Abstract
Klotho is a new anti-aging gene. Genetic mutation of klotho causes multiple premature aging-like phenotypes and strikingly shortens lifespan. Overexpression of the klotho gene in mice suppresses aging and extends lifespan which may involve the mechanism of suppression of insulin signaling and oxidant stress. Klotho functions as a cofactor/coreceptor regulating fibroblast growth factor (FGF) 23 signaling. Klotho acts as a glucuronidase and activates ion channel TRPV5. Klotho protects against endothelial dysfunction and regulates the production of nitric oxide. Klotho also influences intracellular signaling pathways including p53/p21, cAMP, protein kinase C (PKC) and Wnt signaling pathways. The discovery of klotho has a great impact on aging research. The purpose of this review is to provide the recent progress and future directions of klotho research. Specifically, this review will cover: klotho and aging, structure and expression of the klotho gene, localization of klotho expression, source of circulating klotho, current understanding of klotho functions, and signaling pathways of klotho.
Keywords: klotho, aging, insulin signaling, fibroblast growth factor, glucuronidase, vitamin D
Klotho and aging
Aging is defined as the age-related decline in physiological functions necessary for survival and fertility. The aging process is multi-factorial, with genetic background and environmental stress as two critical components. Recent studies revealed that mutation of a single gene in chromosome 13 causes extensive aging phenotypes including arteriosclerosis, vascular calcifications, soft tissue calcifications, emphysema, hypoactivity, gonadal dysplasia, infertility, skin atrophy, ataxia, hypoglycemia and severe hyperphosphatemia (Table 1) that is associated with increased concentrations of 1,25(OH)2D3, an essential vitamin for calcium metabolism (Kuro-o et al., 1997). This gene was named after the purported Greek goddess, Klotho, who spins the thread of life. The life-span of klotho-deficient mice is only about 5-6% of that of wild-type mice (Nabeshima, 2006). The defect in klotho involves the process of randomly integrating a foreign gene into the mouse genome (Kuro-o et al., 1997). In this process, the klotho gene is disrupted in its 5′-flanking promoter region (about 6 kb upstream of the transcription start site) by the random insertion of an exogenously introduced nonfunctional gene. The coding structure of the mutated klotho is still preserved, but klotho gene expression is severely reduced, which results in a strong hypomorphic allele and leads to aging-like phenotypes (Kuro-o et al., 1997). On the other hand, overexpression of klotho extends life span in mice (by 20 and 30% in females and males, respectively) (Table 1) (Kurosu et al., 2005). Klotho functions as a hormone that represses intracellular signals of insulin and insulin-like growth factor (IGF-1), an evolutionarily conserved mechanism for extending life span. Disruption of the insulin and IGF-1 signaling attenuates aging-like phenotypes in klotho-deficient mice (Kurosu et al., 2005), suggesting that klotho suppresses aging probably via inhibition of insulin and IGF-1 signaling. Therefore, klotho functions as an anti-aging gene in mammals. Table 1 summarizes the phenotypes of klotho deficient and klotho overexpression mice.
Table 1.
Comparison of phenotypes between klotho deficient and klotho overexpression mice
| Parameters | Klotho deficient mice | Klotho overexpression mice |
|---|---|---|
| Body weight | Showing growth retardation and becoming inactive and marantic at 3 to 4 weeks of age (Kuro-o et al., 1997). | Normal (Kurosu et al., 2005) |
| Average lifespan | About 2 months (vs 2.5 to 3 years for wild-type mice) (Kuro-o et al., 1997). | About 20–30% longer than wild-type mice (Kurosu et al., 2005). |
| Maximal lifespan | Less than 100 days (Kuro-o et al., 1997). | More than 936 days (Kurosu et al., 2005). |
| Insulin | Decreased insulin secretion and enhanced insulin sensitivity (Kuro-o et al., 1997). | Increased resistance to insulin and IGF-1 signaling (Kurosu et al., 2005). |
| Phosphorus homeostasis | Hyperphosphatemia (Kuro-o et al., 1997). | Normal (Kurosu et al., 2005). |
| Calcium homeostasis | Ectopic calcification in various organs (Kuro-o et al., 1997). | Normal (Kurosu et al., 2005). |
| Diseases | Hypogonadism, infertility, premature thymic involution, ectopic calcification, decreased bone mineral density, skin and muscle atrophy, ataxia, emphysema, cognitive impairment, hearing loss, vascular calcification (Kuro-o et al., 1997). Reduction of NO synthesis in vascular endothelial cells (Saito et al., 1998). | Protection of the angiotensin II-induced renal damage (Mitani et al., 2002). Suppression of H2O2-induced apoptosis and cellular senescence in vascular cells (Ikushima et al., and 2006). Reduction of risk factors for atherosclerosis. Enhanced hearing ability (Bektas et al., 2004) |
The structure and expression of the klotho gene
The mouse klotho gene is composed of 5 exons and 4 introns and resides on chromosome 13q12 with a size of over 50 kb (Kuro-o et al., 1997). The transcript of the mouse klotho gene is about 5.2 kb. The size of the human and rat klotho gene transcript is similar to that of the mouse (5.2 kb) (Matsumura et al., 1998; Nabeshima, 2002b; Ohyama et al., 1998). Its promoter region lacks a TATA-box and contains four potential binding sites for Sp1 (a human transcription factor involved in gene expression in the early development of an organism) (Matsumura et al., 1998). In the third exon, there is an alternative splicing at an internal splice donor site. Two transcripts arise from this alternative RNA splicing: a transmembrane and a secreted form of klotho protein. The transmembrane form of klotho has a full-length transcript and encodes 1014 amino acids. Once the short transmembrane domain is removed, this fragment can be released into the circulation (secreted form). An alternative mRNA splicing generates another transcript - secreted form of klotho protein (Matsumura et al., 1998; Ohyama et al., 1998; Shiraki-Iida et al., 1998). It has a half-length of the transcript and encodes 550 amino acids, which is a truncated klotho protein (Table 2).
Table 2.
Expression of the klotho gene in mouse, rat, and human
| mouse klotho protein 1,6 | rat klotho protein 5 | human klotho protein 4 | |
|---|---|---|---|
| Gene location | 13q12 | 12q12 | 13q12 |
| membrane form (ORF) | 3042bp | 3042bp | 3036bp |
| secreted form (ORF) | 1650bp | 1647bp | |
| membrane protein length | 1014-aminoacid | 1014-aminoacid | 1012-aminoacid |
| secreted protein length | 550-aminoacid | 549-aminoacid |
ORF : open reading frame
In the mouse, the expression of the transmembrane klotho predominates over the secreted protein in all tissues examined (Matsumura et al., 1998). In humans however, the major klotho gene product is the secreted protein (Li et al., 2004). The mouse klotho is highly homologous to that of rat (Shiraki-Iida et al., 1998) (Table 3). For example, a full length mouse klotho cDNA has 93% homology and a mouse klotho protein has 94% homology, respectively, with those of rats. In contrast, rat and mouse klotho cDNA and protein have about 80% homology with those of humans (Table 3).
Table 3.
The homology of klotho between mouse, rat, and human
| Mouse with rat | Mouse with human | Rat with human | |
|---|---|---|---|
| Kotho cDNA homology | 93% | 80% | 83% |
| klothoProtein homology | 94% | 86% | 85% |
Mouse transmembrane klotho protein has the typical structure of membrane proteins. It has 1014-aminoacid with a molecular weight of 130 kDa. A signal sequence is located at its N-terminus and a single trans-membrane helix domain is near its C-terminus. The transmembrane klotho consists of an N-terminal signal sequence, an extracellular domain with two internal repeats (KL1 and KL2), a single transmembrane domain, and a short intracellular domain. The two internal repeats are ∼ 550 amino acids in length, they share homology with members of the family 1 glycosidases and exhibit 20–40% sequence identity to β-glucosidases (enzymes involved in the digestion of sugar moieties of substrates) from bacteria and plants as well as mammalian lactase glycosylceramidase (Mian, 1998; Tohyama et al., 2004). Between two internal repeats (KL1, KL2), there is a short stretch of basic amino acids (Lys-Lys-Arg-Lys), a possible site for proteolytic cleavage similar to the polybasic proteolytic processing site. This structure is similar among the rat, human and mouse.
Mouse secreted klotho protein is derived from an alternative mRNA splicing. Compared with the transmembrane form protein, it does not have the second internal repeat of the extracellular domain (KL2), the transmembrane domain, or the intracellular domain. It only encodes the N-terminal half of klotho with its extracellular domain (KL1), and has 550 amino acids with a molecular weight of approximately 65–70 kDa (Matsumura et al., 1998; Ohyama et al., 1998; Shiraki-Iida et al., 1998) (Fig. 1).
Figure 1.
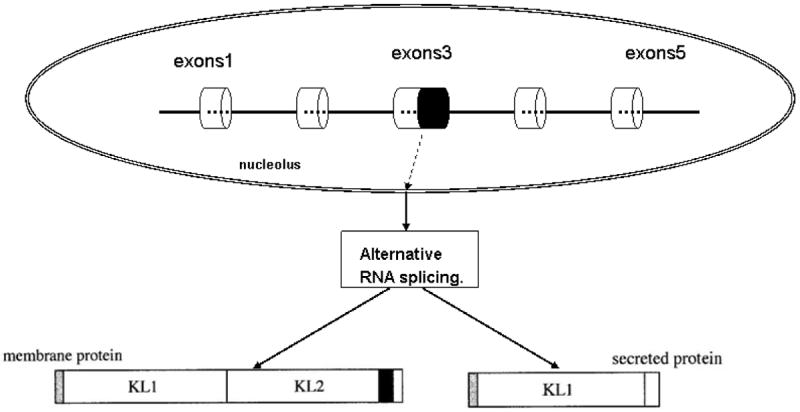
The mouse klotho gene structure and alternative RNA splicing. The mouse klotho gene is composed of 5- exons and 4-intron and resides on chromosome 13. Two transcripts arise from this alternative RNA splicing: a membrane or a secreted form of klotho protein.
Localization of klotho expression
Although genetic mutation of klotho causes multiple aging-related disorders in nearly all organs and tissues, the klotho gene is only expressed in limited tissues in mice, rats and humans. The subcellular localization of klotho is also reviewed, as it may help explain klotho function.
1. Mouse klotho
Mouse klotho is predominantly expressed in the kidney and the epithelium of the choroid plexus in the brain. Slight expression of klotho was also found in the pituitary gland, placenta, skeletal muscle, urinary bladder, pancreas, testis, ovary, colon, and inner ear (Kamemori et al., 2002; Kuro-o et al., 1997). In the kidney, klotho mRNAs and proteins are localized in the distal tubular cells. Despite its encoding as a transmembrane protein, klotho protein is not present on the cell surface of klotho-expressing cells. Instead, it is diffusely expressed in the cytoplasm; its intracellular distribution mostly overlaps the endoplasmic reticulum and Golgi apparatus (Li et al., 2004). In the brain, klotho is expressed at the apical plasma membrane of ependymal cells in the choroids plexus of both the lateral ventricles and the third ventricle. Klotho expression is recognized exclusively in the sinoatrial node region where it plays an essential role in regulating the sinoatrial node function and acts as a dependable pacemaker under stress conditions (Takeshita et al., 2004). Klotho gene deficiency causes cardiac arrhythmia and death (Takeshita et al., 2004). Klotho protein is also expressed in reproductive organs in lower levels. In the testis, klotho is expressed in the inner layers of seminiferous tubules containing elongating spermatids or mature germ cells. It is absent in spermatogonia, primary spermatocytes, round spermatids, and sertoli cells. In the ovary, klotho is expressed exclusively in the most mature follicles; it is absent or weakly expressed in primary and secondary follicles (Li et al., 2004).
2. Rat klotho
Rat klotho is also predominantly expressed in the kidney, and faintly expressed in the brain, lungs, intestines, and gonads. Ohyama et al found that rat klotho expression in the kidney was faint at 18 days of prenatal life until 1 day of age, and markedly augmented after 4 days of age (Ohyama et al., 1998). This result suggests that the kidney klotho may not be important for embryonic development, but essential for survival after birth. On the other hand, considerable klotho expression is detected in the placenta in humans and mice (Kuro-o et al., 1997; Matsumura et al., 1998). Placenta may release klotho into the embryonic circulation. Therefore, the role of klotho produced in the placenta in the embryonic development needs to be evaluated (Ohyama et al., 1998).
3. Human klotho
Human klotho is primarily expressed in the kidney, although it is also detectable in the placenta, prostate, and small intestines. The expression of the secreted form of klotho predominates over the transmembrane form of klotho (Matsumura et al., 1998). To date, the expression of the human klotho has been only partly deciphered. Up to now, the reduced production of klotho protein has been observed in patients with chronic renal failure. Some single nucleotide polymorphisms (SNP) in the human klotho gene are associated with altered lifespan, osteoporosis, stroke and altered risk for coronary artery disease (Arking et al., 2003; Arking et al., 2002; Kawano et al., 2002; Mullin et al., 2005; Ogata et al., 2002; Yamada et al., 2005). The molecular basis of these effects is not yet to be determined.
Regulation of klotho expression
Klotho expression is influenced by many physiological and pathological conditions. (i). Klotho expression is significantly altered with many physiological processes. For example, klotho expression is minimal in prenatal life, but markedly augmented after birth in the rat kidney (Ohyama et al., 1998). With aging, klotho expression decreases in the heart sinoatrial node and the liver (Nabeshima, 2002a; Shih et al., 2007). In the white matter of the rhesus monkey, klotho protein expression is also significantly decreased with age (Duce et al., 2008). By using two microsatellite markers flanking the klotho gene and DNA sequencing, Arking et al demonstrated that a functional variant of klotho (KL-VS) was associated with human survival and longevity (defined as postnatal life expectancy greater than 75 years) (Arking et al., 2002). (ii). Klotho mRNA expression is also significantly decreased in some disease conditions. Because of sustained circulatory and/or oxidant stress, klotho expression is decreased in spontaneously hypertensive rats, deoxycorticosterone acetate–salt hypertensive rats, 5/6 nephrectomized rats, noninsulin-dependent diabetes mellitus rats (Aizawa et al., 1998; Nagai et al., 2000), ischemia–reperfusion injury models (Vonend et al., 2004), and rats with acute myocardial infarction (Aizawa et al., 1998; Amann et al., 1996). In human, klotho also decreases in chronic renal diseases (Koh et al., 2001). (iii). Some endogenous and exogenous factors also influence klotho expression. Oral administration of troglitazone augments renal klotho mRNA expression (Yamagishi et al., 2001) and estrogen deficiency increases klotho protein level in the aromatase-deficient mouse model. Klotho expression is decreased after estradiol therapy. Angiotensin II downregulates renal klotho gene expression in an AT1 receptor-dependent pathway, but a pressor-independent mechanism (Mitani et al., 2002). Klotho expression is also reduced in the kidney by HMG-CoA reductase (Kuwahara et al., 2008) or oxidant stress injury by H2O2 (Mitobe et al., 2005).
Current understanding of klotho functions
The klotho gene plays a pivotal role in regulating aging and the development of age-related diseases in mammals: (i). A loss of klotho results in multiple aging-like phenotypes (Kuro-o et al., 1997). (ii). Overexpression of klotho gene extends lifespan by 20–30% (Kurosu et al., 2005). It is interesting to note that klotho gene expresses in limited tissues, but a defect in klotho gene expression leads to multiple aging-like phenotypes involving almost all organ systems in the mouse (Kuro-o et al., 1997). How does klotho cause systemic aging phenotypes? It is important to understand how klotho exerts its anti-aging effects in tissues not expressing klotho. Recent studies reveal some biological and physiological functions of klotho protein.
1. Klotho acts as a circulating hormone
Klotho protein itself or its metabolites may function as a humoral factor. This is supported by the following facts: (a) Parabiosis (the surgical connection of two animals to allow exchange of humoral factors) between klotho heterozygous mice and wild-type mice results in a complete recovery of endothelial function in klotho mice (Saito et al., 2000). (b) The transmembrane form of klotho protein exists in blood, urine, and cerebrospinal fluid (Imura et al., 2004). (c) In Otsuka Long Evans Tokushima Fatty (OLETF) rats, klotho gene delivery ameliorates renal damage induced by angiotensin II, although transferred klotho expression was mainly observed in the liver (Mitani et al., 2002). Recent studies reveal that klotho protein might enter into the circulation system through three pathways: (i). Alternative RNA splicing. Klotho genes can directly generate the secreted form of klotho protein by an alternative RNA splicing (Matsumura et al., 1998; Ohyama et al., 1998; Shiraki-Iida et al., 1998). The secreted klotho protein is liberated into the extracellular space and subsequently, the circulation. (ii). Proteolytic cleavage. The transmembrane form of klotho protein could be cleaved and released into the circulation system (Chen et al., 2007; Imura et al., 2004). Chen et al recently reported that ADAM10 and ADAM17, the members of the ADAM (a disintegrin and metalloproteinases) family, can cleave the extracellular domain of klotho to generate 130- and 68-kDa klotho fragments in klotho-transfected COS-7 cells (Chen et al., 2007). The cleavage that generates 130-kDa klotho fragments is called α-cut, and the cleavage that generates 68-kDa klotho fragments is β-cut (Chen et al., 2007). Both ADAM10 and ADAM17 can operate the two cleavages and shed the klotho protein with a positive feedback system style. Although these two ADAMs are responsible for both the α- and β-cleavages of klotho, the efficiency of α- and β-cuts is not the same. In most cases, the 130-kDa fragment is the major product of klotho shedding. (iii). Movement of Na+, K+-ATPase (adenosine triphosphatase). Although klotho expresses in kidney cells, it is not present on the cell surface; it is diffusely expressed in the cytoplasma, and mostly overlaps on the endoplasmic reticulum and Golgi apparatus (Li et al., 2004). Recently, Imura, et al. reported that in the endoplasmic reticulum (ER) and Golgi apparatus, the premature form of klotho and the mature form of klotho can bind to Na+, K+ -ATPase. The complex of klotho and Na+, K + -ATPase is then recruited to the cell surface by the combination of ‘the conventional pathway’ and ‘a novel klotho-dependent pathway’ in response to extracellular Ca2+ fluctuation (Imura et al., 2007). In this process, the complex of klotho and Na+, K+-ATPase could be going up to the cell surface where klotho could be cleaved and secreted into the extracellular space and hence the circulation system (Imura et al., 2007) (Fig. 2). However, the major source of the secreted klotho remains unknown.
Figure 2.
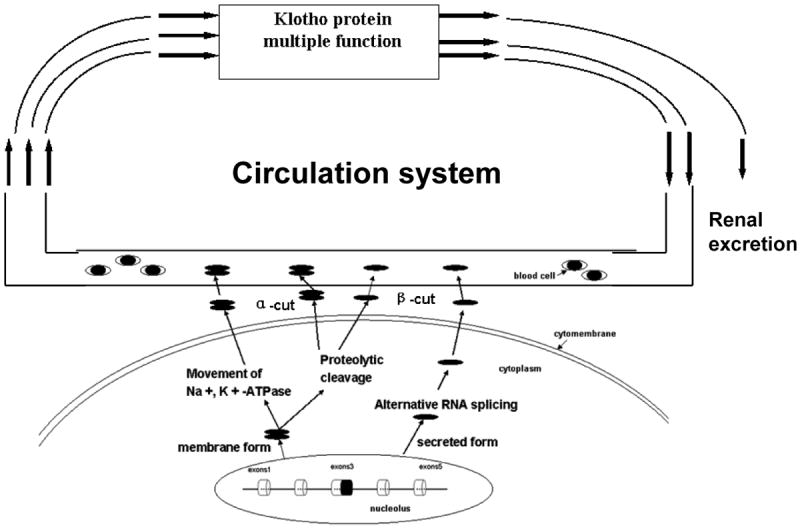
The generation of secreted form klotho. The klotho protein can enter the circulation via three pathways: (1). Alternative RNA splicing. The klotho gene can directly generate secreted form klotho protein by an alternative RNA splicing. The secreted klotho protein is liberated into the extracellular space and subsequently the circulation. (2). Proteolytic cleavage. Klotho protein could be cleaved and released to the circulation system. (3). Na+, K+ -ATPase. Klotho can bind to Na+, K+ -ATPase, the complex of klotho and Na+, K+ -ATPase could be going up to the cell surface where klotho is cleaved and secreted into extracellular space and hence the circulation system.
2. Klotho regulates insulin/insulin-like growth factor 1 (IGF1) signaling and suppresses oxidative stress
Klotho may inhibit insulin/IGF-1 signaling pathway. This is supported by the facts: (i). klotho-/- mice are hypoglycemic and extremely sensitive to insulin (Utsugi et al., 2000). (ii). Mice with over-expression of klotho are resistant to insulin (in males) and to IGF1 (in females), although they maintain normal fasting blood glucose levels (Kurosu et al., 2005). A recent study revealed that moderate inhibition of the insulin/IGF-1 signaling pathway is one of the evolutionarily conserved mechanisms for suppressing aging (Tatar et al., 2003). This viewpoint is supported by many studies in experimental models. In C. elegans, loss-of-function mutations in the genes encoding insulin receptor homolog (daf-2) and PI3-kinase homolog (age-1) extend lifespan of adult worms (Kenyon et al., 1993; Morris et al., 1996). The other models include Drosophila (Clancy et al., 2001), dwarf mice (Bartke et al., 2001), and some insulin receptor knockout mice (Holzenberger et al., 2003; Taguchi et al., 2007). All of these observations support the notion that suppression of insulin/IGF-1 signaling could extend the life span. It is speculated that klotho has high affinity to yet unidentified receptors. The extracellular domain of klotho may bind to its putative receptors that express on the cell surface, and this binding may activate a signaling pathway that cross-talks with the insulin/IGF1 signaling pathway, but the precise mechanism remains to be determined.
However, klotho protein regulates insulin/IGF1 could involve resistance to oxidative stress at the cellular and organismal levels in mammals (Mitani et al., 2002). Because mammalian forkhead box O (FOXO) transcription factors, FOXO1, FOXO3a, and FOXO4, are negatively regulated by insulin/IGF-1 signaling (Brunet et al., 1999), activation of insulin/IGF-1 signaling leads to phosphorylation and activation of a serine-threonine kinase Akt, which in turn phosphorylates FOXOs. Phosphorylated FOXOs are excluded from the nucleus and inactivated. If this process is interrupted, the nuclear FOXOs then directly bind to the promoters of antioxidant enzymes including catalase and mitochondrial manganese-superoxide dismutase (SOD2) (Kops, Dansen et al., 2002), and up-regulate their expression, thereby facilitating removal of reactive oxygen species (ROS) and conferring resistance to oxidative stress.
Klotho-overexpressing transgenic mice can inhibit insulin/IGF-1 signaling, and therefore lead to a decrease in phosphorylated FOXOs, activated FOXOs, and increased SOD2 expression, thus reducing oxidative stress. As proof, a lower level of urinary 8-OHdG, a marker of oxidative damages to DNA, was detected in mice over-expressing klotho (Yamamoto et al., 2005). In addition, klotho-overexpressing transgenic mice can survive following injection of lethal quantities of paraquat, a strong oxidizer that generates a large amount of reactive oxygen species (ROS) (Yamamoto et al., 2005). Thus, klotho overexpression induces resistance to oxidative stress.
3. Klotho acts as a cofactor/coreceptor regulating fibroblast growth factor (FGF) 23 Signaling
FGF23 is a bone derived hormone that functions in the kidney and inhibits phosphate reabsorption and vitamin D biosynthesis, thereby increasing urinary phosphate excretion and suppressing the levels of 1,25-dihydroxyvitamin D3 (Liu et al., 2007). FGF23 belongs to the FGF ligand superfamily; most of the FGF family members exert their functions through interacting with FGFRs. To date, four FGFRs with alternative spliced variants for each of them have been reported (Goldfarb, 2005; Mohammadi et al., 2005). Each of the FGFRs has its subtypes (e.g. FGFR1 has three different subtypes, namely FGFR1a, FGFR1b, and FGFR1c). FGF23 has the ability to interact with FGFR1c, FGFR2c, FGFR3c, and FGFR4 (Yamashita et al., 2002; Yu et al., 2005). Although the FGF23 signals through its receptors, it shows very weak affinity to any FGFRs in vitro (Mohammadi et al., 2005).
Recently, the extracellular domain of klotho was demonstrated to bind directly to multiple FGF receptors and functions as the coreceptor in FGF23 signaling (Kurosu et al., 2006; Urakawa et al., 2006). These corecepteors including klotho–FGFR1c, klotho–FGFR3c and klotho-FGFR4, all bind to FGF23 with much higher affinity than FGFRs or klotho alone. Klotho plays an important role in FGF23 signaling. Klotho-FGF23 signaling stimulates proliferation and prevents vitamin D-induced apoptosis (Medici et al., 2008). Thus, klotho and FGF23 may function in a common signal transduction pathway in maintaining mineral ion homeostasis. In the mouse, klotho deficiency disturbs the klotho-FGF23 signal transduction pathway and results in increased production of 1,25(OH)2D3 (an active metabolite of vitamin D which plays an important role in calcium metabolism) (Kuro-o et al., 1997). High serum levels of 1,25(OH)2D3 promote absorption of dietary phosphate and calcium from the intestine, inducing not only hyperphosphatemia but also hypercalcemia in these mutants (Nabeshima, 2006). Klotho deficiency leads to overproduction of 1,25(OH)2D3 (Kuro-o et al., 1997) and increased oxidative stress (Kurosu et al., 2005). Overproduction of 1,25(OH)2D3 may be primarily responsible for the aging-like phenotypes in klotho-deficient mice. The decreased production of 1,25- dihydroxyvitamin D3 (1,25(OH)2D3) by restricting dietary intake of phosphate and vitamin D (Tsujikawa et al., 2003) or by ablating the 1α-hydorxylase gene that encodes an enzyme essential for biosynthesis of 1,25(OH)2D3 (Nabeshima, 2006), can rescue almost all aging-like phenotypes and increase the survival span (Kurosu et al., 2005; Tsujikawa et al., 2003; Yamamoto et al., 2005). Elevation of calcium levels in the blood is a predisposition to ectopic calcification of blood vessels and soft tissues. This may be the reason that klotho-deficient mice and FGF 23-knockout mice exhibit strikingly similar physical and biochemical phenotypes, including aging-like syndrome, hyperphosphatemia, hypercalcemia and increased 1,25(OH)2 D3 synthesis (Deluca, 1979; Kuro-o et al., 1997; Sitara et al., 2004; Yoshida et al., 2002). Without klotho, the function of FGF23 is literally abolished.
4. Klotho acts as a glucuronidase and activates ion channel TRPV5
The extracellular domain of klotho (KL1, KL2) shares homology with members of the glycosidase family 1 β-glycosidases (Kuro-o et al., 1997; Matsumura et al., 1998; Ohyama et al., 1998). However, β-glucosidase-like enzymatic activity is undetectable in recombinant klotho protein. Instead, klotho protein has been demonstrated to have weak β-glucuronidase activity (Tohyama et al., 2004). The chimeric klotho-human IgG1 Fc protein hydrolyzes the 4-methylumbelliferyl β-D-glucuronide among a series of putative substrates and this enzymatic activity is reduced by addition of the specific inhibitors of β-glucuronidase. Klotho-human IgG1 Fc protein can also hydrolyze some naturally occurring β-glucuronides including β-estradiol 3-β-D-glucuronide, 3-β-D-glucuronide, and estriol 3-β-D-glucuronide. Klotho β-glucuronidase activity is weak, it is considerably lower than that of other glycosidases (Tohyama et al., 2004).
The transient receptor potential vanilloid 5 (TRPV5) ion channel is an epithelial calcium channel expressed in the epithelial cells of distal convoluted tubules. It functions as an entry gate for transcellular calcium reabsorption and participates in the maintenance of calcium homeostasis (Hoenderop et al., 2005; Nijenhuis et al., 2005). Recombinant extracellular klotho protein, when added to the culture medium of HEK293 cells expressing TRPV5, increased the Ca2+ influx and the amount of cell-surface TRPV5 (Chang et al., 2005). Klotho β-glucuronidase activities play an important role in activating the TRPV5 ion channel because inhibition of β-glucuronidase blocks the ability of klotho to activate TRPV5 (Chang et al., 2005). The molecular mechanism of klotho-induced activation of TRPV5 channel is that klotho in urine, as a β-glucuronidase, hydrolyzes the N-linked extracellular sugar residues of TRPV5, then augments retention of TRPV5 on the plasma membrane, and hence increases the influx of Ca2+ in kidneys (Fig. 5). Cha et al recently reported that in a human cell line, the extracellular domain of klotho activates plasma-membrane resident TRPV5 through removing terminal sialic acids from TRPV5 N-glycan chains (Cha et al., 2008). Klotho removal of terminal sialic acids leads to galectin-1 binding to TRPV5, this binding at the extracellular surface leads to decreasing TRPV5′s endocytosis, and enhances retention of TRPV5 at the cell surface. Klotho is exclusively co-expressed with TRPV5 and calbindin-D28K (the vitamin D-sensitive intracellular Ca2+ - transporting protein) in the distal convoluted tubule cells, form a specialized region of the nephron segments where transepithelial Ca2+ reabsorption is actively regulated (de Groot et al., 2008). This co-localization is believed to be important for the homeostatic control of calcium.
5. Klotho protects against endothelial dysfunction and regulates the production of nitric oxide
Klotho gene and protein delivery has anti-apoptotic and anti-senescence effects on vascular endothelial cells and thus protects against endothelial dysfunction in multiple risk factor syndromes. In humans, several single-nucleotide polymorphisms of the klotho gene have been found to be associated with lifespan, coronary artery diseases, stroke, and osteoporosis.
Klotho is considered as a humoral factor, and vascular endothelial cells are continuously exposed to klotho. Vascular endothelium has been known to play a crucial role in the control of vascular tone; endothelial cells release nitric oxide (NO) in response to specific agonists such as acetylcholine (Griffith et al., 1984; Palmer et al., 1987). Klotho gene deficiency significantly attenuates endothelium-dependent vasodilation of aorta and arterioles in response to acetylcholine (Saito et al., 1998). Thus, klotho may up-regulate NO production. This hypothesis is supported by the fact that the urinary excretion of NO metabolites (NO−2 and NO−3) is significantly decreased in klotho-deficient mice. L- NAME, a nitric oxide synthase (NOS) inhibitor, decreased the urinary excretion of NO−2 and NO−3 to the same level in both wild-type and heterozygous klotho mice, suggesting that the klotho deficiency-induced decrease in NO production is due to suppression of endogenous NO formation. It was found that klotho gene delivery increases NO production in Otsuka Long-Evans Tokushima Fatty (OLETF) rats which manifest multiple atherogenic risk factors (e.g., hypertension, diabetes mellitus, hyperlipidemia, and obesity) (Saito et al., 2000). It is interesting that parabiosis between klotho heterozygous mice and wild-type mice restored endothelial function of klotho-deficient mice, indicating that the klotho protein may act as a humoral factor to maintain normal endothelial function. Klotho protects against endothelial dysfunction and regulates the production of NO in severe calcified aorta and in some arterioles that calcification was not evident. The protective effect of klotho was decreased in klotho heterozygous mice to a smaller extent, despite the fact that they have normal blood phosphate and vitamin D levels. Thus, the decreased NO synthesis in klotho-deficient mice may not be secondary to hyperphosphatemia or hypervitaminosis D. The mechanism of NO synthesis mediated by klotho protein is unknown. However, klotho deficiency may down-regulate eNOS and decrease NO formation. Klotho protein may act as an enzyme converting inactive precursor to a biological active form. Further studies will be needed to investigate how the klotho protein regulates NO synthesis. On the other hand, the klotho deficiency-induced decrease in NO level may be attributed to accelerated degradation of NO by scavengers such as superoxide anions because an increase in superoxide production has been shown in aging (Okuwaki, 1990; Song et al., 1999).
Klotho influences intracellular signaling pathways
Although klotho was identified eleven years ago, its exact biological role and the underlying molecular mechanism are yet to be determined. Recent studies reveal some novel klotho signal pathways, which may contribute to the understanding of the molecular mechanism of klotho. As discussed above, klotho can inhibit the insulin/IGF-1 signal pathway (Kurosu et al., 2005). Klotho may also influence the following signaling pathways.
1. Klotho influences the p53/p21 signal pathway
Non-transformed animal cells have a finite proliferative capacity in cultures, known as the Hayflick limit, which results in irreversible proliferative arrest, called cellular senescence (Rubin, 1997). Phenotypic changes of senescent cultured cells include enlargement, flattened morphology, expression of acidic β-galactosidase (β-Gal) and a permanent cell cycle arrest at G1 phase (Ben-Porath et al., 2005). The pro-apoptotic protein p53 regulates the transcription of genes involved in cell-cycle arrest and apoptosis and is a well-characterized regulator of cellular senescence (Wahl et al., 2001). Transient or irreversible p53-mediated cell cycle arrest in the transition from the G1 to S phase of cell replication is mediated by the transcriptional activation of the cyclin dependent kinase (CDK) inhibitors such as p16 and p21 (Campisi, 2005). As cells exhaust their replicative potential, the increased p53 activity activates CDK (the p53/p21 pathway is activated), and triggers the growth arrest and senescence (K. Suzuki et al., 2001). After DNA damage, klotho can reduce the cellular senescence phenotype in MRC-5 primary human fibroblast cells and human umbilical vascular endothelial cells (HUVECs) through the p53/p21 pathway. In MRC-5 primary human fibroblast cells, loss of klotho activity by RNAi results in a dramatic premature senescence phenotype. RNAi inhibition of klotho increased β-Gal activity by 15-fold and resulted in senescence (cells were flattened and enlarged) (de Oliveira, 2006). This early senescence is fully dependent upon the cell cycle regulatory factor p53 which up-regulates a downstream p53-dependent CDK factor p21. Thus, klotho reduces senescence by the p53/p21 dependent pathway (de Oliveira, 2006). It was also demonstrated that klotho reduces senescence through the p53/p21 pathway in HUVECs. In order to reduce cellular senescence, klotho recombinant protein was added to HUVECs in 50 uM H2O2 for 1 week. Senescence-associated β-gal was reduced in klotho-treated HUVECs. The expression levels of p53 and p21 were lower in klotho-treated HUVECs than in the control cells. In this study, klotho not only inhibited cellular senescence, but also suppressed apoptosis because the caspase-3 and caspase-9 activities were decreased in HUVECs treated with klotho recombinant protein than in control HUVECs (Ikushima et al., 2006). Thus, the p53/p21 signal pathway is an important intracellular signal pathway in senescence. It was reported that Akt (an important molecule in mammalian cellular signaling) also negatively regulates the in vitro lifespan of HUVECs via the p53/p21-dependent pathway (Miyauchi et al., 2004). Thus, klotho may inhibit cellular senescence through inactivation of Akt. This hypothesis, however, needs to be validated.
2. Klotho influences the cAMP signal pathway
Cyclic adenosine monophosphate (cAMP) is a nucleotide generated from ATP through the action of the enzyme adenylate cyclase. The intracellular concentration of cAMP is increased or decreased by a variety of hormones and such fluctuations affect a variety of cellular processes. One prominent and important effect of elevated concentrations of cAMP is activation of a cAMP-dependent protein kinase, protein kinase A (PKA). PKA is normally in a catalytically-inactive state, but becomes active when it binds to cAMP. Upon activation, PKA phosphorylates a number of other proteins, many of which are enzymes that are either activated or suppressed by being phosphorylated. Such changes in enzymatic activity within the cell clearly alter its state (Antoni, 2000).
Recently, Yang et al demonstrated that klotho protein can function as a circulating hormone, trigger and upregulate the second messenger cAMP in endothelial cells. They first found that klotho recombinant protein can increase angiotensin I-converting enzyme (ACE) activity in HUVECs (Yang et al., 2003). Klotho also induced time- and dose-dependent enhancement of cAMP production in HUVECs. Thus, they further studied the cAMP-PKA signal pathway in this process because it was reported that activation of cAMP upregulates ACE activity in the endothelium (Xavier-Neto et al., 1999). The study showed that Rp-cAMP, an inhibitor of cAMP-dependent PKA, completely reversed the increasing effect of klotho protein on ACE activity in HUVECs, demonstrating that this effect is cAMP–PKA-dependent (Yang et al., 2003). These results suggest that klotho protein acts as a humoral factor to increase ACE activity in HUVECs via a cAMP–PKA-dependent pathway.
In addition, this research group demonstrated that klotho recombinant protein increases Mn-SOD activity and NO production in HUVECs via the cAMP–PKA-dependent pathway, and has a protective effect against angiotensin II-induced ROS production (Rakugi et al., 2007). Angiotensin II was demonstrated to play an important role in endothelial dysfunction associated with aging via activation of superoxide anions (Mukai et al., 2002). Thus, klotho protein may improve endothelial dysfunction by regulating the homeostatic balance between reactive oxygen species (ROS) and antioxidant agents. This hypothesis is supported by the result that klotho protein reduced hydrogen peroxide (H2O2)-induced apoptosis and cellular senescence in cultured endothelial cells (Ikushima et al., 2006).
3. Klotho influences the PKC signal pathway
Protein kinase C (PKC) transduces the cellular signals that promote lipid hydrolysis. This 80 kDa enzyme is recruited to the plasma membrane by diacylglycerol and, in many cases, by calcium. The enzyme is activated by diacylglycerol and phospholipid and is thought to undergo a conformational change upon binding to the membrane. PKC phosphorylates a variety of target proteins which control cell growth and differentiation (Dehvari et al., 2007; Mellor et al., 1998; Nishizuka, 1995; Perez et al., 2002; A. Suzuki et al., 2003). cAMP and PKC are known to elevate 25-hydroxyvitamin D3 1α-hydroxylase gene expression. However, recent studies revealed that klotho protein upregulated the cAMP and PKC pathway, but suppressed the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in kidney cells (Imai et al., 2004). Therefore, suppression of 25-hydroxyvitamin D3 1α-hydroxylase by klotho is independent of the cAMP or PKC pathway (Imai et al., 2004). Because klotho plays an important role in calcium metabolism (Yoshida et al., 2002), klotho deficiency might increase calcium concentration by upregulating the 25-hydroxyvitamin D3 1α-hydroxylase gene. However, activation of the PKC pathway by klotho was observed only in the kidney and testis where the klotho gene is abundantly expressed (Imai et al., 2004).
4. Klotho influences the Wnt signal pathway
Wnt is one of the secreted factors essential for stem cell proliferation and maintenance, chronic stimulation of Wnt signaling can lead to rapid exhaustion and depletion of stem cells and stem cell dysfunction potentially contribute to aging processes (Kirstetter et al., 2006; Scheller et al., 2006). It was recently reported that the extracellular domain of klotho protein binds to multiple Wnt ligands and inhibits their ability to activate Wnt signaling (H. Liu et al., 2007). That klotho inhibits Wnt signaling may explain several aging-like phenotypes observed in klotho-deficient mice. The changes in the skin were accompanied by a reduction in the number of epidermal stem cells within the hair follicles, which may also explain a deficit in wound healing in klotho-deficient mice. Epidermal stem cells in klotho-deficient mice had higher Wnt signaling activity and higher expression of an endogenous cell-senescence marker (SAβ-galactosidase) than those in wild-type mice, suggesting that continuous activation of Wnt signaling caused by klotho deficiency may lead to senescence of stem cells. Abnormal bone mineral density observed in klotho-deficient mice may be partly attributed to the altered Wnt signaling as well (Kawaguchi et al., 1999; Yamashita et al., 1998). In contrast to a marked decrease in cortical bone thickness in the tibia, trabecular bone density at epiphysis is rather increased in klotho-deficient mice. Since augmented Wnt signaling in osteoblasts promotes their proliferation/survival and is associated with increased bone formation, loss of the inhibitory effect of klotho on Wnt may increase bone mass. Indeed, the Wnt signaling activity was increased selectively in the epiphysial trabecular bones of klotho-deficient mice carrying a Wnt reporter transgene (a β-galactosidase reporter under the control of Wnt-responsive elements). The mechanism underlying the regional difference in bone density remains to be determined.
Manya reported that klotho-deficiency results in activation of μ-calpain, a calcium-dependent protease that cleaves various cellular proteins including β-catenin (Manya et al., 2002). Activation of the Wnt signaling leads to accumulation of cytoplasmic β-catenin, which in turn translocates into the nucleus and interacts with transcription factors of the T cell factor (TCF) family to activate their target genes. The cytoplasmic β-catenin level is regulated primarily by proteasomal degradation. Recent studies indicated that calpain, activated by calcium release from intracellular stores, also participates in β-catenin degradation (G. Li et al., 2002). Activation of calpain may be a compensatory response to the augmented Wnt signaling caused by klotho deficiency. β-catenin can bind not only to TCFs but also to FOXOs and enhances their transcriptional activity (Essers et al., 2005). In general, TCFs stimulate cell cycle progression, while FOXOs inhibit it and promote DNA repair (Kops et al., 2002a, 2002b). Interestingly, the interaction between β-catenin and FOXOs is enhanced in cells under oxidative stress, which potentially shifts β-catenin from TCFs to FOXOs and induces cell cycle arrest and DNA repair (Kuro-o, 2008).
Conclusions and perspectives
Klotho gene plays a critical role in regulating aging and the development of age-related diseases in mammals. Klotho can shed into the circulation and function as a multi-functional humoral factor that influences multiple biological processes. (i). Klotho functions as a co-receptor for FGF23 in FGF23 signaling, which down-regulates the expression of 1,25-dihydroxyvitamin D3 and phosphate reabsorption. (ii). Klotho inhibits insulin and IGF-1 signaling. (iii). Klotho increases the resistance to oxidative stress. (iv). Klotho acts as a β-glucuronidase that activates the ion channel TRPV5 by trimming its sugar moiety, leading to the up-regulation of Ca2+ reabsorption. Klotho may protect the cardiovascular system by increasing NO production and inhibiting oxidative stress. However, the mechanism mediating the role of klotho in aging-related diseases (e.g., hypertension) remains to be found. Whether the klotho gene delivery can serve as a new therapeutic approach for rejuvenation and aging-related diseases is potentially an interesting topic to pursue.
Klotho influences several intracellular signaling pathways which underlie the molecular mechanism of klotho function. Klotho can suppress the insulin/IGF-1 signal pathway. In addition, klotho influences p53/p21, cAMP, PKC and Wnt signaling pathways. Understanding of the klotho signaling pathways may reveal a new understanding of aging and aging-related diseases.
It should be mentioned that the exact biological functions of klotho and the underlying molecular mechanisms are not fully understood. The klotho gene expresses in limited tissues, but klotho deficiency causes extensive aging phenotypes in nearly all tissues and organs. Thus, klotho may function as a hormone that acts on tissues that do not express klotho. However, the major source of the circulating klotho is still not known. Perhaps, the unknown receptor of klotho is a critical barrier for understanding the function of klotho. Thus, it is imperative to clone and characterize receptors that mediate the effect of klotho.
Figure 3.
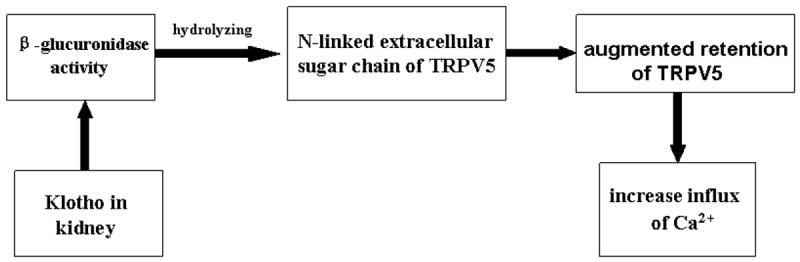
The functional relationship of klotho and transient receptor potential vanilloid 5 (TRPV5) in kidneys. The β-glucuronidase activity plays an important role in activating TRPV5. Klotho in urine, as a β-glucuronidase, hydrolyzes the N-linked extracellular sugar residues of TRPV5, and then augments the retention of TRPV5 on the plasma membrane hence increase the influx of Ca2+ in kidneys.
Acknowledgments
This work was supported by NIH R01 NHLBI 077490 (to Z. S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- Amann K, Nichols C, Tornig J, Schwarz U, Zeier M, Mall G, Ritz E. Effect of ramipril, nifedipine, and moxonidine on glomerular morphology and podocyte structure in experimental renal failure. Nephrol Dial Transplant. 1996;11:1003–1011. [PubMed] [Google Scholar]
- Antoni FA. Molecular diversity of cyclic AMP signalling. Front Neuroendocrinol. 2000;21:103–132. doi: 10.1006/frne.1999.0193. [DOI] [PubMed] [Google Scholar]
- Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Krebsova A, Macek M, Sr, Macek M, Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C. Prolonged longevity of hypopituitary dwarf mice. Exp Gerontol. 2001;36:21–28. doi: 10.1016/s0531-5565(00)00205-9. [DOI] [PubMed] [Google Scholar]
- Bektas A, Schurman SH, Sharov AA, Carter MG, Dietz HC, Francomano CA. Klotho gene variation and expression in 20 inbred mouse strains. Mamm Genome. 2004;15:759–767. doi: 10.1007/s00335-004-2375-3. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- de Groot T, Bindels RJ, Hoenderop JG. TRPV5: an ingeniously controlled calcium channel. Kidney Int. 2008 doi: 10.1038/ki.2008.320. [Epub ahead of print] PMID: 18596722. [DOI] [PubMed] [Google Scholar]
- de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Dehvari N, Cedazo-Minguez A, Isacsson O, Nilsson T, Winblad B, Karlstrom H, Benedikz E, Cowburn RF. Presenilin dependence of phospholipase C and protein kinase C signaling. J Neurochem. 2007;102:848–857. doi: 10.1111/j.1471-4159.2007.04571.x. [DOI] [PubMed] [Google Scholar]
- Deluca HF. Vitamin D-resistant rickets. A prototype of nutritional management of a genetic disorder. Curr Concepts Nutr. 1979;8:3–32. [PubMed] [Google Scholar]
- Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR. Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 2008;56:106–117. doi: 10.1002/glia.20593. [DOI] [PubMed] [Google Scholar]
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- Goldfarb M. Fibroblast growth factor homologous factors: evolution, structure, and function. Cytokine Growth Factor Rev. 2005;16:215–220. doi: 10.1016/j.cytogfr.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TM, Edwards DH, Lewis MJ, Newby AC, Henderson AH. The nature of endothelium-derived vascular relaxant factor. Nature. 1984;308:645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- Imai M, Ishikawa K, Matsukawa N, Kida I, Ohta J, Ikushima M, Chihara Y, Rui X, Rakugi H, Ogihara T. Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression. Endocrine. 2004;25:229–234. doi: 10.1385/ENDO:25:3:229. [DOI] [PubMed] [Google Scholar]
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- Kamemori M, Ohyama Y, Kurabayashi M, Takahashi K, Nagai R, Furuya N. Expression of Klotho protein in the inner ear. Hear Res. 2002;171:103–110. doi: 10.1016/s0378-5955(02)00483-5. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104:229–237. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Ogata N, Chiano M, Molloy H, Kleyn P, Spector TD, Uchida M, Hosoi T, Suzuki T, Orimo H, Inoue S, Nabeshima Y, Nakamura K, Kuro-o M, Kawaguchi H. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17:1744–1751. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002a;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, Lam EW, Burgering BM. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002b;22:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara N, Sasaki S, Kobara M, Nakata T, Tatsumi T, Irie H, Narumiya H, Hatta T, Takeda K, Matsubara H, Hushiki S. HMG-CoA reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int J Cardiol. 2008;123:84–90. doi: 10.1016/j.ijcard.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Li G, Iyengar R. Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/beta -catenin-regulated cell proliferation. Proc Natl Acad Sci U S A. 2002;99:13254–13259. doi: 10.1073/pnas.202355799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Liu S, Gupta A, Quarles LD. Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr Opin Nephrol Hypertens. 2007;16:329–335. doi: 10.1097/MNH.0b013e3281ca6ffd. [DOI] [PubMed] [Google Scholar]
- Manya H, Inomata M, Fujimori T, Dohmae N, Sato Y, Takio K, Nabeshima Y, Endo T. Klotho protein deficiency leads to overactivation of mu-calpain. J Biol Chem. 2002;277:35503–35508. doi: 10.1074/jbc.M206033200. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, Goetz R, Mohammadi M, Kuro OM, Olsen BR, Lanske B. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol. 2008;182:459–465. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian IS. Sequence, structural, functional, and phylogenetic analyses of three glycosidase families. Blood Cells Mol Dis. 1998;24:83–100. doi: 10.1006/bcmd.1998.9998. [DOI] [PubMed] [Google Scholar]
- Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838–843. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. 2005;101:e67–74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. Embo J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Mukai Y, Shimokawa H, Higashi M, Morikawa K, Matoba T, Hiroki J, Kunihiro I, Talukder HM, Takeshita A. Inhibition of renin-angiotensin system ameliorates endothelial dysfunction associated with aging in rats. Arterioscler Thromb Vasc Biol. 2002;22:1445–1450. doi: 10.1161/01.atv.0000029121.63691.ce. [DOI] [PubMed] [Google Scholar]
- Mullin BH, Wilson SG, Islam FM, Calautti M, Dick IM, Devine A, Prince RL. Klotho gene polymorphisms are associated with osteocalcin levels but not bone density of aged postmenopausal women. Calcif Tissue Int. 2005;77:145–151. doi: 10.1007/s00223-004-0291-x. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y. Ectopic calcification in Klotho mice. Clin Calcium. 2002a;12:1114–1117. [PubMed] [Google Scholar]
- Nabeshima Y. Klotho: a fundamental regulator of aging. Ageing Res Rev. 2002b;1:627–638. doi: 10.1016/s1568-1637(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y. Toward a better understanding of Klotho. Sci Aging Knowledge Environ. 2006;2006:pe11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738–746. doi: 10.1007/s000180050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis T, Hoenderop JG, Bindels RJ. TRPV5 and TRPV6 in Ca(2+) (re)absorption: regulating Ca(2+) entry at the gate. Pflugers Arch. 2005;451:181–192. doi: 10.1007/s00424-005-1430-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. Faseb J. 1995;9:484–496. [PubMed] [Google Scholar]
- Ogata N, Matsumura Y, Shiraki M, Kawano K, Koshizuka Y, Hosoi T, Nakamura K, Kuro OM, Kawaguchi H. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone. 2002;31:37–42. doi: 10.1016/s8756-3282(02)00786-x. [DOI] [PubMed] [Google Scholar]
- Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- Okuwaki K. Chronic organophosphorus intoxication and aging in rats. Nippon Ganka Gakkai Zasshi. 1990;94:723–730. [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Perez P, Calonge TM. Yeast protein kinase. C J Biochem. 2002;132:513–517. doi: 10.1093/oxfordjournals.jbchem.a003250. [DOI] [PubMed] [Google Scholar]
- Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, Maekawa Y, Kida I, Miyazaki J, Ogihara T. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–87. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- Rubin H. Cell aging in vivo and in vitro. Mech Ageing Dev. 1997;98:1–35. doi: 10.1016/s0047-6374(97)00067-5. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Bao M, Li D, Li YM. Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev. 1999;108:239–251. doi: 10.1016/s0047-6374(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Akimoto K, Ohno S. Protein kinase C lambda/iota (PKClambda/iota): a PKC isotype essential for the development of multicellular organisms. J Biochem. 2003;133:9–16. doi: 10.1093/jb/mvg018. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Mori I, Nakayama Y, Miyakoda M, Kodama S, Watanabe M. Radiation-induced senescence-like growth arrest requires TP53 function but not telomere shortening. Radiat Res. 2001;155:248–253. doi: 10.1667/0033-7587(2001)155[0248:rislga]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279:9777–9784. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. [Google Scholar]
- Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, Aizawa H, Itoh H, Kurabayashi M, Kawazu S, Tomono S, Oka Y, Suga T, Kuro-o M, Nabeshima Y, Nagai R. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49:1118–1123. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- Vonend O, Apel T, Amann K, Sellin L, Stegbauer J, Ritz E, Rump LC. Modulation of gene expression by moxonidine in rats with chronic renal failure. Nephrol Dial Transplant. 2004;19:2217–2222. doi: 10.1093/ndt/gfh374. [DOI] [PubMed] [Google Scholar]
- Wahl GM, Carr AM. The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol. 2001;3:E277–286. doi: 10.1038/ncb1201-e277. [DOI] [PubMed] [Google Scholar]
- Xavier-Neto J, Pereira AC, Junqueira ML, Carmona R, Krieger JE. Rat angiotensin-converting enzyme promoter regulation by beta-adrenergics and cAMP in endothelium. Hypertension. 1999;34:31–38. doi: 10.1161/01.hyp.34.1.31. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of the androgen receptor and klotho genes with bone mineral density in Japanese women. J Mol Med. 2005;83:50–57. doi: 10.1007/s00109-004-0578-4. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Saito Y, Nakamura T, Takeda S, Kanai H, Sumino H, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. Troglitazone improves endothelial function and augments renal klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with multiple atherogenic risk factors. Hypertens Res. 2001;24:705–709. doi: 10.1291/hypres.24.705. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Konishi M, Miyake A, Inui K, Itoh N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:28265–28270. doi: 10.1074/jbc.M202527200. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Nifuji A, Furuya K, Nabeshima Y, Noda M. Elongation of the epiphyseal trabecular bone in transgenic mice carrying a klotho gene locus mutation that leads to a syndrome resembling aging. J Endocrinol. 1998;159:1–8. doi: 10.1677/joe.0.1590001. [DOI] [PubMed] [Google Scholar]
- Yang J, Matsukawa N, Rakugi H, Imai M, Kida I, Nagai M, Ohta J, Fukuo K, Nabeshima Y, Ogihara T. Upregulation of cAMP is a new functional signal pathway of Klotho in endothelial cells. Biochem Biophys Res Commun. 2003;301:424–429. doi: 10.1016/s0006-291x(02)03056-5. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology. 2002;143:683–689. doi: 10.1210/endo.143.2.8657. [DOI] [PubMed] [Google Scholar]
- Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]


