ABSTRACT
This study's goals were twofold: (1) to analyze the author's experience with the petro-occipital trans-sigmoid (POTS) approach for the resection of tumors arising in or adjacent to the jugular foramen, and (2) to define the anatomical sites exposed by this approach. A retrospective review was conducted of 61 patients with jugular fossa tumors that included lower cranial nerve schwannomas, paragangliomas, meningiomas, chordomas, cholesteatomas, and other benign or low-grade malignant tumors. Outcome measures were mortality, morbidity, and long-term outcomes. No deaths were found in this study. The major morbidity was deficits of the glossopharyngeal, vagus, and accessory nerves. Hearing and facial nerve function were largely preserved. The resections were undertaken as single-stage procedures regardless of whether the tumor was entirely extradural or both intra- and extradural. None of the patients had central nervous system complications. Good outcomes were achieved for schwannomas, meningiomas, chondrosarcomas, and papillary adenoma. Chordomas tended to recur, and only class C1 paragangliomas could be removed using this approach. The study found that the POTS approach should be considered the approach of choice for many tumors in the region of the jugular foramen, particularly schwannomas. It is not suitable for the resection of malignant tumors and most paragangliomas because it offers limited access to the skull base between the jugular fossa and carotid canal.
Keywords: Jugular foramen, tumors of jugular foramen, surgical procedures, petro-occipital trans-sigmoid approach
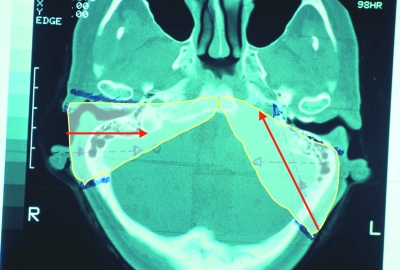
The petro-occipital trans-sigmoid (POTS) approach has been developed for removing tumors situated in the jugular foramen together with their local extension into the adjacent parts of the skull base, the cerebellopontine angle (CPA), and the parapharyngeal space (PPS). Compared with the infratemporal type A (IFT-A) approach, with its direct lateral exposure and medially directed surgical axis, the POTS approach involves a posterolateral exposure and an anteromedially directed surgical axis (Fig. 1). This allows the surgeon to leave the external and middle ear complex, together with the facial nerve, entirely undisturbed. Single-stage removal of intra- and extradural lesions extending from the CPA to the PPS can be achieved with minimal risk of cerebrospinal fluid (CSF) leak.
Figure 1.
Extent and direction of the infratemporal type A (short arrow) and petro-occipital trans-sigmoid (long arrow) approaches as shown in an axial computed tomography of the skull base.
OPERATIVE TECHNIQUE AND PATIENTS
Operative Technique
In essence, the POTS approach is the combination of a retrolabyrinthine petrosectomy and retrosigmoid craniotomy. The external ear canal, tympanic cavity, and facial nerve are left in situ. Removal of the inferior aspect of the mastoid and petro-occipital bone gives access to the PPS. Opening the dura gives wide access to the CPA. This procedure1 is a development of the jugulopetrosectomy2 described for the resection of tympanojugular paragangliomas.
After induction of general anesthesia, a lumbar drain is inserted to facilitate control of the intracranial pressure. The patient is placed in the supine position with the head turned toward the opposite side. A C-shaped postauricular incision is made ~6 cm behind the sulcus, extending from above the ear into the neck to the level of C1, midway between the mastoid process and the angle of the mandible. The skin and subcutaneous tissues are raised so that a second, U-shaped, soft tissue flap can be raised that is composed of musculoaponeurotic tissue. This flap is based inferiorly at the craniocervical junction. The superior incision for this soft tissue flap is at or above the temporal line, and it runs close to the spine of Henle anteriorly; posteriorly it runs on the retrosigmoid occipital squama. This flap is elevated to expose the mastoid, temporal, and occipital squama.
Initial bone work involves a mastoidectomy that uncovers the sigmoid sinus down to the jugular bulb and a narrow strip of retrosigmoid dura. The jugular vein is exposed in the neck and ligated. Closure of the sigmoid sinus can be achieved in several ways, but we open its lumen and pack it with Surgicel. This is pushed carefully upstream as far as the junction of the sigmoid and transverse sinuses. In this way, the Labbé vein remains patent. Surgicel is also packed downstream into the sinus toward the jugular bulb.
A 4 × 4–cm retrosigmoid craniotomy is fashioned immediately adjacent to the mastoidectomy. The dura is gently raised from the undersurface of the petrous bone and retracts away from the occipital condyle spontaneously as CSF is drained. Further bone removal is then undertaken from beneath the labyrinth and from the occipital bone so that the lesion and jugular fossa are fully exposed. At this point, the anatomical boundaries of the exposure are the fallopian canal anteriorly and the posterior semicircular canal superiorly. The endolymphatic sac is preserved up to its entry into the vestibular aqueduct. The tympanic cavity may be exposed through a posterior tympanotomy fashioned both above and below the fallopian canal. Bone removal then continues around the jugular foramen as necessary to expose, debulk, and remove the tumor, dissecting it from local neurovascular structures.
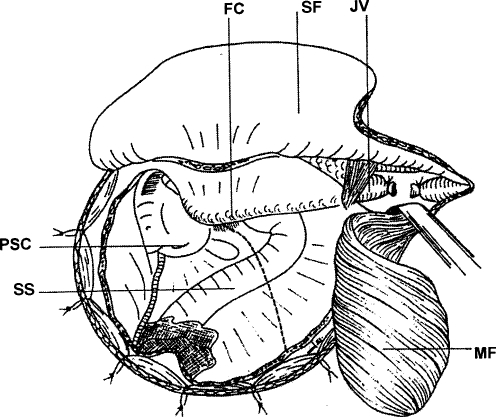
The operative field can be extended into the neck by removing the inferior aspect of the mastoid and styloid process. Resection of the lateral mass of C1 as far as the vertebral foramen gives additional exposure. The tumor is then mobilized in the PPS and dissected clear of the carotid arteries and associated fascial planes (Fig. 2).
Figure 2.
The surgical field from the skin to the dura. The skin flap (SF) is raised anteriorly, and the musculoaponeurotic flap (MF) is turned caudally. The bone removal includes a retrosigmoid craniotomy and a posterolateral petrosectomy up to the posterior semicircular canal, the antrum, fallopian canal (FC), and the posterior wall of the outer ear canal. The sigmoid sinus (SS) runs across the field, and the dotted line shows the dura incision. The jugular vein (JV) is ligated in the upper neck. PSC, posterior semicircular canal. (Reprinted from Mazzoni A, Sanna M. A posterolateral approach to the skull base: the petro-occipital transsigmoid approach. Skull Base Surg 1995;5:157–167.)
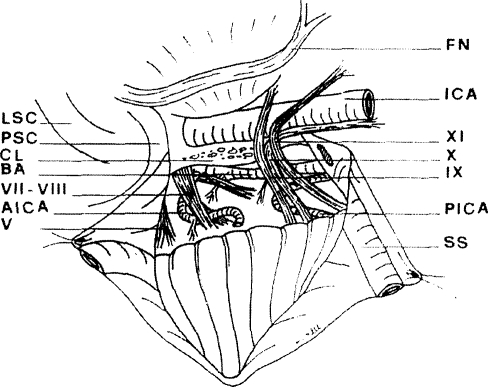
The dura is opened through an incision that lies caudal to the endolymphatic sac but, if necessary, may transect the sac as it enters the aqueduct. In this way, the CPA is entered. Displacement of the lower cranial nerves opens a route to the lower clivus. Dura may need to be resected in patients with meningioma (Figs. 3 and 4).
Figure 3.
Dural flaps have been retracted and cerebellopontine angle (CPA) exposed to show cranial nerves V and VII to XI, anteroinferior cerebellar artery (AICA), posteroinferior cerebellar artery (PICA), and basilar artery (BA) with perforating branches to the brainstem. Following removal of jugular vein and bulb, the low clivus (CL) is exposed. The occipital condyle is covered by caudal dural flap. The facial nerve (FN) can be raised from fallopian canal, and tympanic bone removed as far as the tympanic annulus. This improves access to infratubal portion of carotid canal. ICA, internal carotid artery; LSC, lateral semicircular canal; PSC, posterior semicircular canal; SS, sigmoid sinus; V, trigeminal nerve; VII, facial nerve; VIII, cochleovestibular nerve; IX, glossopharyngeal nerve; X, vagus nerve; XI, spinal accessory nerve. (Reprinted from Mazzoni A, Sanna M. A posterolateral approach to the skull base: the petro-occipital transsigmoid approach. Skull Base Surg 1995;5:157–167.)
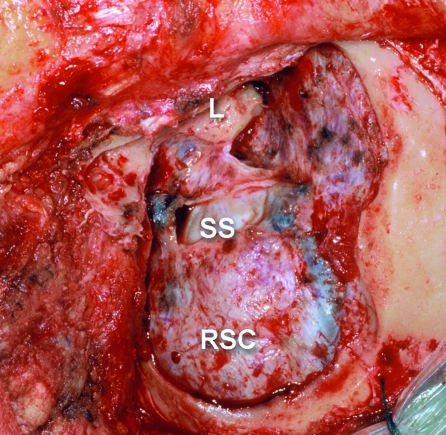
Figure 4.
Surgical view after posterior petrosectomy and retrosigmoid craniotomy (RSC), left side. L, labyrinth and petrous dura; SS, sigmoid sinus medial wall.
Closure must be meticulous to avoid a CSF leak. The dura is sutured. A free fat graft is used to obliterate the bone defect. It is secured by replacement of the inferiorly based, soft tissue flap, which also covers the dural closure. The skin is closed in two layers.
Patients
Our experience consists of a series of 61 patients who had tumors that had arisen in the jugular foramen or adjacent part of the skull base and underwent surgery between 1985 and 2004 (Table 1). A part of these cases has been reported in previous articles.1,11 There were 20 patients with schwannomas. Sixteen of these had developed from the glossopharyngeal, vagus, or accessory nerves, and four had extended into the jugular fossa from other sites. In this latter group, two schwannomas had arisen from the mastoid portion of the facial nerve, one was a residual or recurrent vestibular schwannoma, and one had developed in the condylar canal. There were 11 paragangliomas, 7 meningiomas, 6 cholesteatomas, 4 chordomas, 6 chondrosarcomas, and 7 other tumors. In every case, the lesion was situated caudal to the labyrinth and tympanic cavity with variable extension into the CPA, neck, and skull base. In 35 patients, the tumor was entirely extradural, and 26 patients had an intradural extension. The posterior part of the labyrinth was removed to improve access in 10 patients, all of whom had a preoperative hearing loss. Table 2 details the pathological nature of the tumors, their clinical extent or stage, and their preoperative and postoperative cranial nerve deficits. Follow-up consisted of a clinical examination that documented cranial nerve function, hearing acuity, computed tomography (CT), and/or magnetic resonance imaging (MRI) at 6 months, 1 year, 2 or 3 years, and 4 or 5 years postoperatively.
Table 1.
Case Material of 61 Cases
| Schwannomas 9–10–11 n | 16 |
| Schwannomas 7–8–12 n | 4 |
| Paragangliomas | 6 |
| Residual paragangliomas | 5 |
| Meningiomas | 7 |
| Cholesteatomas | 6 |
| Cholest. granul. | 1 |
| Chordomas | 4 |
| Chondrosarcomas | 6 |
| Papillary adenomas | 3 |
| Epithelioid hemangioma | 1 |
| Teratoma | 1 |
| Petrous apex cyst | 1 |
Table 2.
Case Material (Preoperative and Postoperative Features)
| Case | Age/Sex | Histology | Preop Nerve Losses (VII [I–VI HB]; IX,X,XI,XII) | Preop Hearing PTA, (% discr.) | Extent of Lesion | Procedure and Date (mm/yy) | Postop Nerve Changes (VII; IX,X,XI,XII) | Postop Hearing (Change; Follow-up) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| / = Preoperative normal or postoperative unchanged. | ||||||||||||
| Preop, before surgery; HB, House-Brackmann grade; PTA, pure tone average; discr., discrimination; postop, after surgery; JF, jugular fossa; CPA, cerebellopontine angle; POTS, petro-occipital trans-sigmoid approach; i, intradural; e, extradural; NED, no evidence of disease; n, nerve; C, clivus; P, petrous bone; Co, occipital condyle; A, petrous apex; CC, carotid canal; TL, translabyrinthine; PPS, parapharyngeal space; T, tympanum; M, mastoid; 7th graft, graft on the facial nerve; Tpl, tympanoplasty; IAC, internal auditory canal; Tp, transpetrous; DOD, dead of disease; AWD, alive with disease; G1, grade of differentiation. | ||||||||||||
| 1 | 40/F | Schwannoma | / | / | 29.0% | JF, CPA | POTS, i + e | 09/85 | / | IX,X | / | NED 15 y |
| 2 | 16/F | Schwannoma 12th n. | / | XII | 0, 100% | C, Co, JF | POTS, e | 09/86 | / | IX,X,XI | / | NED 18 y |
| 3 | 48/F | Schwannoma | / | IX,X,XII | deaf | JF, A, CC, CPA, Co | POTS + TL, i + e | 07/87 | / | / | / | NED 10 y |
| 4 | 49/M | Schwannoma | / | XII | 20, 100% | JF, CC, PPS | POTS, e | 10/89 | / | / | / | NED 10 y |
| 5 | 25/M | Schwannoma | II | / | deaf | JF, A, CC, T, CPA | POTS, i + e | 06/90 | / | / | / | NED 10 y |
| 6 | 48/F | Schwannoma | / | /IX,X,XI,XII | 20, 100% | JF, PPS | POTS, e | 09/90 | / | / | / | NED 10 y |
| 7 | 37/M | Schwannoma | / | // | deaf | JF, CPA | POTS + TL, i + e | 09/90 | / | / | / | NED 10 y |
| 8 | 39/M | Schwannoma | / | /IX,X,XI | 40, 70% | JF, CPA, PPS | POTS, i + e | 01/91 | / | / | / | NED 10 y |
| 9 | 23/M | Schwannoma | / | /IX,X,XI,XII | 10, 100% | JF, CC, CPA, Co | POTS, i + e | 09/92 | / | / | / | NED 10 y |
| 10 | 40/F | Schwannoma | / | /IX,X,XI | 15, 100% | JF, CPA, PPS | POTS, i + e | 08/93 | II | / | / | NED 10 y |
| 11 | 42/F | Schwannoma | / | X,XII | / | JF, PPS, CC | POTS, e | 11/96 | / | / | / | NED 5 y |
| 12 | 58/M | Schwannoma | / | / | deaf | JF, CPA,PPS | POTS + TL, i + e | 02/96 | / | IX,X | / | NED 5 y |
| 13 | 45/F | Schwannoma | / | /IX,X,XI,XII | / | JF, CC | POTS, e | 01/99 | / | / | / | NED 5 y |
| 14 | 50/M | Schwannoma | / | / | deaf | JF, CPA, PPS | POTS, i + e | 12/99 | / | IX,X | / | NED 5 y |
| 15 | 26/M | Schwannoma 7th n. | V | / | / | M, JF, P | POTS, e | 06/96 | III | / | / | NED 5 y |
| 16 | 51/M | Schwannoma | / | IX,X | 55, 60% | JF, M, 7N. | POTS, e | 09/00 | III | / | / | NED 5 y |
| 17 | 22/M | Schwannoma | / | IX,X | JF, Co, CPA | POTS, i + e | 10/00 | / | / | / | NED 5 y | |
| 18 | 41/M | Schwannoma | / | IX,X | JF, Co | POTS, e | 05/04 | NED 1 y | ||||
| 19 | 40/M | Schwannoma 8th n. | III | / | deaf | JF, A | POTS, i + e | 05/95 | / | / | / | NED 9 y |
| 20 | 45/M | Schwannoma 7th n. | II | / | 50, 100% | JF, T, PPS | POTS, e + 7th graft | 06/92 | III | / | / | 20, 100% |
| 21 | 68/F | Paragangl. C1 D1 | / | IX,X,XI,XII | 35, 90% | JF, CPA, C | POTS, i + e | 11/89 | / | / | / | NED 10 y |
| 22 | 39/F | Paragangl. C1 D1 | / | IX,X,XII | 15, 100% | JF, CPA, Co | POTS, i + e | 11/89 | / | XI | / | NED 10 y |
| 23 | 65/F | Paragangl. C1 | VI | IX,X,XI,XII | 15, 100% | JF | POTS, e + 7th graft | 07/90 | IV | / | / | NED 10 y |
| 24 | 69/F | Paragangl. C1 | / | / | 45, 80% | JF | POTS, e, deliberate partial removal | 11/91 | / | / | / | AWD,DOD C 7 y |
| 25 | 51/M | Paragangl. C1 D1 | III | IX,X | deaf | JF, A, Co, CPA | POTS + TL, i + e | 05/92 | / | / | / | NED 10 y |
| 26 | 66/F | Paragangl. C1( + mening.) | / | / | 50, 50% | JF, CPA | POTS, i + e | 04/99 | / | / | / | NED 5 y |
| 27 | 44/F | Meningioma | / | / | 24, 90% | JF, CPA | POTS, i + e | 07/90 | / | IX,X,XI | / | NED 15 y |
| 28 | 42/F | Meningioma | / | / | deaf | JF, CPA, IAC | POTS, i + e | 06/91 | / | XI,X | / | NED 8 y |
| 29 | 60/M | Meningioma | / | / | 55, 80% | JF, CPA | POTS, i + e | 09/91 | / | IX,X,XI | 60, 50% | LOST |
| 30 | 42/F | Meningioma | / | / | 20, 100% | JF, Co, CPA, CC | POTS, i + e | 12/93 | / | IX,X | deaf | NED 10 y |
| 31 | 35/F | Meningioma | / | / | 10, 100% | JF, T, A, CC, Co, CPA, PPS | POTS, i + e | 06/94 | / | IX,X,XI,XII | deaf | NED 10 y |
| 32 | 44/F | Meningioma | / | / | 60, 40% | JF, CPA | POTS, i + e | 03/98 | / | IX,X,XI | deaf | NED 5 y |
| 33 | 39/F | Meningioma | / | / | 10, 100% | JF, CPA, SS, PPS | POTS, i + e | 06/02 | / | IX,X,XI,XII | deaf | NED 3 y |
| 34 | 30/M | Cholesteatoma | / | / | deaf | JF, A | POTS, e | 01/85 | / | / | / | NED 10 y |
| 35 | 76/M | Cholesteatoma | VI | / | 55, 70% | JF, l, T, CPA | POTS, e | 02/93 | / | / | deaf | NED 10 y |
| 36 | 46/F | Cholesteatoma | / | / | 10, 100% | JF, T, CC | POTS + petrosectomy, e | 06/94 | / | / | 45, 50% | NED 10 y |
| 37 | 61/M | Cholesteatoma | / | / | 45, 50% | JF, M | POTS, e | 12/00 | / | / | deaf | NED 3 y |
| 38 | 40/F | Cholesteatoma | III | / | deaf | JF, M | POTS + TL, e | 08/85 | / | / | / | NED 10 y |
| 39 | 28/M | Cholesteatema | / | / | deaf | JF, M, T | POTS + Tpl, e | 04/98 | / | / | / | NED 5 y |
| 40 | 22/M | Cholest. Granuloma | VI | / | deaf | JF, CPA, A, CC, IAC | POTS, Tp + Graft, i + e | 07/90 | III | / | / | NED 10 y |
| 41 | 40/F | Chordoma | / | IX,X,XII | 27, 100% | JF, CC, Co, T, PPS | POTS, e | 12/90 | / | / | / | DOD 5 y |
| 42 | 16/F | Chordoma | / | IX,X,XI,XII | / | JF, PPS | POTS, e | 06/98 | / | / | / | AWD 8 y |
| 43 | 56/M | Chordoma | / | IX,X,XI,XII | / | JF | POTS, e | 01/96 | / | / | / | DOD 8 y |
| 44 | 47/F | Chordoma | / | IX,XII | / | JF, Cl, Co, A | POTS, e | 12/02 | / | / | deaf | AWD 3 y |
| 45 | 22/F | Chondrosar. G1 | / | / | 19, 100% | JF, A, CC | POTS, e | 11/97 | / | / | / | NED 7 y |
| 46 | 23/F | Chondrosarc. G1 | / | / | 10, 100% | JF, A, Cl, CC | POTS, e | 05/98 | / | / | / | NED 6 y |
| 47 | 60/M | Chondrosarc. G1 | / | IX,X,XI,XII | 50, 40% | JF, PPS | POTS, e | 02/98 | / | / | deaf | DOD 4 y |
| 48 | 54/F | Chondrosarc. G1 | VIth n. / | / | 15, 100% | JF, A,Cl, Co | POTS, e | 12/00 | / | IX,X | deaf | NED 3 y |
| 49 | 36/M | Chondrosarc. G1 | / | / | 30, 90% | JF, Co, CC, PPS | POTS, e | 03/01 | / | / | / | NED 3 y |
| 50 | 37/M | Chondrosarc. G1 | / | IX,X,XI,XII | 45, 70% | JF, Co, Cl, CC | POTS, e | 11/01 | / | / | deaf | NED 3 y |
| 51 | 26/M | Papillary aden. | / | IX,X | deaf | JF, CC | POTS + TL, i + e | 05/95 | / | / | / | NED 7 y |
| 52 | 56/M | Papillary aden. | III | / | deaf | JF, CPA, A | POTS, i + e | 03/97 | V | / | / | NED 5 y |
| 53 | 25/M | Papillary aden. | / | / | deaf | JF, CPA | POTS, i + e | 11/99 | / | / | / | NED 4 y |
| 54 | 61/F | Epithelioid hemangioma | / | IX,X,XI,XII | 35, 70% | JF, Co, C | POTS, e | 07/96 | / | / | 90, 0% | NED 8 y |
| 55 | 24/M | Teratoma | / | / | 60, 90% | JF, PPS | POTS, e | 10/89 | / | / | / | NED 15 y |
| 56 | 32/M | Petrous Cyst | / | / | deaf | JF, A, CC, PPS | POTS, e | 01/92 | / | / | / | NED 10 y |
| 57 | 49/M | Residual Paraganglioma | III | XII | deaf | JF, CPA, Co | POTS, i + e | 09/87 | / | / | / | AWD 15 y |
| 58 | 39/M | Residual Paraganglioma | / | / | 70, 40% | JF,CC, T | POTS, e | 09/88 | / | IX,X,XII | deaf | AWD 15 y |
| 59 | 50/F | Residual Paraganglioma | / | IX,X,XI,XII | 10, 90% | CC, C | POTS, e | 04/89 | / | / | 20, 80% | NED 15 y |
| 60 | 56/M | Residual Paraganglioma | / | IX,X,XI,XII | / | JF | POTS, e | 01/96 | / | IX,X,XII | / | NED 12 y |
| 61 | 62/F | Residual Paraganglioma | III | / | deaf | Co | POTS + TL, e | 04/90 | / | IX,XI | / | NED 12 y |
RESULTS
Complete excision was achieved in all patients except those with chordoma and in two of the five patients with residual paragangliomas. There were no deaths and no untoward effects on the brainstem or central nervous system problems related to the surgery. No CSF leaks developed that required surgical repair, but four patients required a compressive head dressing for a short while.
Transient facial weakness developed in four patients in whom the mastoid segment of the facial nerve had been temporarily lifted from the fallopian canal. Acquired deficits of the lower cranial nerves (Table 3) seemed to be related to the underlying pathological nature of the tumor. Seventeen patients acquired glossopharyngeal nerve deficits, and in 15 patients both the glossopharyngeal and the vagus were lost, mainly in association with resection of meningiomas and schwannomas. An accessory deficit was acquired by nine patients who had surgery for either meningiomas or residual paragangliomas. The hypoglossal nerve palsy was sustained by five patients, again in association with meningioma and residual paraganglioma resections.
Table 3.
Acquired Cranial Nerve Deficits
| Pathology | No. | VII | VIII | IX | X | XI | XII |
|---|---|---|---|---|---|---|---|
| One case each of epithelioid hemang., cholest. granul., teratoma, petrous cyst; no postop losses. | |||||||
| Schwannoma | 17 | 1/2 | 7/8 | 4/9 | 5/10 | 5/6 | 7/7 |
| Schwannoma 7, 8 n | 3 | 3/3 | 2/2 | 0/0 | 0/0 | 0/0 | 0/0 |
| Meningioma | 7 | 0/0 | 4/11 | 0/7 | 0/7 | 0/5 | 0/2 |
| Paraganglioma | 6 | 2/3 | 5/6 | 4/4 | 2/3 | 2/2 | 2/2 |
| Residual paraganglioma | 5 | 2/2 | 3/4 | 1/4 | 1/3 | 1/3 | 2/4 |
| Cholesteatoma | 6 | 2/2 | 4/6 | 0/0 | 0/0 | 0/0 | 0/0 |
| Chordoma | 4 | 0/0 | 0/1 | 3/3 | 3/3 | 3/3 | 3/3 |
| Chondrosarcoma | 6 | 0/0 | 3/6 | 2/3 | 2/3 | 2/2 | 1/2 |
| Papillary adenoma | 3 | 1/1 | 3/3 | 1/1 | 1/1 | 0/0 | 0/0 |
| Total | 57 | 11/13 | 32/47 | 16/32 | 15/31 | 14/22 | 16/21 |
Postoperative deafness was recorded in 17 patients who had either meningioma or chondrosarcoma. Loss of hearing was the result of extensive bone removal around the labyrinth with its consequent interruption of the aqueducts and veins.
Vocal cord augmentation with Teflon paste injection was required in nine patients with glossopharyngeal or vagal palsies. A temporary tracheostomy was necessary in one patient (case 32). Bronchopneumonia developed in four patients and meningitis in another (case 26).
DISCUSSION
Jugular foramen tumors have been one of the major impetuses in the development of skull base surgery. Lying midway between the surface of the head and the center skull, tumors at that site are relatively common and often very extensive. Indeed, some extend from the CPA to the neck and also infiltrate the bone of the skull base.
Transpetrous approaches to the jugular fossa were first described for thrombophlebitis of the sigmoid sinus and jugular bulb.3 Glomus jugulare tumors have been approached either by relatively extensive techniques4,5,6,7,8 or by more conservative procedures.2,9 Over the years, the type A infratemporal fossa type A (IFT-A) approach has become accepted as the approach of choice for most jugular paragangliomas.10
The goal of the POTS approach1 is to preserve the anatomy and function of the facial nerve, external auditory canal, tympanic cavity, and labyrinth. A secondary consideration is to maintain the natural shape of the head. Our experience suggests that whether this is possible is determined by the nature and extension of the underlying tumor. Some lesions can be removed completely using this approach, and others cannot. From an aesthetic standpoint, the natural appearance of the head both in profile and from in front is unchanged. There is a small depression where the mastoid tip was, and this can become more pronounced if there is muscle wasting caused by an accessory deficit. In this respect, the appearance after a POTS approach is certainly different from the depressed profile that develops following an IFT-A approach.
Preservation of function is best considered on the basis of the pathology of the underlying tumor. In jugular foramen schwannomas, no patients incurred hearing loss or facial weakness with the POTS approach. Combined vagal and glossopharyngeal deficits were acquired in three patients but were unavoidable as they were related to the underlying disease process. The other benign lesions that involved the jugular bulb and the bone of the jugular fossa that had an equally good outcome were infralabyrinthine cholesteatoma, cholesterol granuloma, and cystic masses. Similarly, chondrosarcoma and papillary adenocarcinoma of the endolymphatic sac had almost no surgical morbidity and an equally good long-term outcome. That this was so could be attributed to careful surgical planning and the intraoperative control of the lesion that the POTS approach achieved. Patients with meningiomas did not fare as well. Preservation of lower cranial nerve function and hearing acuity was the exception for these patients rather than the rule. In these patients, the jugular fossa is filled with tumor, and both the soft tissues and bone are infiltrated with tumor. Deafness was almost inevitable when the cochlear and/or vestibular aqueducts and veins were included in extensive bone removal around the jugular fossa. The only advantage of POTS approach for this disease seemed the preservation of a normal cranial contour. There were no approach-related difficulties with the resection of meningiomas that extended into the CPA and PPS.
In our opinion, it is most unusual for the POTS approach to be appropriate for resection of a jugulotympanic paraganglioma, as the small number of our cases in this series emphasizes. Our past experience with a conservative procedure similar to this2 resulted in a high rate of residual tumor because of inadequate control of the tympanic bone and the area of bone lying between the fallopian canal and internal carotid artery (ICA). This approach simply does not allow satisfactory removal of bone around the tumor to effect a radical resection. The POTS approach can occasionally be used for C1 class paragangliomas that extend posteriorly into the jugular fossa and CPA but have minimal anterior extension into the tympanic cavity. Tumor infiltration around the carotid canal cannot be addressed; therefore, tumor, whether it is a meningioma or paraganglioma, will almost certainly be left behind. We believe that anterior extension of tumor to involve the petrous carotid artery is a contraindication to this approach. It cannot be emphasized too strongly that it is the histopathological nature of the tumors that dictates the most suitable surgical approach for resection. Tumors can only be resected using the POTS approach if there is a clean and safe margin along the boundaries of the approach—that is, the posterior aspect of the horizontal carotid, the vertical part of the carotid artery below the level of the eustachian tube, and the tympanic bone,. This was only found in patients with a schwannoma, meningioma, chondrosarcoma, or papillary adenocarcinoma.
In this series of patients, no postoperative CSF leak developed in those where a transdural resection was necessary, even those that extended from the CPA to the PPS. To avoid a CSF leak, we closed the dural incision carefully with sutures, obliterating the resection cavity with fat, and deployed the pedicled musculoaponeurotic flap to retain the fat graft and seal the cavity.
CONCLUSIONS
The key feature of the POTS approach is the posterolateral access to the skull base, which gives an oblique route to the jugular foramen. This leaves the external, middle, and inner ear, together with the facial nerve, totally undisturbed. In other words, neither hearing nor facial nerve function is impaired. In addition, the natural contour of the head is preserved. The following anatomical sites are exposed in continuity with the jugular foramen: CPA, occipital condyle, lower clivus, posterior aspect of the ICA, petrous apex, and PPS. Transdural tumors that extend from the CPA to the neck can be removed in a single stage.
This approach is most suitable for removal of jugular foramen schwannomas, infralabyrinthine cholesteatomas, chondrosarcomas of the petroclival junction, and papillary adenocarcinomas of the endolymphatic sac. Meningiomas that extend from the CPA to the PPS can also be resected satisfactorily. The only contraindication is paragangliomas. Only C1 paragangliomas without any significant anterior extension can be removed satisfactorily with the POTS approach and these are uncommon. Our experience with a range of skull base tumors suggests that careful preoperative planning with detailed imaging will help determine whether the POTS approach is appropriate. It is possible to convert the POTS approach to a larger exposure intraoperatively, for example to IFT-A, translabyrinthine, transotic approaches. More general contraindications include the absence of a collateral venous drainage and a contralateral lesion that has caused or might inflict glossopharyngeal or vagal palsies. The POTS approach is a valuable alternative to the IFT-A approach for the mixed group of nonparaganglioma tumors of the jugular foramen.
REFERENCES
- Mazzoni A, Sanna M. A posterolateral approach to the skull base: the petro-occipital transsigmoid approach. Skull Base Surg. 1995;5:157–167. doi: 10.1055/s-2008-1058930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A. Jugulo-petrosectomy. Arch Ital Otol Rinol Laringol. 1974;2:20–25. [Google Scholar]
- Gruenert L. Die operative Ausraumung des Bulbus Venae Jugularis (Bulbusoperation) Arch Ohrenheilk. 1884;36:71–77. [Google Scholar]
- Capps F CW. Glomus jugulare tumors of the middle ear. J Laryngol Otol. 1952;66:302–314. doi: 10.1017/s002221510004771x. [DOI] [PubMed] [Google Scholar]
- Gaillard J, Rebattu J P, Morgan A, Guy F. Note de technique sur la chirurgie des tumeurs glomiques tympano-jugulaires: la dèroutation du nerve facial. J Fr Otorhinolarangol. 1960;9:969–980. [Google Scholar]
- Shapiro M J, Neues D K. Technique for removal of glomus jugulare tumors. Arch Otolaryngol. 1964;79:219–224. doi: 10.1001/archotol.1964.00750030226003. [DOI] [PubMed] [Google Scholar]
- Kempe L G, VanderArk G D, Smith D R. The neurosurgical treatment of glomus jugulare tumors. J Neurosurg. 1971;35:59–64. doi: 10.3171/jns.1971.35.1.0059. [DOI] [PubMed] [Google Scholar]
- Hilding D A, Greemberg A. Surgery for large glomus jugulare tumors: the combined suboccipital, transtemporal approach. Arch Otolaryngol. 1971;93:227–231. doi: 10.1001/archotol.1971.00770060365001. [DOI] [PubMed] [Google Scholar]
- Glasscock M E, Harris P F, Newsome G. Glomus tumors: diagnosis and treatment. Laryngoscope. 1974;84:2006–2032. doi: 10.1002/lary.5540841116. [DOI] [PubMed] [Google Scholar]
- Fisch U. Infratemporal fossa approach for glomus tumors of the temporal bone. Ann Otol Rhinol Laryngol. 1982;91:474–479. doi: 10.1177/000348948209100502. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Sanna M, Saleh E, Achilli V. Lower cranial nerve schwannomas involving the jugular foramen. Ann Otol Rhinol Laryngol. 1997;106:370–379. doi: 10.1177/000348949710600503. [DOI] [PubMed] [Google Scholar]