Abstract
Introduction
Diet and exercise affect endothelial function in the penis, but the molecular mechanisms underlying their effects are not understood.
Aims
We evaluated endothelial nitric oxide synthase (eNOS) interaction with its negative regulator caveolin-1 and eNOS uncoupling as molecular targets in the penis associated with the beneficial effects of low-fat diet and chronic exercise.
Methods
The penes were obtained from adult male Yucatan pigs fed a normal-fat or high-fat diet on exercised or sedentary regimen for 24 weeks. Markers of endothelial function (guanosine 3′,5′-monophosphate [cGMP] production), endothelial dysfunction (eNOS uncoupling and eNOS interaction with caveolin-1), and oxidative stress (thiobarbituric acid reactive substances [TBARS]) were measured in the penes. The concentrations of cGMP and TBARS were determined using commercial kits. eNOS uncoupling was determined by low-temperature sodium dodecyl sulfate polyacrylamide gel electrophoresis. eNOS binding to caveolin-1, eNOS phosphorylation (Ser-1177), and protein expression of eNOS and caveolin-1 were measured by Western blot analysis in penes purified for NOS and in homogenates, respectively.
Main Outcome Measures
Molecular parameters of endothelial function including eNOS regulatory function.
Results
Relative to normal-fat diet, high-fat diet significantly (P < 0.05) reduced cGMP levels and significantly (P < 0.05) increased eNOS uncoupling, eNOS binding to caveolin-1, and TBARS production in the penis of sedentary pigs. Exercise of pigs on high-fat diet reversed (P < 0.05) the abnormalities in cGMP levels, eNOS uncoupling, and eNOS binding to caveolin-1, but not TBARS levels. Exercise of pigs on normal-fat diet did not affect any of these parameters. Protein expressions of caveolin-1, phosphorylated (Ser-1177), and total eNOS were unaffected by diet or exercise.
Conclusion
Low-fat diet and chronic exercise preserve endothelial function in the pig penis by sustaining active eNOS in its dimeric form and by limiting eNOS interaction with its negative regulator caveolin-1.
Keywords: eNOS Uncoupling, Caveolin-1, Oxidative Stress, cGMP, Cholesterol
Introduction
High-fat diet and hypercholesterolemia are associated with endothelial dysfunction in the penis and erectile dysfunction (ED [1,2]). Decreased nitric oxide (NO) signaling and increased oxidative stress have been postulated to be major molecular factors contributing to hypercholesterolemia-induced ED [1,2]. Human and animal studies have shown that ED associated with hypercholesterolemia is accompanied by decreased NO synthase (NOS) activity and NO availability [1-8] and decreased function of the endothelial NO/guanosine 3′,5′-monophosphate (cGMP) signal transduction pathway [9]. Corpus cavernosal tissue of cholesterol-fed rabbits exhibits increased production of superoxide anion [4,9], possibly mediated by nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase [9]. The exact molecular mechanisms for endothelial dysfunction and endothelial NOS (eNOS) defects in a hypercholesterolemic penis are, however, unclear and require further investigation. Studies in isolated endothelial cells indicate that the oxidized form of low-density lipoprotein (LDL) cholesterol (ox-LDL) plays a pivotal role in endothelial dysfunction associated with initiation and progression of atherosclerosis [10,11]. ox-LDL affects eNOS trafficking and eNOS–protein interactions, reducing eNOS activity [10]. ox-LDL itself increases superoxide anion generation via the induction of NAD(P)H oxidase, xanthine oxidase, mitochondrial electron transport chain, and uncoupled eNOS [11].
Hypercholesterolemia and exercise have opposing effects on endothelial function. Beneficial effects of exercise are mostly because of its direct effect on the vasculature through an increase in shear stress and the maintenance or restoration of NO bioavailability [12,13] and decrease in oxidative stress [14-16]. In animal models of vascular injury [17-20] and in patients with coronary artery disease [21,22], exercise-induced improvement of endothelial function in the arterial circulation has been attributed to increases in eNOS protein expression and its phosphorylation at Ser-1177. Conversely, physical inactivity impairs endothelial function in the aorta of healthy [23] and hypercholesterolemic [17] mice through the reduction in eNOS gene expression, increased vascular production of reactive oxygen species (ROS), and increased NAD(P)H oxidase activity.
Several epidemiologic studies have shown that physical activity is inversely associated with ED [24,25]. Lifestyle changes, such as reduction in calorie intake and increase in physical activity, lead to improved sexual function in obese men with ED [26]. In patients with chronic heart failure and ED, short-term exercise improves brachial artery endothelial function in correlation with improvement in sexual activity [27]. Very few studies have evaluated the mechanism for the effect of exercise training on the penis. Eight weeks of run training of healthy rats increased the relaxation response of the corpus cavernosum by endothelium-independent mechanisms, that is not accompanied by changes in contractile sensitivity [28]. In rats made hypertensive by chronic NO blockade, the same training regimen restored the reduced relaxing response of corpus cavernosum by NO-dependent mechanisms [29]. However, the precise molecular mechanisms underlying the effects of exercise on endothelial function in the penis in general, and specifically the hypercholesterolemic penis, are largely unknown.
Our aim in the present study was to investigate the molecular basis for the impairment of endothelial function, specifically the role of eNOS uncoupling and eNOS interaction with caveolin-1 in the penis under high-fat dietary conditions. Furthermore, we sought to evaluate the molecular mechanisms underlying the effects of chronic exercise training on endothelial function in the penis under conditions of both low-fat and high-fat diet. For this investigation, we used a well-established porcine model of early stage atherosclerosis [14,20,30], that models early human atherosclerosis [30]. A well-controlled exercise regimen in this animal model has provided a rigorous experimental approach to assess chronic exercise effects in the progression of atherosclerotic vascular disease [14,31,32].
Materials and Methods
Animal Model
Fresh frozen whole pig penes were obtained from the University of Missouri. The study was approved and conducted in conformance with the University of Missouri Animal Care and Use Committee's guidelines on animal research. The experimental animals were adult (8–12 months of age) male Yucatan miniature swine weighing 25–40 kg. The pigs were provided a normal-fat diet (Purina Lab Mini-pig Chow; PMI LabDiet, St. Louis, MO, USA) or high-fat diet in which 8% and 46% of daily caloric intake was derived from fat, respectively [30]. Four weeks after the diet was initiated, the pigs were exercise trained on a motorized treadmill 5 days/week once a day or remained sedentary for 16 weeks. Exercise progressed in intensity and duration until the 12th week from 15 to 60 minutes. During this time period, pigs continued to consume the high-fat or normal-fat diet. Previous studies using this animal model have established that high-fat diet significantly elevated plasma levels of cholesterol, triglycerides, and LDL cholesterol [30]. As in previous studies with this model, exercise training efficacy was demonstrated by increased skeletal muscle oxidative capacity, improved treadmill performance tests, and increased heart weight/body weight ratios [14,20,32,33].
cGMP Assay
Pig penis was homogenized in ice-cold 5% trichloroacetic acid (TCA) and centrifuged at 10,000 × g for 10 minutes at 4°C. The TCA was extracted with water-saturated diethyl ether. The concentration of cGMP was determined with a commercially available competitive enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA). The results were expressed as pmol cGMP/mg proteins.
Thiobarbituric Acid Reactive Substances (TBARS) Production
Lipid peroxidation was evaluated as TBARS. The pig penis was pulverized and resuspended at a concentration of 100 mg/mL in phosphate buffered solution. TBARS production was measured according to the manufacturer's instructions using a TBARS assay kit (ZeptoMetrix Corp., Buffalo, NY, USA). Samples were analyzed spectrofluorometrically in a microplate reader (BMG Labtech, Durham, NC, USA) and values were expressed as nmol TBARS/g wet tissue.
Western Blot Analysis
Minced penile tissue was homogenized and partially purified for NOS as described [34]. Purified NOS samples or penile homogenates were resolved on 4–20% Tris gels and transferred to polyvinylidene difluoride membrane. Membranes with purified NOS were probed with polyclonal rabbit anti-phospho (P)-eNOS (Ser-1177) antibody (Cell Signaling Technology, Beverly, MA) at 1:450 dilution (for P-eNOS analysis), rabbit anti-caveolin-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:6,000 dilution (for caveolin-1 bound to eNOS analysis), or rabbit anti-eNOS antibody (BD Transduction Laboratories, San Diego, CA, USA) at 1:1,000 dilution (for eNOS uncoupling analysis). Membranes used for P-eNOS (Ser-1177) analysis were then stripped and probed with monoclonal anti-eNOS antibody (BD Transduction Laboratories) at 1:1,000 dilution. For the immunoblot analysis of the dimeric and monomeric forms of eNOS, purified samples in Laemmli buffer were not heated, and the temperature of the gel was maintained below 15°C during electrophoresis (low-temperature sodium dodecyl sulfate polyacrylamide gel electrophoresis) [35]. P-eNOS and caveolin-1 densities were normalized relative to those of eNOS in purified samples. eNOS uncoupling was expressed as the ratio of active eNOS dimers to inactive eNOS monomers. For the analysis of total caveolin-1 and total eNOS expression by Western immunoblot, a separate set of penile homogenates (10–50 μg) was used without purification and standardized to β-actin. The ratio was determined in terms of arbitrary units and expressed relative to the ratio for normal-fat fed sedentary animals.
Immunohistochemistry of eNOS and Caveolin-1
Immunohistochemistry was performed on paraffin-embedded penile samples using eNOS and caveolin-1 antibodies at 1:800 dilutions, as described previously [32]. Primary antibodies were incubated with tissue sections overnight at 4°C. The sections were examined and photographed with the Olympus BX40 photomicroscope (Olympus, Center Valley, PA, USA).
Plasma Cholesterol and Lipoprotein Analyses
LDL subfractions were isolated by density gradient ultracentrifugation and analyzed for cholesterol enzymatically using the Sigma Diagnostic kit (Infinity, Procedure #402, St. Louis, MO, USA) and quantitated on a spectrophotometer Beckman DU-530 (Beckman, Fullerton, CA, USA) at 500 nm, as described [36].
Statistical Evaluation
Statistical analysis was performed by using oneway analysis of variance, followed by Newman–Keuls multiple comparison test or by t-test when appropriate. The data were expressed as the mean ± standard error of the mean (SEM). A value of P < 0.05 was considered to be statistically significant.
Results
Blood Lipid Concentrations
In both sedentary and exercised animals included in this study, the high-fat diet increased total plasma cholesterol, total LDL, very low-density lipoprotein (VLDL), and intermediate density lipoprotein-C (IDL-C) and IDL-B (P < 0.05) (Table 1).
Table 1.
Plasma cholesterol and lipoprotein levels in normal-fat fed sedentary (NFSe), normal-fat fed exercised (NFEx), high-fat fed sedentary (HFSe), and high-fat fed exercised (HFEx) pigs
| Total cholesterol (mg/dL) | Total LDL (mg/dL) | VLDL (mg/dL) | IDL-C (mg/dL) | IDL-B (mg/dL) | |
|---|---|---|---|---|---|
| NFSe | 61.2 ± 3.9 | 31.6 ± 2.5 | 17.6 ± 1.4 | 3.2 ± 0.2 | 5.0 ± 1.7 |
| NFEx | 59.9 ± 3.6 | 26.0 ± 1.1 | 11.8 ± 3.2 | 5.2 ± 0.6 | 7.2 ± 0.7 |
| HFSe | 409.7 ± 101* | 154.4 ± 23.5* | 72.8 ± 15.6* | 64.0 ± 7.9* | 78.4 ± 15.2* |
| HFEx | 559.4 ± 95.7* | 108.8 ± 18.8* | 64.0 ± 26.5* | 52.8 ± 9.7* | 50.4 ± 9.0* |
P < 0.05 vs. NFSe and NFEx.
LDL = low-density lipoprotein; VLDL = very low-density lipoprotein; IDL-C = intermediate density lipoprotein-C; IDL-B = intermediate density lipoprotein-B.
Effect of Diet and Exercise on cGMP Levels
cGMP levels, as an indicator of endothelial function [37], were significantly (P < 0.05) reduced in the penes of high-fat fed sedentary pigs relative to levels found in normal-fat fed sedentary pig penes (Figure 1). Exercise training of high-fat fed pigs significantly (P < 0.05) increased cGMP levels in the penis to the levels similar to those of pigs on normal-fat diet. Exercise of pigs on normal-fat diet did not affect cGMP levels in the penis.
Figure 1.
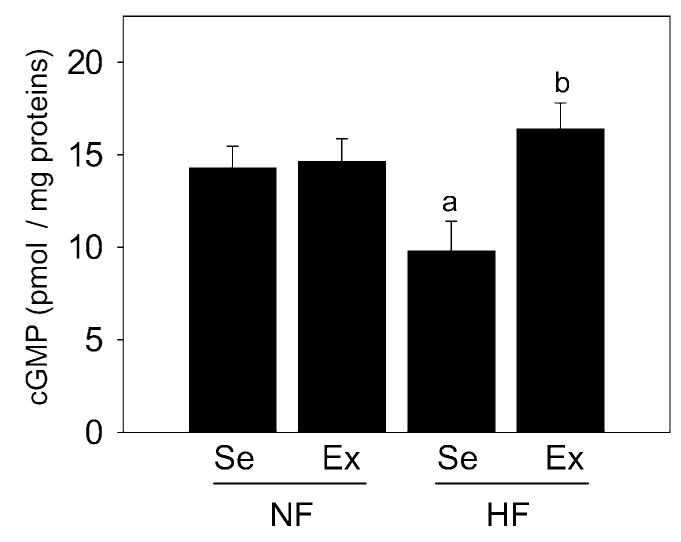
Effect of diet and exercise on guanosine 3′,5′-monophosphate (cGMP) levels in the penis. HFSe pig penes exhibited reduced cGMP levels compared to NFSe pig penes, which was prevented by exercise. Each bar represents the standard error of the mean (SEM). a = P < 0.05 vs. NFSe; b = P < 0.05 vs. HFSe; N = 7; NF = normal-fat diet; HF = high-fat diet; Se = sedentary; Ex = exercised.
Localization of eNOS and Caveolin-1
We localized eNOS and caveolin-1 immunohistochemically in serial sections of pig penis of the four experimental groups (Figure 2). eNOS and caveolin-1 colocalized in the vascular endothelium.
Figure 2.
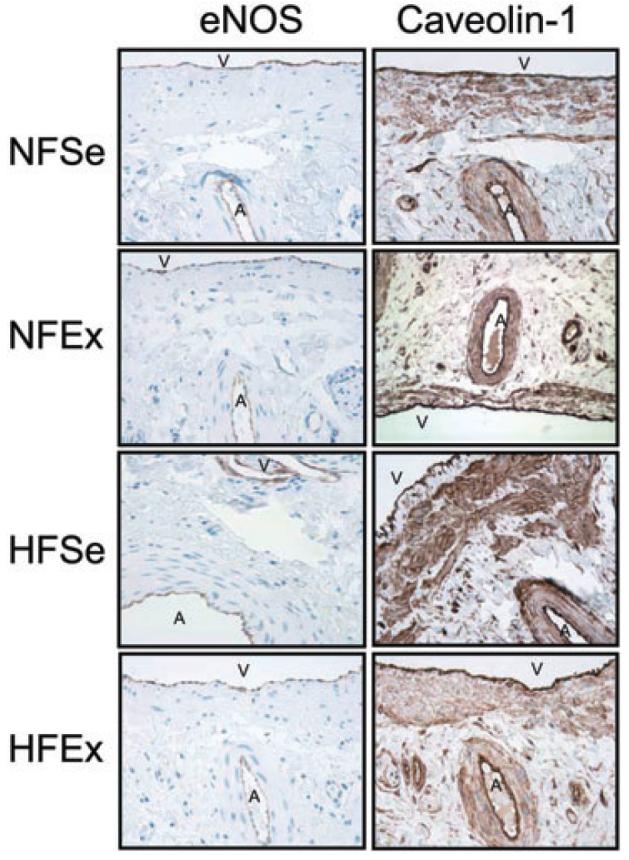
Representative immunohistochemical localization of endothelial nitric oxide synthase (eNOS) and caveolin-1 in serial sections of NFSe, NFEx, HFSe, and HFEx pig penes show staining in the vascular endothelium. Magnification 400×. NF = normal-fat diet; HF = high-fat diet; Se = sedentary; Ex = exercised; V = Vein; A = artery.
Effect of Diet and Exercise on eNOS Uncoupling
eNOS uncoupling, which refers to eNOS transition from its dimeric to monomeric form that is a source of ROS [35], can be represented inversely by a calculated ratio of eNOS dimers/monomers. This ratio was significantly (P < 0.05) decreased in the penes of high-fat fed sedentary pigs relative to levels found in normal-fat fed sedentary pig penes (Figure 3). Exercise training of high-fat fed pigs significantly (P < 0.05) increased the dimer/monomer ratio in the penes to the levels found in pigs on normal-fat diet. Exercise of pigs on normal-fat diet did not affect this ratio.
Figure 3.
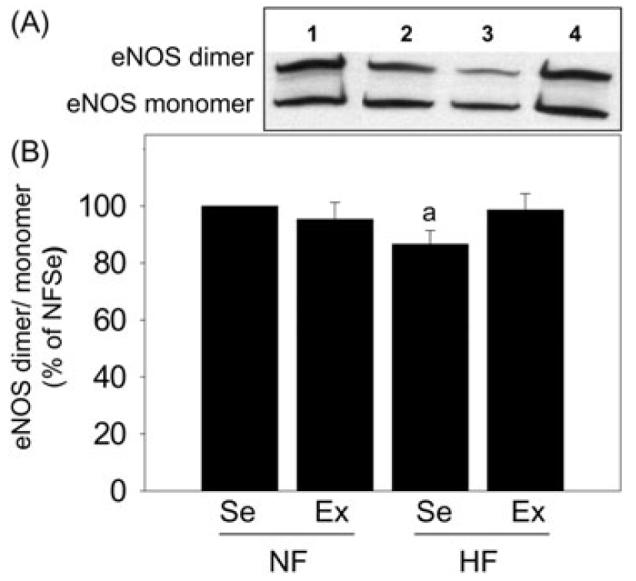
Effect of diet and exercise on endothelial nitric oxide synthase (eNOS) dimers/monomers in the penis. (A) Representative Western immunoblot of eNOS dimers and eNOS monomers in the penes of NFSe (line 1), NFEx (line 2), HFSe (line 3), and HFEx (line 4) pigs. (B) Quantitative analysis of eNOS dimers/monomers in the penes of NFSe, NFEx, HFSe, and HFEx pigs. The ratio of eNOS dimers to monomers was reduced in HFSe pig penes compared to NFSe pig penes, and this was prevented by exercise. Each bar represents the standard error of the mean (SEM). a = P < 0.05 vs. NFSe; N = 8; NF = normal-fat diet; HF = high-fat diet; Se = sedentary; Ex = exercised.
Effect of Diet and Exercise on eNOS Binding to Caveolin-1
Binding of eNOS and caveolin-1, a negative regulator of eNOS function [38], was evaluated in penile samples partially purified for NOSs, thus allowing the detection of eNOS and proteins bound to eNOS using specific antibodies. The ratio of caveolin-1/eNOS was significantly (P < 0.05) increased in the penes of high-fat fed sedentary pigs compared to that of pigs on normal-fat diet (Figure 4). Exercise significantly (P < 0.05) decreased the ratio of caveolin-1/eNOS in the penes of pigs on high-fat diet to levels comparable to that found in pigs on normal-fat diet. Although exercise led to a decrease in caveolin-1/eNOS levels in the penes of pigs on normal-fat diet, the extent was not statistically significant (P > 0.05).
Figure 4.
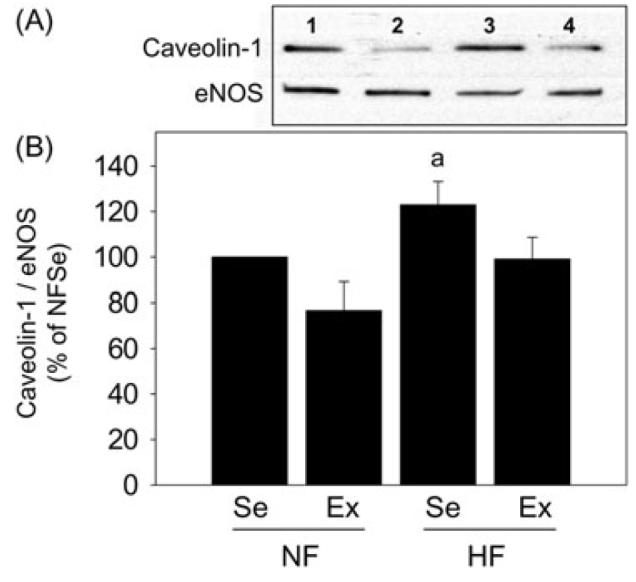
Effect of diet and exercise on caveolin-1 binding to endothelial nitric oxide synthase (eNOS) in the penis. (A) Representative Western immunoblot of caveolin-1 and eNOS in samples purified for NOSs in the penes of NFSe (line 1), NFEx (line 2), HFSe (line 3), and HFEx (line 4) pigs. (B) Quantitative analysis of caveolin-1 binding to eNOS in the penes of NFSe, NFEx, HFSe, and HFEx pigs. Binding of eNOS to caveolin-1 was increased in HFSe pig penes compared to NFSe pig penes, and this was prevented by exercise. Each bar represents the standard error of the mean (SEM). a = P < 0.05 vs. NFSe; N = 5; NF = normal-fat diet; HF = high-fat diet; Se = sedentary; Ex = exercised.
Effect of Diet and Exercise on TBARS Production
TBARS assay serves as an indicator of oxidative stress activity in tissues. The levels of TBARS were significantly (P < 0.05) elevated in the penes of high-fat fed sedentary pigs relative to levels in normal-fat fed sedentary pig penes. These levels remained elevated in the penes of high-fat exercised pigs (Figure 5). TBARS production was unaffected by exercise in the penes of pigs on normal-fat diet.
Figure 5.
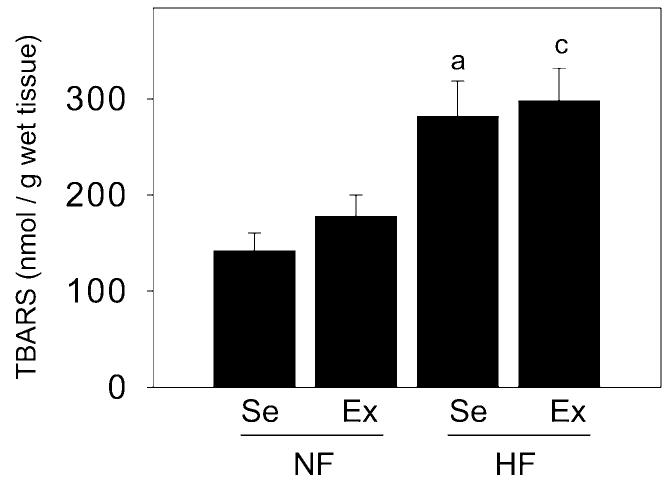
Effect of diet and exercise on thiobarbituric acid reactive substances (TBARS) production in the penis. The levels of TBARS were increased in HFSe pig penes compared to NFSe pig penes, and these were unaffected by exercise. Each bar represents the standard error of the mean (SEM). a = P < 0.05 vs. NFSe; c = P < 0.05 vs. NFEx; N = 6–13; NF = normal-fat diet; HF = high-fat diet; Se = sedentary; Ex = exercised.
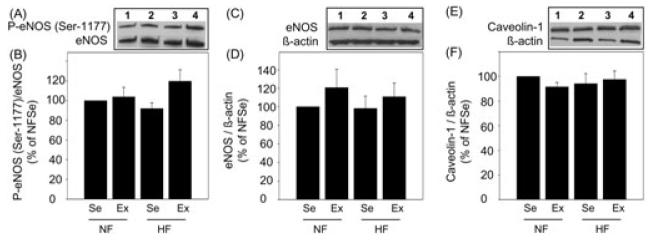
Effect of Diet and Exercise on Protein Expression of P-eNOS (Ser-1177), eNOS, and Caveolin-1
Protein expressions of P-eNOS (Ser-1177), eNOS, and caveolin-1 were not significantly affected by diet or exercise in the penes of any experimental group (Figure 6).
Figure 6.
Effect of diet and exercise on protein expression of phospho endothelial nitric oxide synthase (P-eNOS) (Ser-1177), eNOS, and caveolin-1 in the penis. Representative Western immunoblots of P-eNOS (Ser-1177) and eNOS (A), total eNOS and β-actin (C), and caveolin-1 and β-actin (E) in the penes of NFSe (line 1), NFEx (line 2), HFSe (line 3), and HFEx (line 4) pigs. Quantitative analysis of P-eNOS (Ser-1177) (B), eNOS (D), and caveolin-1 (F) in the penes of NFSe, NFEx, HFSe, and HFEx pigs. No statistically significant differences were found among treatment groups. Each bar represents the standard error of the mean (SEM). N = 5–11; NF = normal-fat diet; HF = high-fat diet; Se = sedentary; Ex = exercised.
Discussion
The present study demonstrated that high-fat diet and associated hypercholesterolemia impair endothelial function in the pig penis by increasing eNOS interaction with its negative protein regulator caveolin-1, inducing eNOS uncoupling and increasing oxidative stress. Chronic exercise training preserved endothelial function in the hypercholesterolemic penis by decreasing the eNOS–caveolin-1 interaction and by preventing eNOS uncoupling. Such training did not influence the results of normal-fat dietary conditions. The localization of eNOS and caveolin-1 in the vascular endothelium further confirms that those changes occur at the level of endothelium.
The decrease in the abundance of the active dimeric form of eNOS (eNOS uncoupling) in the hypercholesterolemic pig penis is associated with endothelial dysfunction (downregulation of cGMP) and increased oxidative stress (upregulation of TBARS production). eNOS uncoupling refers to both the impaired NO-producing activity of the enzyme as well as its increased capacity to produce superoxide anion. This molecular reaction has been attributed to the oxidation of the zinc thiolate cluster of eNOS and the cofactor tetrahydrobiopterin, and the decreased availability of the NOS substrate L-arginine, destabilizing the eNOS dimer, which results in the increased production of ROS and reduced NO production by eNOS [39]. Elevated oxidative stress may also cause damage to the DNA, proteins and lipids, promote the release of vasoconstrictors, increase apoptosis, and cause tissue injury. The beneficial effects of supplementation with L-arginine and tetrahydrobiopterin on endothelium-dependent relaxation have been documented in the aorta of hypercholesterolemic mice [40-42] and in the brachial artery of patients with familial hypercholesterolemia [43,44]. Our data showing that eNOS uncoupling contributes to the endothelial dysfunction in the penes of high-fat fed pigs elucidate the molecular mechanisms of vascular ROS production in the penis and hypercholesterolemia-induced impairment of eNOS function in the penis. The findings also suggest a new molecular target for pharmacologic therapies for vasculogenic ED.
eNOS uncoupling results in the decreased functional activity, but not necessarily catalytic activity or protein expression, of eNOS [45,46]. In the human umbilical vein endothelial cells and bovine aortic endothelial cells, LDL and peroxynitrite actually increase eNOS (Ser-1177) phosphorylation [46,47]. Accelerated electron flux from the reductase to the oxygenase domain of the enzyme increases the activation status of eNOS, although this results in increased production of superoxide anion instead of NO by the uncoupled enzyme [46]. Our data showing that protein levels of P-eNOS (Ser-1177) and total eNOS in the penis did not change with hypercholesterolemia are in accordance with this concept. We contrast our findings with those of Ryu et al. [6,7] and Xie et al. [8] who demonstrated decreased eNOS phosphorylation at Ser-1177 in the penes of cholesterol fed rats and rabbits. This disparity may pertain to different stages of atherogenesis progression and use of different animal models.
Growing evidence indicates that hypercholesterolemia may impair the caveolae structure, eNOS trafficking, and eNOS-protein interactions, and further potentiate superoxide anion generation [11]. In bovine aortic endothelial cells [10], hypercholesterolemic serum increases caveolin-1 expression and its association with eNOS, without affecting eNOS protein expression. Binding of eNOS to caveolin-1 inhibits the enzyme's activity by occupying its calmodulin-binding site and by interfering with other caveolin-associated receptors [38]. The protein expression of caveolin-1 was reportedly increased in the penes [48] and coronary arteries [14] of hypercholesterolemic rats and pigs, respectively, or not altered in the brachial arteries [33] of hypercholesterolemic pigs. Little information is, however, available regarding eNOS interaction with caveolin-1 in the vasculature in general, and no studies have addressed it in the penis. Our present study shows that, although the protein expression of caveolin-1 did not change, the levels of caveolin-1 bound to eNOS were increased in the hypercholesterolemic pig penis. Increased interaction between eNOS and caveolin-1 apparently prevents eNOS activation and contributes to endothelial dysfunction in the penis. These findings provide a mechanism whereby eNOS–protein interaction can affect eNOS function in the penis in the setting of hypercholesterolemia.
Previous studies using this porcine model of early stage atherosclerotic vascular disease have shown that chronic exercise training preserves endothelial function in the coronary and brachial arteries [14,32,33]. We used this animal model to evaluate specific molecular mechanisms of endothelial function in the penis, which are regulated by exercise. We found that chronic exercise training of pigs on high-fat diet improved specific indices of endothelial function in the penis; it decreased eNOS uncoupling and decreased eNOS interaction with caveolin-1. This exercise regimen did not affect P-eNOS (Ser-1177), eNOS, or caveolin-1 protein expressions, and it did not reduce elevated lipid peroxidation in the penis of high-fat fed pigs. Thus, the beneficial effects of exercise on eNOS function in this animal model are apparently not mediated by increased eNOS protein content or eNOS phosphorylation. While the mechanisms of exercise effect on eNOS activity have been mostly attributed to its genomic effects on eNOS and antioxidants, and on eNOS phosphorylation [17-22], other nongenomic effects affecting eNOS activity may also be involved, such as decreased mechanisms of vasoconstriction [49]. The exact mechanism by which exercise affects eNOS function is not clear and awaits further investigation. In addition to its effect on eNOS and endothelial NO, exercise also produces NO-independent and endothelium-independent effects. The former include enhanced production of prostacyclin and endothelium-derived hyperpolarizing factor [33], and the latter include, among others, vascular remodeling, such as an increase in the number and the diameter of blood vessels, growth factors, hormones, and cytokine production [50].
The improvement in eNOS function apparently preserves NO bioavailability (cGMP production) in the penis. The fact that exercise did not improve lipid peroxidation, although it did prevent eNOS uncoupling in the penis of high-fat fed pigs, suggests that other sources of ROS in the penis may not be downregulated by exercise in this animal model. Exercise training did not affect endothelial function in the “healthy” penis, which is consistent with human and animal studies showing that impaired endothelial function is more predisposed to improvement by exercise training than is intact endothelial function [22,49].
The limitation of our study is the lack of erectile response measurements in correlation with the indices of endothelial function. Physiologic data would conceivably complement emerging evidence that proper eNOS/cGMP signaling, including eNOS “coupling” and its optimal interaction with caveolin-1, are critically involved in penile erection. The pig model may be advantageous for such additional experiments to the extent that it permits inferences about human physiology, given the close resemblance of the pig cardiovascular system and that of humans. Smaller animal models such as rodents and rabbits have been used regularly for erection studies, possibly because of economic reasons. Understandably, no animal model precisely mimics the human condition.
Conclusion
Normal-fat diet maintains endothelial function in the pig penis by preserving eNOS in its active, dimeric form, by limiting eNOS interaction with its main negative protein regulator caveolin-1, and by preventing oxidative stress. While chronic exercise does not enhance endothelial function in the penes of pigs on normal-fat diet, it improves endothelial function in the penes of pigs on high-fat diet through specific molecular mechanisms, which include decreased eNOS uncoupling and decreased eNOS interaction with caveolin-1 (Figure 7).
Figure 7.
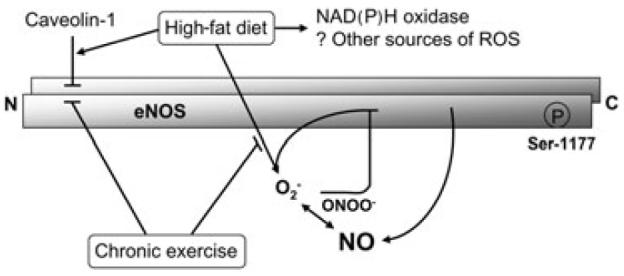
A model of high-fat diet and chronic exercise effect on endothelial nitric oxide synthase (eNOS) function in the penis. Hypercholesterolemia associated with high-fat diet impairs endothelial function in the penis by increasing eNOS interaction with its negative protein regulator caveolin-1, inducing eNOS uncoupling, and increasing oxidative stress from uncoupled eNOS, nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase, and possibly other sources of reactive oxygen species (ROS). Chronic exercise training preserves endothelial function in the hypercholesterolemic penis by decreasing eNOS–caveolin-1 interaction and by preventing eNOS uncoupling. → = stimulatory effect; ⊣ = inhibitory effect.
Footnotes
Conflict of Interest: None declared.
References
- 1.Seo KK, Yun HY, Kim H, Kim SC. Involvement of endothelial nitric oxide synthase in the impaired endothelium-dependent relaxation of cavernous smooth muscle in hypercholesterolemic rabbit. J Androl. 1999;20:298–306. [PubMed] [Google Scholar]
- 2.Azadzoi KM, Saenz de Tejada I. Hypercholesterolemia impairs endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J Urol. 1991;146:238–40. doi: 10.1016/s0022-5347(17)37759-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Klyachkin ML, Svendsen E, Davies MG, Hagen PO, Carson CC., 3rd Experimental hypercholesterolemia in rabbits induces cavernosal atherosclerosis with endothelial and smooth muscle cell dysfunction. J Urol. 1994;151:198–205. doi: 10.1016/s0022-5347(17)34916-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim SC, Kim IK, Seo KK, Baek KJ, Lee MY. Involvement of superoxide radical in the impaired endothelium-dependent relaxation of cavernous smooth muscle in hypercholesterolemic rabbits. Urol Res. 1997;25:341–46. doi: 10.1007/BF01294663. [DOI] [PubMed] [Google Scholar]
- 5.Behr-Roussel D, Darblade B, Oudot A, Compagnie S, Bernabe J, Alexandre L, Giuliano F. Erectile dys-function in hypercholesterolemic atherosclerotic apolipoprotein E knockout mice. J Sex Med. 2006;3:596–603. doi: 10.1111/j.1743-6109.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 6.Ryu JK, Cho CH, Shin HY, Song SU, Oh SM, Lee M, Piao S, Han JY, Kim IH, Koh GY, Suh JK. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol Ther. 2006;13:705–15. doi: 10.1016/j.ymthe.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Ryu JK, Shin HY, Song SU, Oh SM, Piao S, Han JY, Park KW, Suh JK. Downregulation of angiogenic factors and their downstream target molecules affects the deterioration of erectile function in a rat model of hypercholesterolemia. Urology. 2006;67:1329–34. doi: 10.1016/j.urology.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Xie D, Kontos CD, Donatucci CF, Annex BH. Cholesterol feeding reduces vascular endothelial growth factor signaling in rabbit corporal tissues. J Sex Med. 2005;2:634–40. doi: 10.1111/j.1743-6109.2005.00111.x. [DOI] [PubMed] [Google Scholar]
- 9.Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol. 2005;517:224–31. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest. 1999;103:897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 12.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–53. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 13.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–60. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 14.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol. 2004;96:1114–26. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- 15.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–9. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–87. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 17.Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M, Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–14. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 18.Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103:2839–44. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- 19.Indolfi C, Torella D, Coppola C, Curcio A, Rodriguez F, Bilancio A, Leccia A, Arcucci O, Falco M, Leosco D, Chiariello M. Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ Res. 2002;91:1190–7. doi: 10.1161/01.res.0000046233.94299.d6. [DOI] [PubMed] [Google Scholar]
- 20.Henderson KK, Turk JR, Rush JW, Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: Effect of exercise. J Appl Physiol. 2004;97:1159–68. doi: 10.1152/japplphysiol.00261.2004. [DOI] [PubMed] [Google Scholar]
- 21.Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–8. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 22.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol. 2004;44:1320–7. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: Can lifestyle changes modify risk? Urology. 2000;56:302–6. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]
- 25.Nicolosi A, Glasser DB, Moreira ED, Villa M. Prevalence of erectile dysfunction and associated factors among men without concomitant diseases: A population study. Int J Impot Res. 2003;15:253–7. doi: 10.1038/sj.ijir.3901010. [DOI] [PubMed] [Google Scholar]
- 26.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, D'Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: A randomized controlled trial. JAMA. 2004;291:2978–84. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 27.Belardinelli R, Lacalaprice F, Faccenda E, Purcaro A, Perna G. Effects of short-term moderate exercise training on sexual function in male patients with chronic stable heart failure. Int J Cardiol. 2005;101:83–90. doi: 10.1016/j.ijcard.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Claudino MA, Priviero FB, Teixeira CE, de Nucci G, Antunes E, Zanesco A. Improvement in relaxation response in corpus cavernosum from trained rats. Urology. 2004;63:1004–8. doi: 10.1016/j.urology.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Claudino MA, Priviero FB, Camargo EA, Teixeira CE, De Nucci G, Antunes E, Zanesco A. Protective effect of prior physical conditioning on relaxing response of corpus cavernosum from rats made hypertensive by nitric oxide inhibition. Int J Impot Res. 2007;19:189–95. doi: 10.1038/sj.ijir.3901511. [DOI] [PubMed] [Google Scholar]
- 30.Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. Int J Exp Pathol. 2005;86:335–45. doi: 10.1111/j.0959-9673.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turk JR, Laughlin MH. Physical activity and atherosclerosis: Which animal model? Can J Appl Physiol. 2004;29:657–83. doi: 10.1139/h04-042. [DOI] [PubMed] [Google Scholar]
- 32.Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. J Appl Physiol. 2003;94:2017–26. doi: 10.1152/japplphysiol.01025.2002. [DOI] [PubMed] [Google Scholar]
- 33.Woodman CR, Thompson MA, Turk JR, Laughlin MH. Endurance exercise training improves endothelium-dependent relaxation in brachial arteries from hypercholesterolemic male pigs. J Appl Physiol. 2005;99:1412–21. doi: 10.1152/japplphysiol.00293.2005. [DOI] [PubMed] [Google Scholar]
- 34.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2002;99:4061–6. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F1144–52. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 36.Thomas TR, Pellechia J, Rector RS, Sun GY, Sturek MS, Laughlin MH. Exercise training does not reduce hyperlipidemia in pigs fed a high-fat diet. Metabolism. 2002;51:1587–95. doi: 10.1053/meta.2002.36313. [DOI] [PubMed] [Google Scholar]
- 37.Burnett AL. Role of nitric oxide in the physiology of erection. Biol Reprod. 1995;52:485–9. doi: 10.1095/biolreprod52.3.485. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 39.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–26. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aji W, Ravalli S, Szabolcs M, Jiang XC, Sciacca RR, Michler RE, Cannon PJ. L-arginine prevents xanthoma development and inhibits atherosclerosis in LDL receptor knockout mice. Circulation. 1997;95:430–7. doi: 10.1161/01.cir.95.2.430. [DOI] [PubMed] [Google Scholar]
- 41.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTPcyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–50. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 42.Hattori Y, Hattori S, Wang X, Satoh H, Nakanishi N, Kasai K. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:865–70. doi: 10.1161/01.ATV.0000258946.55438.0e. [DOI] [PubMed] [Google Scholar]
- 43.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–6. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, RabelInk TJ. 5-Methyltetrahydrofo-late the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97:237–41. doi: 10.1161/01.cir.97.3.237. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Wallerath T, Munze T, Forstermann U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide. 2002;7:149–64. doi: 10.1016/s1089-8603(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 46.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–7. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 47.Pritchard KA, Ackerman AW, Ou J, Curtis M, Smalley DM, Fontana JT, Stemerman MB, Sessa WC. Native low-density lipoprotein induces endothelial nitric oxide synthase dysfunction: Role of heat shock protein 90 and caveolin-1. Free Radic Biol Med. 2002;33:52–62. doi: 10.1016/s0891-5849(02)00851-1. [DOI] [PubMed] [Google Scholar]
- 48.Bakircioglu ME, Hsu K, El-Sakka A, Sievert KD, Lin CS, Lue TF. Effect of a Chinese herbal medicine mixture on a rat model of hypercholesterolemic erectile dysfunction. J Urol. 2000;164:1798–801. [PubMed] [Google Scholar]
- 49.Jasperse JL, Laughlin MH. Endothelial function and exercise training: Evidence from studies using animal models. Med Sci Sports Exerc. 2006;38:445–54. doi: 10.1249/01.mss.0000191187.24525.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–97. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]