensatory mechanisms regulate prostaglandin production differently in alternative tissues [19, 20]. These studies suggest that COX-2 expression is adjusted, in a tissue/cell type specific manner, by the prostaglandins whose synthesis it mediates.
To investigate Cox-2 gene expression and to understand the role of this gene in the context of the living animal, we generated knock-in mice in which firefly luciferase is expressed at the translation start site of the endogenous Cox-2 gene [21]. The Cox-2 luciferase knock-in allele is also a functional Cox-2 knockout allele. Loss of functional COX-2 expression from the Cox-2luc allele provides a tool to investigate whether prostanoid products resulting from Cox-2 gene expression affect subsequent Cox-2 promoter transcription. Here we use Cox-2luc/+ and Cox-2luc/- macrophage and fibroblasts to examine effects of COX-2 regulated prostanoids on subsequent Cox-2 transcription in culture. We also address Cox-2 feedback regulation in living animals, using in vivo optical imaging of Cox-2luc mice.
Materials and methods
Mice carrying the Cox-2 luciferase and knockout alleles were described previously [21]. LPS (Lipopolysaccharide; Escherichia coli serotype 0111:B4), Zymosan and TPA (tetradecanoylphorbol acetate) were from Sigma (MO, USA). PGE2, PGD2 and NS398 were from Cayman Chemicals (Ann Arbor, MI, USA).
Macrophages were isolated by peritoneal lavage three days after peritoneal injection (three ml) of 3% thioglycolate medium (Sigma). Cells were washed, resuspended and plated in DMEM/10% FBS. Fibroblasts from Cox-2luc/+ and Cox-2lux/- embryos (12.5 dpc) were prepared as described [21]. LPS zymosan, TPA, PGE2, and PGD2 treated cells were assayed for luciferase activity as described [21].
Inflammation was induced by intraplantar zymosan (2.0% in PBS, 30 μl) injection into the right hind paw, as described [22]. The left hind paw received PBS. For in vivo imaging, mice were anesthetized, injected with luciferin, and imaged with an IVIS imaging system (Xenogen) as described [21].
Results
To investigate whether COX-2 protein, or any of the prostanoid products resulting from COX-2 induction, affect transcriptional activation of the Cox-2 gene we used mice carrying a Cox-2luc knock-in allele [21]. Because the Cox-2luc allele is also a Cox-2 knockout allele, luciferase activity of Cox-2luc/- cells or mice reflects Cox-2 promoter transcriptional activity in the absence of COX-2 protein or any of its downstream products.
Macrophages generate prostaglandins in response to endotoxin. However, peritoneal macrophages from Cox-2-/- homozygous knockout mice completely lack endotoxin-induced PGE2 synthesis [23]. Cox-2luc/- mice are homozygous for their inability to express functional COX-2 and should, therefore, similarly not produce PGE2. Cox-2luc/+ luciferase knock-in mice were crossed with Cox-2+/- mice and peritoneal macrophages were isolated from Cox-2luc/+ and Cox-2luc/- progeny. Cox-2luc/+ and Cox-2luc/- macrophages were treated with LPS and assayed for luciferase activity (Fig. 1A). Luciferase activity is induced at two hours after LPS addition and reaches a maximum at four hours in Cox-2luc/+ macrophages. Cox-2luc/- macrophages also show luciferase induction at two and four hours. However, luciferase activity in Cox-2luc/- macrophages is only 30 - 50% of that in Cox-2luc/+ macrophages, despite the fact that both macrophage populations have one Cox-2luc allele.
Figure 1.
(A) Peritoneal macrophages were isolated from two Cox-2luc/+ and two Cox-2luc/- mice, treated with LPS (100 ng/ml) for times shown and assayed for luciferase activity. (B) Zymosan-treated macrophage from Cox-2luc/+ and Cox-2luc/- mice were assayed for luciferase activity. Data are averages ± SD for three independent cultures for each mouse at each time point in (A) and (B). (C) NS398 suppresses Cox-2luc/+ peritoneal macrophage luciferase activity. Data are averages ± SD for three independent cultures.
Cox-2 expression is also induced by zymosan in peritoneal macrophages [24]. Cox-2luc/+ and Cox-2luc/- macrophages were treated with zymosan (Fig. 1B). Luciferase expression is induced in macrophages of both genotypes. However, once again, luciferase activity of Cox-2luc/- macrophages is only about 50% of that in Cox-2luc/+ macrophages.
The activating effect of COX-2 protein expression on subsequent Cox-2 promoter activity in peritoneal macrophages was also observed in an experiment using the COX-2 specific inhibitor, NS398 (Fig. 1C). Cox-2luc/+ macrophage luciferase activity is decreased to 50% and 30% with 10 nM and 100 nM NS398, respectively. These genetic (Figs 1A, 1B) and pharmacologic (Fig. 1C) results suggest that downstream COX-2 products have a positive feedback effect on Cox-2 transcription in peritoneal macrophages.
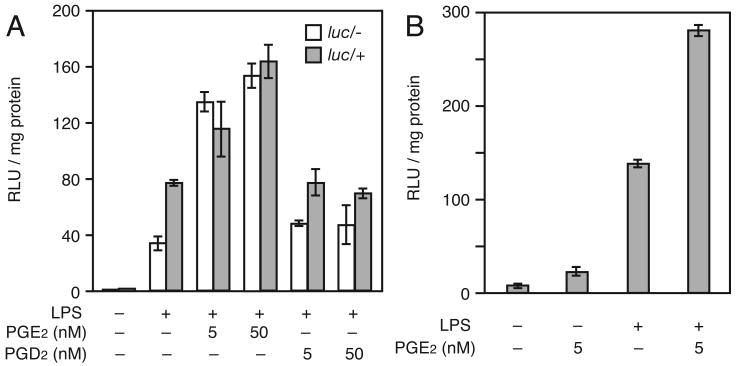
The major downstream COX-2 macrophage product is PGE2. We examined whether exogenous PGE2 would restore transcriptional activity of the Cox-2 promoter in Cox-2luc/- macrophages (Fig. 2A). Although, once again, endotoxin-treated Cox-2luc/- macrophage expressed only half the Cox-2 promoter-directed luciferase activity observed in Cox-2luc/+ macrophages, luciferase activity in Cox-2luc/- macrophages is comparable with activity in Cox-2luc/+ macrophages when the cells receive PGE2 in addition to LPS. PGE2 also increased luciferase activity of LPS-treated Cox-2luc/+ macrophages; greater induction is observed with both 5 nM and 50 nM PGE2 in macrophages of both genotypes. In contrast, PGD2 (5 nM or 50 nM) does not rescue Cox-2 promoter activity in Cox-2luc/- macrophages or increase luciferase activity in Cox-2luc/+ macrophages.
Figure 2.
(A) PGE2 enhances Cox-2 driven luciferase expression in Cox-2luc/+ and Cox-2luc/- macrophages. Macrophages were treated with LPS (100 ng/ml), LPS + PGE2 (5 or 50 nM) or LPS + PGD2 (5 or 10 nM) for 4 hours. (B) PGE2 plus LPS induce an increased transcriptional response. Cox-2luc/- macrophages were treated with LPS (100 ng/ml), PGE2 (5 nM) or LPS + PGE2 for 4 hours. Data are averages ± SD for three independent cultures for (A) and (B).
To compare the effect of PGE2 alone and the combination of PGE2 plus LPS on Cox-2 promoter activity, Cox-2luc/- macrophages, (which cannot produce any COX-2) were examined for luciferase activity following treatment with each ligand alone and in combination (Fig. 2B). Luciferase activity is induced approximately three-fold by PGE2 alone, while LPS alone induced a 20 fold increase in luciferase activity. When LPS was added together with PGE2 the luciferase induction was 40 fold, indicating that PGE2 and LPS co-operate to elevate Cox-2 transcriptional activity in murine macrophages.
A number of studies suggest that feedback regulation of Cox-2 transcription by prostanoid products is varied and dependent on cell type. We previously reported that the absence of Cox-2 protein expression does not modulate transcriptional activation for the Cox-2 gene in MEFs; no difference in luciferase activity was observed between Cox-2luc/+ and Cox-2luc/- MEFs induced with TPA [21]. In Figure 3A, we demonstrate this result again, to emphasize the difference in this regard between macrophages (Figs. 1A and 1B) and fibroblasts. To examine the direct effect of PGE2 on Cox-2 promoter activity in fibroblasts unable to express any COX-2 enzyme, Cox-2luc/- MEFs were treated with TPA and PGE2 (Fig. 3B). TPA alone induces luciferase activity from the Cox-2 promoter approximately nine fold. PGE2 alone does not induce luciferase activity. In contrast to the results for macrophage treated with the combination of LPS and PGE2, where additional stimulation of LPS-induced Cox-2 promoter activity was observed in the presence of PGE2 (Fig. 2), PGE2 suppresses the TPA-induced luciferase activity elicited from the Cox-2 promoter in MEFs (Fig. 3B). LPS alone, or the combination of LPS plus PGE2, does not induce luciferase activity from the Cox-2 promoter in MEFs, unlike results for macrophage.
Figure 3.
(A) Luciferase induction from the Cox-2 promoter in Cox-2luc/+ and Cox-2luc/- MEFs treated with TPA (50 ng/ml). (B) Luciferase induction from the Cox-2 promoter in MEFs by TPA is inhibited by exogenous PGE2. Cox-2luc/- MEFs isolated from two different mice were treated with TPA (50 ng/ml), PGE2 (50 nM), TPA+ PGE2, LPS (100 ng/ml), and LPS+ PGE2 for 4 hours. Data are averages ± SD for three independent cultures for each time point for (A) and B).
Our results with cells isolated from Cox-2luc/+ and Cox-2luc/- mice support the conclusions, established in many cell culture models, that Cox-2 promoter activity can be modulated by prostanoid products and that the nature of this modulation is cell-type dependent. The use of Cox-2luc/+ and Cox-2luc/- mice, in which luciferase reporter gene expression permits us to monitor non-invasively the Cox-2 gene transcriptional activity [21], provides a hither-to unavailable opportunity to monitor prostanoid-dependent modulation of Cox-2 promoter activity/gene expression in living animals. To investigate this feedback system in living mice, we used in vivo imaging to compare Cox-2 promoter-dependent luciferase activity in Cox-2luc/+ and Cox-2luc/- mice following an inflammatory stimulus.
Previously, we optimized a zymosan-induced paw inflammation model for mice, and characterized luciferase reporter responsiveness in Cox-2luc/+ mice during this inflammation process [21]. To extend this system to the study of prostanoid feedback on Cox-2 gene expression in living mice, Cox-2luc/+ mice (which produce COX-2 dependent prostanoids in response to this inflammatory stimulus) and Cox-2luc/- mice (which cannot produce COX-2 dependent prostanoids) were injected with zymosan into the right rear paws and with saline into the left rear paws. Luciferase expression was analyzed by non-invasive bioluminescent imaging [21] before zymosan injection and at 6, 9, 12, 24 and 48 hrs following zymosan (Fig. 4A). In Cox-2luc/+ heterozygous mice, elevated Cox-2 promoter-driven luciferase signal was observed at six hours in the zymosan-injected paw, reached a maximum level around 9 – 12 hours, and decreased to baseline levels by 24 hours (Fig. 4, and data from [21]). In Cox-2luc/- mice, luciferase activity is also induced at nine hours following zymosan injection and starts decreasing at 12 hours (Fig. 4). However, at 24 hours, the luciferase signal increases again and shows a second, higher peak of luciferase activity not seen in Cox-2luc/+ mice. The second wave of Cox-2 transcriptional activity in Cox-2luc/- mice decreases by 48 hours.
Figure 4.

(A) In vivo imaging of Cox-2luc/+ and Cox-2luc/- mice. Each mouse was injected with zymosan in the right rear paw and PBS in the left paw, then anesthetized, injected with luciferin, and imaged at time shown following zymosan injection. (B) Luciferase activities, as photon number, are shown for zymosan-injected paws of each mouse.
Discussion
In this study we demonstrated the effect of COX-2 products on Cox-2 promoter activity, using a Cox-2 luciferase knock-in/knock-out allele. Luciferase reporter gene expression is regulated by the endogenous chromosomal Cox-2 promoter region for the Cox-2luc knock-in allele, in same manner as the wild-type Cox-2 gene. Promoter-mediated transcriptional activation of the Cox-2luc knockout and Cox-2+ wild-type alleles should be identical, in cultured cells and in vivo. The induced luciferase activity of Cox-2luc/luc homozygous mouse embryo fibroblasts is approximately double the amount of luciferase activity found in Cox-2luc/+ fibroblasts, suggesting a simple gene dosage relationship for expression from the Cox-2luc allele in those cells [21]. However, Cox-2luc/- cells, which are heterozygous for luciferase expression but are homozygous Cox-2 knockouts, have one Cox-2luc allele – like Cox-2luc/+ cells. As a result, one can study the transcriptional activation of the Cox-2luc allele in the presence or absence of a wild-type, functional Cox-2+ allele.
The Cox-2 gene contains a number of “AREs” (AU-rich elements) in the 3′-untranslated regions (UTR) [25, 26]. AREs regulate the stability and translation of many mRNAs, including Cox-2 mRNA [27]. For example, in IL-1β treated human synovial fibroblasts, PGE2 regulates Cox-2 stability post-transcriptionally through the effect of p38 MAP kinase on the 3′-UTR [13]. However, the Cox-2luc allele was constructed with an SV40 polyA signal at its 3′ region [21] instead of the original Cox-2 3′ non-coding sequence, which contains AREs. Thus, by using the Cox-2luc allele, one can monitor the transcriptional activity of the endogenous Cox-2 gene promoter, eliminating the Cox-2 gene 3′-UTR effects on message stability and translation.
In peritoneal macrophage, Cox-2luc/- cells show less Cox-2 promoter activity after LPS induction than do Cox-2luc/+ cells. This difference is rescued by the addition of PGE2, but not by PGD2. PGE2 induces only a slight increase in Cox-2 transcription by itself, however, in contrast to its significantly enhancing effect on the transcriptional activation induced by LPS. These results indicate the existence of positive feedback regulation by PGE2 on Cox-2 promoter activity in murine macrophages. Mouse peritoneal macrophages express PGE receptor subtypes EP2 and EP4. PGE2 binding to these receptors increases intracellular cAMP levels, in both untreated and LPS treated mouse peritoneal macrophages [28]. Similarly, in murine bone marrow derived macrophages, Cox-2 expression is up-regulated by PGE2 or by dibutyryl cAMP (a cell permeable analogue of cAMP) in association with TNFα [29]. In RAW 264.7 macrophages, PGE2 up-regulates Cox-2 expression with LPS; this up-regulation is mediated by adenylyl cyclase [14]. These studies suggest that the positive feedback effect of PGE2 on Cox-2 expression in cultured macrophages is mediated by cAMP. Elevated cAMP activates protein kinase A, which phosphorylates CREB, in bone marrow derived macrophages [30]. Although CREB elevation in response to PGE2 is not by itself able to activate Cox-2 gene expression, the data suggest that phosphorylated CREB contributes to the enhancement of LPS- or TNFα- induced Cox-2 gene transcription. Positive feedback regulation of PGE2 has also been reported in human monocytes, lung fibroblast cells, human cholangiocarcinoma cells, prostate cancer cell lines and mouse cultured podocytes [14-16, 31, 32].
Transcriptional regulation of the Cox-2 gene is very complex; substantial feedback variation appears to exist in different cell types and in response to distinct stimuli in individual cell types. Moreover, multiple signaling pathways appear to be interacting with one another. In contrast to PGE2 effects on COX-2 induction in macrophages, we observe no difference in luciferase induction between Cox-2luc/- and Cox-2luc/+ embryonic fibroblasts treated with TPA. However, exogenous PGE2 suppresses the TPA-induced Cox-2 transcription with TPA, suggesting a potential PGE2 negative feedback regulation in these cells. PGE2 also down-regulates Cox-2 expression in human umbilical vein endothelial cell (HUVEC) [33].
Cox-2 expression is also regulated by other prostanoids. PGF2α, 15d-PGJ2 and 6-keto PGF1α enhance COX-2 protein in mouse lung fibroblast cells [15], however, 15d-PGJ2 negatively regulates COX-2 in the macrophage-like differentiated cell line (U937) and rheumatoid synovial fibroblasts [17, 18]. These studies, and our results, indicate that feedback regulation in cultured cells is dependent on cell type.
Because Cox-2 expression can be induced in many types of cells, and alternative prostanoids have been shown to regulate Cox-2 expression in cell culture systems, it is quite likely that Cox-2 expression in vivo is also regulated by positive and negative feedback mechanisms in response to prostaglandins produced by various types of cells. Using Cox-2luc/- and Cox-2luc/+ mice makes it possible to analyze, in living animals, COX-2 product-dependent feedback regulation of Cox-2 gene expression. To investigate whether the transcriptional effects on the Cox-2 gene by COX-2 dependent products could be monitored in living animals by utilizing Cox-2luc mice, we examined luciferase expression during paw inflammation induced by zymosan injection, a model previously described and investigated in our laboratory [21], in Cox-2luc/+ and Cox-2luc/- mice. In this inflammation model, Cox-2luc/+ mice exhibit a single peak of Cox-2 promoter driven luciferase activity following zymosan injection. In contrast, Cox-2luc/- mice have a second peak of luciferase activity. This second peak of luciferase activity in the zymosan-injected, inflamed paw is not observed in Cox-2luc/+ mice. This result suggests the existence of negative feedback regulation of Cox-2 transcriptional activity by one of the COX-2 downstream products; COX-2 dependent prostanoid production appears to prevent the second peak of Cox-2 promoter activity in the zymosan-injected paw.
In our homogeneous macrophage and fibroblast cell culture systems, the effects of COX-2 dependent prostanoid synthesis on subsequent Cox-2 transcriptional activity are clearly occurring in the same cell type. However, in the in vivo paw inflammation model, it is quite possible – and even likely – that COX-2 dependent prostanoid production in one cell type will modulate Cox-2 transcriptional activity in a second cell type. Thus the second peak of Cox-2 promoter transcriptional activity observed in the paw following zymosan injection may, in fact, occur in a population of cells different from the cells in which the initial Cox-2 transcriptional activity is seen. We previously demonstrated the local accumulation of COX-2 protein following zymosan injection occurs in epidermal cells and infiltrating inflammatory cells of the zymosan injected paw, using anti-COX-2 immunohistochemistry [22]. We have not yet identified the cell type(s) expressing the initial and subsequent peaks of luciferase activity in Cox-2luc/- mice; available anti-luciferase antibodies do not perform well in immunohistochemical studies. Using a different reporter gene such as GFP (e.g. in Cox-2gfp/+ and Cox-2gfp/- mice) or cell-type specific conditional Cox-2 knockout mice [34] should make it possible to overcome this problem and identify the cells in which Cox-2 expression is regulated by this feedback mechanism in vivo.
Acknowledgments
We thank Art Catapang for technical assistance. These studies were supported by Grants NCI CA084572, NCI CA123055 and P50 CA086306 to HRH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68-69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang LH, Hajibeigi A, Xu XM, Loose-Mitchell D, Wu KK. Characterization of the promoter of human prostaglandin H synthase-1 gene. Biochem Biophys Res Commun. 1993;190:406–411. doi: 10.1006/bbrc.1993.1062. [DOI] [PubMed] [Google Scholar]
- 3.Herschman HR. Historical aspects of COX-2: Cloning and characterization of the cDNA, protein and gene. In: Harris RE, editor. COX-2 blockade in cancer prevention and therapy. Jumana Press; Clifton, NJ: 2003. pp. 13–32. [Google Scholar]
- 4.Herschman HR. Regulation and function of prostaglandin synthase 2/cyclooxygenase. In: Curtis-Prior P, editor. The eicosanoids. John Wiley & Sons, Ltd; 2004. pp. 43–52. [Google Scholar]
- 5.Kang YJ, Mbonye UR, DeLong CJ, Wada M, Smith WL. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res. 2007;46:108–125. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 7.Herschman HR, Talley JJ, Dubois R. Cyclooxygenase 2 (COX-2) as a target for therapy and noninvasive imaging. Mol Imaging and Biol. 2003;5:286–303. doi: 10.1016/j.mibio.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Duque J, Fresno M, Iñiguez MA. Expression and function of the nuclear factor of activated T cells in colon carcinoma cells: involvement in the regulation of cyclooxygenase-2. J Biol Chem. 2005;280:8686–8693. doi: 10.1074/jbc.M413076200. [DOI] [PubMed] [Google Scholar]
- 9.Joo M, Park GY, Wright JG, Blackwell TS, Atchison ML, Christman JW. Transcriptional regulation of the cyclooxygenase-2 gene in macrophages by PU.1. J Biol Chem. 2004;279:6658–6665. doi: 10.1074/jbc.M306267200. [DOI] [PubMed] [Google Scholar]
- 10.Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK, Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Espel E. The role of the AU-rich elements of mRNAs in controlling translation. Semin Cell Dev Biol. 2005;16:59–67. doi: 10.1016/j.semcdb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Dean JL, Sully G, Clark AR, Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–1121. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Faour WH, He Y, He QW, de Ladurantaye M, Quintero M, Mancini A, Di Battista JA. Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1 beta-treated human synovial fibroblasts. J Biol Chem. 2001;276:31720–31731. doi: 10.1074/jbc.M104036200. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B, Brune K, Pahl A. Cyclooxygenase-2 expression in lipopolysaccharide-stimulated human monocytes is modulated by cyclic AMP, prostaglandin E(2), and nonsteroidal anti-inflammatory drugs. Biochem Biophys Res Commun. 2000;278:790–796. doi: 10.1006/bbrc.2000.3885. [DOI] [PubMed] [Google Scholar]
- 15.Vichai V, Suyarnsesthakorn C, Pittayakhajonwut D, Sriklung K, Kirtikara K. Positive feedback regulation of COX-2 expression by prostaglandin metabolites. Inflamm Res. 2005;54:163–172. doi: 10.1007/s00011-004-1338-1. [DOI] [PubMed] [Google Scholar]
- 16.Tjandrawinata RR, Hughes-Fulford M. Up-regulation of cyclooxygenase-2 by product-prostaglandin E2. Adv Exp Med Biol. 1997;407:163–170. doi: 10.1007/978-1-4899-1813-0_25. [DOI] [PubMed] [Google Scholar]
- 17.Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem. 2000;275:28028–28032. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- 18.Tsubouchi Y, Kawahito Y, Kohno M, Inoue K, Hla T, Sano H. Feedback control of the arachidonate cascade in rheumatoid synoviocytes by 15-deoxy-Delta(12,14)-prostaglandin J2. Biochem Biophys Res Commun. 2001;283:750–755. doi: 10.1006/bbrc.2001.4847. [DOI] [PubMed] [Google Scholar]
- 19.Reese J, Zhao X, Ma WG, Brown N, Maziasz TJ, Dey SK. Comparative analysis of pharmacologic and/or genetic disruption of cyclooxygenase-1 and cyclooxygenase-2 function in female reproduction in mice. Endocrinology. 2001;142:3198–3206. doi: 10.1210/endo.142.7.8307. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Goorha S, Raghow R, Ballou LR. The tissue-specific, compensatory expression of cyclooxygenase-1 and -2 in transgenic mice. Prostaglandins Other Lipid Mediat. 2002;67:121–135. doi: 10.1016/s0090-6980(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa T, Jain NK, Taketo MM, Herschman HR. Imaging cyclooxygenase-2 (Cox-2) gene expression in living animals with a luciferase knock-in reporter gene. Mol Imaging Biol. 2006;8:171–187. doi: 10.1007/s11307-006-0034-7. [DOI] [PubMed] [Google Scholar]
- 22.Jain NK, Ishikawa T, Spigelman I, Herschman HR. COX-2 expression and function in the hyperalgesic response to paw inflammation in mice. Prostaglandins Leukot Essent Fatty Acids. 2008 doi: 10.1016/j.plefa.2008.08.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 24.Rouzer CA, Tranguch S, Wang H, Zhang H, Dey SK, Marnett LJ. Zymosan-induced glycerylprostaglandin and prostaglandin synthesis in resident peritoneal macrophages: roles of cyclooxygenase-1 and -2. Biochem J. 2006;399:91–99. doi: 10.1042/BJ20060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cok SJ, Morrison AR. The 3′-untranslated region of murine cyclooxygenase-2 contains multiple regulatory elements that alter message stability and translational efficiency. J Biol Chem. 2001;276:23179–23185. doi: 10.1074/jbc.M008461200. [DOI] [PubMed] [Google Scholar]
- 26.Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J Biol Chem. 2000;275:11750–11757. doi: 10.1074/jbc.275.16.11750. [DOI] [PubMed] [Google Scholar]
- 27.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 28.Ikegami R, Sugimoto Y, Segi E, Katsuyama M, Karahashi H, Amano F, Maruyama T, Yamane H, Tsuchiya S, Ichikawa A. The expression of prostaglandin E receptors EP2 and EP4 and their different regulation by lipopolysaccharide in C3H/HeN peritoneal macrophages. J Immunol. 2001;166:4689–4696. doi: 10.4049/jimmunol.166.7.4689. [DOI] [PubMed] [Google Scholar]
- 29.Fournier T, Fadok V, Henson PM. Tumor necrosis factor-alpha inversely regulates prostaglandin D2 and prostaglandin E2 production in murine macrophages. Synergistic action of cyclic AMP on cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Biol Chem. 1997;272:31065–31072. doi: 10.1074/jbc.272.49.31065. [DOI] [PubMed] [Google Scholar]
- 30.Fournier T, Riches DW, Winston BW, Rose DM, Young SK, Noble PW, Lake FR, Henson PM. Divergence in macrophage insulin-like growth factor-I (IGF-I) synthesis induced by TNF-alpha and prostaglandin E2. J Immunol. 1995;155:2123–2133. [PubMed] [Google Scholar]
- 31.Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-delta and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem. 2006;281:33982–33996. doi: 10.1074/jbc.M600135200. [DOI] [PubMed] [Google Scholar]
- 32.Faour W, Gomi K, Kennedy C. PGE(2) induces COX-2 expression in podocytes via the EP(4) receptor through a PKA-independent mechanism. Cell Signal. 2008;20:2156–2164. doi: 10.1016/j.cellsig.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Akarasereenont P, Techatrisak K, Chotewuttakorn S, Thaworn A. The induction of cyclooxygenase-2 in IL-1beta-treated endothelial cells is inhibited by prostaglandin E2 through cAMP. Mediators Inflamm. 1999;8:287–294. doi: 10.1080/09629359990298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa T, Herschman HR. Conditional knockout mouse for tissue-specific disruption of the cyclooxygenase-2 (Cox-2) gene. Genesis. 2006;44:143–149. doi: 10.1002/gene.20192. [DOI] [PubMed] [Google Scholar]